Abstract
Regulation of autoreactive CD4 T cells is essential to maintain self-tolerance and prevent autoimmune disease. Although CD8 T regulatory (Treg) cells that recognize self-peptides restricted by Qa-1 (HLA-E in humans) inhibit autoreactive CD4 cells and attenuate experimental autoimmune encephalomyelitis (EAE), the mechanism of this interaction is unclear. We generated Qa-1 mutant knock-in mice that impair Qa-1 binding to the T cell receptor (TCR) and CD94/NKG2A receptors. Analysis of these mice showed that TCR-dependent recognition of Qa-1–peptide complexes on target CD4 cells is essential for suppression by CD8 Treg cells. Further analysis revealed that genetic disruption of the Qa-1–CD94/NKG2A interaction unleashes robust CD8 Treg cell activity that completely abolishes development of EAE.
Keywords: autoimmunity, CD8 Treg, suppression
There is considerable evidence that a regulatory sublineage of CD4 cells can inhibit the development of autoimmune disease in several murine models (1). However, less attention has been given to the potential contribution of regulatory sublineages of CD8 cells to the prevention and treatment of autoimmunity. A subpopulation of CD8 cells that recognizes the MHC class Ib product Qa-1 (HLA-E in man) suppresses the development and relapse of experimental autoimmune encephalomyelitis (EAE) in a mouse model of multiple sclerosis (MS) (2, 3), and mice lacking this CD8 subpopulation develop exaggerated immune responses to self- antigen (4).
Analysis of Qa-1–deficient mice has not fully clarified the contribution of Qa-1 to immunologic suppression due to the ability of the Qa-1 molecule to interact with two distinct receptors. First, engagement of the T cell receptor (TCR) by Qa-1–peptide complexes can lead to activation and expansion of antigen-specific CD8 cells. Second, engagement of the CD94/NKG2A receptor expressed by CD8 cells and natural killer (NK) cells by Qa-1/Qdm peptide ligands attenuates the activities of these cells (5–7). Thus, the immune response phenotype of Qa-1-deficient mice reflects these functionally distinct interactions. Diminished interactions between Qa-1-restricted CD8 cells and Qa-1-deficient CD4 cells results in enhanced CD4 responses, whereas increased NK cell lysis of activated CD4 cells reflects a loss of the inhibitory NKG2A–Qa-1/Qdm interaction.
We isolated Qa-1-dependent engagement of the TCR and NKG2A for analysis and exploited the fact that Qa-1-dependent binding to each receptor used distinct Qa-1 contact residues. We thus generated knock-in mice that express a Qa-1 amino acid exchange mutation (Qa-1 D227K) that disrupts binding of Qa-1 to the CD8 coreceptor (8). Cells that express this Qa-1 D227K point mutation fail to present Qa-1-restricted peptides to CD8 cells but retain the ability to engage NKG2A receptors on CD8 cells and NK cells. A second Qa-1 knock-in strain (Qa-1 R72A) expresses a Qa-1 aa exchange mutation (R72A) that fails to bind to NKG2A receptors on NK cells and CD8 cells but spares Qa-1-dependent peptide presentation to the TCR (7). Expression of this Qa-1 aa exchange mutant by CD4 cells renders them vulnerable to NK and CD8 lysis. These two Qa-1 point mutations were backcrossed to C57BL/6 mice for analysis of CD8 T regulatory (Treg) cell activity.
We report that engagement of Qa-1 by the CD8 coreceptor is essential for Qa-1-restricted Treg activity, as indicated by findings that Qa-1 D227K knock-in mice fail to develop regulatory CD8 activity and display enhanced proteolipid protein (PLP)-induced EAE. Analysis of Qa-1 R72A knock-in mice revealed that genetic disruption of the NKG2A–Qa-1 interaction releases the brakes on CD8-dependent suppressive activity, allowing the development of robust CD8+ Treg activity, which results in complete resistance to the development of EAE.
Results and Discussion
Analysis of B6.Qa-1 D227K and R72A Knock-In Mice.
A genomic 4-Kb Qa-1 fragment containing a D → K amino acid exchange mutation at position 227 after site-directed mutagenesis (Fig. 1A) was cloned into a replacement vector and transfected into the TC1 ES cell line. After injection of positive homologous recombinant clones into blastocysts to produce germline chimeras (Fig. 1B), and deletion of the Neor gene after crossing to B6-EIIα-CRE mice, progeny were backcrossed to C57BL/6 (B6) for seven generations and intercrossed to produce homozygous B6.Qa-1 D227K mutant mice. Expression of cell surface Qa-1 by activated (ConA-stimulated) CD4 cells from B6.Qa-1 D227K knock-in mice was indistinguishable from Qa-1 WT T cells (Fig. 1C). Expression of the Qa-1 D227K mutation by L cells (Fig. 1D, Left) or activated CD4 cells (Fig. 1D, Right) failed to target these cells for lysis by Qa-1-restricted cytolytic T cells (CTLs). Qa-1/Qdm-dependent resistance of activated B6.Qa-1 D227K CD4 cells to lysis by NKG2A+ NK cells was unimpaired in vitro (Fig. 1E, Left) and in vivo, as judged by homeostatic expansion in Rag-2−/− hosts (Fig. 1E, Right).
Fig. 1.
Generation and analysis of Qa-1 D227K mutant knock-in mice. (A) Qa-1 genomic locus and targeting strategy. Boxes represent exons; exon 4 (gray box) indicates the mutation site. The loxP sites are represented by black triangles. TK, thymidine kinase gene; neor, neomycin-resistance gene. (B) Southern blot analysis of ES cell genomic DNA. The upper band (12.5 kb) corresponds to the WT allele; the lower band (7.2 kb) represents the knock-in allele. Right: PCR genotyping of knock-in mice. WT (280 bp) and knock-in (430 bp) products represent the addition of base pairs from the remaining loxP site and surrounding sequence (WT/WT, Qa-1 WT; DK/DK, homozygous mutant knock-in mice; WT/DK, heterozygous). (C) ConA-induced Qa-1 expression of WT and D227K mutant. Splenocytes from littermates (Qa-1-deficient [KO], Qa-1 WT [WT], and Qa-1 D227K knock-in [D227K]) were individually stimulated with ConA for 40 h and analyzed for surface Qa-1 expression by FACS analysis using anti-Qa-1 Ab (BD Bioscience). (D) L cells transfected with GFP, WT Qa-1 or Qa-1 D227K, and ConA blasts from WT or Qa-1 D227K mice were used as targets in a CTL assay by CD8 cells from B6.Tla mice immunized with Qa-1 ConA blasts. Percent lysis is shown at the indicated E:T ratios. (E) Left: NK cell susceptibility of Qa-1 R72A mutant CD4 cells. CD4+ T cells from Qa-1 WT, Qa-1-deficient, Qa-1 D227K, and Qa-1-R72A mutant mice were activated by ConA for 48 h; labeled with 51Cr; and used as targets for IL-2-activated NKG2A+ NK cells in a standard 4-h killing assay. Percent lysis at an E:T ratio is shown. Data shown represents mean ± SD. (n = 3). Right: Homeostatic expansion of Qa-1 mutant CD4 cells. A total of 106 CD4+ T cells isolated from Qa-1 WT, Qa-1-deficient, Qa-1 D227K, and Qa-1 R72A mice were transferred into syngeneic Rag2−/− hosts. Fourteen days later, CD4+ T cells from spleen and lymph nodes were enumerated. Data, shown as mean ± SD, represent one of two independent experiments (n = 3). (F) CD4 T cells from OT-2 TCR-transgenic B6 mice activated with ConA were used to vaccinate B6 mice. Fourteen days later, purified CD8 T cells (1.5 ×106) from OT-2-immunized mice were transferred with OT-2 CD4 T cells (from Qa-1 WT, Qa-1−/−, Qa-1 D227K, and Qa-1 R72A; 1 × 106) into Rag2−/−Prf1−/− mice before challenge with OVA peptide (50 μg) in CFA. Expansion of OT-2 T cells was quantitated by enumerating OT-2 cells in both lymph nodes and spleen 2 weeks after transfer. Data are representative of two independent experiments (n = 3).
Cells from B6 Qa-1 knock-in mice that expressed the Qa-1 R72A mutation were unable to bind to NKG2A and, as a consequence, displayed increased susceptibility to NK lysis in vitro (Fig. 1E, Left) and failed to expand in Rag-2−/− hosts in vivo (Fig. 1E, Right). Taken together, these observations indicate that the interaction of Qa-1 with the TCR–CD8 complex can be experimentally separated from its interaction with NKG2A using B6.Qa-1 D227K and B6.Qa-1 R72A knock-in mice.
We next monitored the effect of the Qa-1 D227K point mutation on the inhibitory interaction between CD8 Treg cells and CD4 Th cells (4). CD8 cells obtained from B6 mice immunized with irradiated activated OT-2 CD4 T cells 2 weeks earlier were transferred along with OT-2 CD4 cells into Rag2−/−Prf1−/− hosts immunized with OT-2 peptide. Expansion of Qa-1 WT and Qa-1 R72A OT2+ CD4 cells was substantially inhibited by CD8 cells, whereas expansion of D227K OT-2 cells was unimpaired (Fig. 1F). These findings indicate that the Qa-1-restricted suppressive interaction between CD8 and CD4 cells requires CD8 coreceptor binding to the Qa-1 molecule on CD4 cells, but not binding to the NKG2A receptor.
Enhanced Susceptibility of Qa-1 D227K Knock-In Mice to EAE.
As noted previously (4), preimmunization of mice with myelin oligodendrocyte glycoprotein (MOG) peptide before induction of EAE with MOG/complete Freund's adjuvant (CFA) and pertussis toxin induces CD8+ Treg cells, which inhibit the development of EAE (Fig. 2A). In contrast, preimmunization of B6.Qa-1 D227K mice with MOG peptide is followed by robust disease development (Fig. 2A). Moreover, CD4 cells from B6.Qa-1 D227K mice, but not from B6 control littermates, mediate vigorous anti-MOG recall responses, as indicated by production of the IFN-γ and IL-17 proinflammatory cytokines (Fig. 2B). Enhanced IFN-γ/IL-17 responses were not accompanied by diminished levels of inhibitory cytokines, including IL-10 and IL-4, which were not detectable in these supernatants (not shown).
Fig. 2.
MOG-induced EAE in Qa-1 D227K mice. (A) EAE was induced into WT (n = 5) and D227K (n = 5) mice by injecting 100 μg of MOG in CFA (50 ng of MTb) with pertussis toxin on days 0 and 2. The mice were reimmunized with 100 μg of MOG in CFA on day 7. EAE disease score was determined as described in Methods. (B) Recall response. At the end of the EAE experiments, spleen and lymph node cells were pooled from three mice in each group, and the cells were restimulated with the indicated doses of MOG peptides in vitro. IFN-γ and IL-17 production was measured by ELISA using 48 h culture supernatants. Data shown are representative of two independent experiments. (C and D) Effects of preimmunization in PLP-induced EAE development. B6 Qa-1 WT and B6 Qa-1 D227K mice were preimmunized with PLP peptide (20 μg) in CFA and boosted 10 days later with PLP peptide in CFA plus pertussis toxin. Clinical score (C, Left) and incidence (C, Right) were determined after the boost (time, horizontal axes; 5–8 mice per group). (D) Lymph nodes and spleen were harvested from mice at the end of the EAE experiments, and single-cell suspensions were prepared; 4 × 105 pooled draining lymph node and splenic cells were restimulated in vitro with the indicated concentrations of PLP peptide. Production of IFN-γ was measured 48 h after culture. Data shown represent two independent experiments.
We then investigated whether the Qa-1 D227K mutation prevented the development of CD8 Treg cells to a second self-antigen, PLP. Preimmunization of B6 mice with PLP peptide (without pertussis toxin) induces PLP-specific CD8+ Treg cells, which inhibit EAE on a subsequent challenge with PLP/CFA and pertussis toxin (4). In contrast, preimmunization of B6.Qa-1 D227K littermates with PLP/CFA failed to prevent the development of severe EAE on challenge with PLP/CFA plus pertussis toxin (Fig. 2C). Loss of protection by Qa-1 D227K knock-in mice was associated with substantially increased anti-PLP IFN-γ responses by CD4 cells on challenge with PLP (Fig. 2D). In summary, expression of the Qa-1 D227K point mutation that prevents Qa-1-restricted recognition by CD8 cells results in markedly enhanced CD4 response to PLP self-antigens and the consequent development of EAE.
Suppression by CD8+ Treg Cells Is Attenuated by Engagement of NKG2A.
In contrast to B6 mice, which express the Qa-1 D227K mutation, B6.Qa-1 R72A knock-in mice were completely protected from induction of EAE by preimmunization with PLP. Protection was accompanied by markedly reduced anti-PLP IFN-γ responses on restimulation of CD4 cells in vitro (Fig. 2D), suggesting that engagement of Qa-1/Qdm on CD4 cells by NKG2A on CD8 Treg cells impairs their suppressive activity.
To directly test this hypothesis, we investigated the susceptibility of MOG-immune CD4 cells expressing the R72A mutation to Qa-1-restricted inhibition by CD8+ Treg cells in adoptive Rag2−/−Prf1−/− hosts. Because activated CD4 T cells that fail to engage inhibitory NKG2A receptors on NK cells are also susceptible to NK cell lysis (7), we eliminated NK activity using adoptive Rag2−/−Prf1−/− hosts. Cotransfer of CD8 cells with MOG-immune Qa-1 WT CD4 cells into Rag2−/−Prf1−/− hosts resulted in modest inhibition of EAE (Fig. 3A, Left). In contrast, transfer of EAE by MOG-immune CD4 cells from R72A donors was completely abolished by cotransfer of CD8 cells, despite the fact that R72A CD4 cells alone induced EAE levels that were at least as robust as those induced by CD4 cells from Qa-1 WT littermate donors (Fig. 3B, Left). Analysis of the in vitro recall response to MOG at 3 weeks revealed that cotransfer of CD8 cells with R72A CD4 cells virtually abolished the anti-MOG recall response, as indicated by IFN-γ secretion (Fig. 3 A and B, Right).
Fig. 3.
MOG-induced EAE development in Qa-1 R72A mice. Left: CD4 cells were purified from MOG-immunized EAE mice [WT (A) and R72A (B)] and transferred into Rag2−/−Prf1−/− hosts with or without CD8 cells from the EAE mice. Mice were then immunized with MOG peptide with pertussis toxin, and the development of EAE was monitored daily. Data shown represent two independent experiments (n = 5). Right: Draining lymph nodes and spleens were collected 35 days after EAE induction. Single-cell suspensions were pooled and stimulated with the indicated concentrations of MOG peptide. IFN secretion was measured by ELISA after 48 h of culture. To ensure complete removal of NK cells from donor T cell populations, NK cells were depleted as described in Methods. (C–E) 2D2-induced EAE. (C) 2D2 CD4+ T cells (106) enriched from Qa-1 WT and R72A 2D2 TCR-transgenic mice were transferred into syngeneic Rag2−/−Prf1−/− hosts (n = 5) with or without CD8 Treg cells generated as described in Methods. Hosts were then immunized with 10 μg of MOG/CFA and 200 μg of pertussis toxin (on days 0 and 2). Development of EAE was scored daily as described in Methods. (D) In vivo suppression of R72A 2D2 expansion by CD8 regulatory cells. 2D2 CD4+ T cells (106) from Qa-1 WT or R72A mice were transferred into syngeneic Rag2−/−Prf1−/− hosts (n = 3) with or without CD8 Treg cells generated as described in Methods. Hosts were then immunized with 10 μg of MOG/CFA. 2D2 CD4 T cells in the draining lymph nodes and spleens were enumerated after 14 days. Data are shown as mean ± SD (n = 3). (E) Single-cell suspensions were prepared from draining lymph nodes collected as described above and pooled according to each group. The lymph node cells were stimulated with the indicated concentrations of MOG peptide in the presence of irradiated splenocytes as antigen-presenting cells. IL-2 secretion was measured by ELISA 48 h after culture.
A more stringent test of the affect of the Qa-1 R72A mutation on the susceptibility of CD4 cells to CD8 Treg cell activity was based on an analysis of the ability of CD8 cells to inhibit CD4 cells that express the MOG-specific 2D2 TCR transgene. Transfer of 2D2+ CD4 cells into Rag2−/− Prf1−/− hosts initiates a progressive and lethal form of EAE that results in death from fulminant disease within 14–16 days after transfer (Fig. 3C). If CD8-dependent suppression of 2D2+ CD4 cells is normally attenuated by an inhibitory interaction between Qa-1/Qdm expressed by CD4 target cells and NKG2A receptors expressed by CD8 Treg cells, then genetic disruption of this interaction might be expected to suppress virulent EAE. Transfer of 106 2D2+ CD4 cells (five times the lethal dose) from B6 or B6 Qa-1 R72A donors into Rag2−/−Prf1−/− hosts provoked lethal EAE by day 15 (Fig. 3C, Left); however, cotransfer of CD8+ Treg cells completely abrogated disease induced by R72A CD4 cells, whereas cotransfer of CD8+ Treg cells had no effect on Qa-1 WT CD4 cells (Fig. 3C, Right).
We next determined whether abolition of EAE development by cotransfer of CD8 cells was associated with suppression of 2D2+ CD4 T cell expansion in Rag2−/−Prf1−/− hosts. Expansion of R72A 2D2 CD4 T cells was almost completely (> 95%) suppressed by cotransfer of CD8 cells, whereas expansion of Qa-1 WT 2D2 CD4 T cells was reduced only slightly by MOG-immune CD8 T cells (Fig. 3D). Moreover, a residual amount (5%) of Qa-1 R72A 2D2+ CD4 cells displayed almost no response to MOG in vitro (Fig. 3E). Thus, failure of EAE development is attributed to CD8-dependent suppression of the anti-MOG response of 2D2+ CD4 cells bearing the MOG-specific TCR.
Interruption of the Qa-1–NKG2A Interaction Unmasks Potent CD8+ Treg Cell Activity In Vitro.
The analysis summarized in Fig. 3 suggests that CD4 T cells expressing a Qa-1 point mutation that impairs engagement of NKG2A expressed by CD8+ Treg cells releases the brakes on suppressive activity inflicted by CD8+ Treg cells. Analysis of the interaction between Qa-1 R72A mutant CD4 cells and CD8 Treg cells in vitro revealed that the MOG-specific response of Qa-1 R72A 2D2+ CD4 T cells was highly susceptible to dose-dependent suppression by CD8 Treg cells in vitro, as indicated by diminished proliferation and IL-2 secretion. In contrast, CD4 cells bearing the Qa-1 D227K mutation were fully resistant to CD8 Treg cell activity (Fig. 4A). The dependence of CD8 Treg cell activity on Qa-1 recognition was confirmed by the finding that anti-Qa-1 Ab blocked CD8-dependent suppression of the CD4 cell IL-2 response (Fig. 4B).
Fig. 4.
Susceptibility of Qa-1 R72A 2D2 cells to CD8+ Treg cells. CD8 cells were purified from the draining lymph nodes of R72A 2D2 and CD8 suppressor cell cotransferred mice. 2D2 CD4 T cells were isolated from Qa-1 WT, D227K, and R72A mice and stimulated in vitro with different doses of MOG peptide in the presence of irradiated splenocytes as antigen-presenting cells with or without purified CD8 suppressor cells. (A) Proliferation of CD4 T cells was measured 60 h later by 3H-thymidine incorporation. Different CD4:CD8 ratios were used in similar experiments, and proliferation of CD4 T cells on stimulation with 30 μg of MOG peptide was measured (Lower). (B) IL-2 secretion was measured by ELISA 48 h after culture; relative IL-2 secretion is shown (normalized to no CD8 control cultures). To confirm specificity of Qa-1-restricted suppression, anti-Qa-1 Ab was included in one group at a concentration of 10 μg/ml. The data shown represent three independent experiments.
Perforin Expression Contributes to Suppressive Activity.
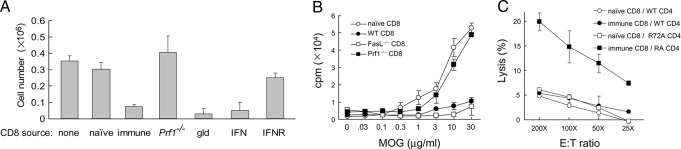
We tested the contribution of perforin and Fas ligand to Treg-mediated suppression. CD8 T cells from immunized perforin-deficient (Prf1−/−) or Fas ligand-deficient generalized lymphoproliferative disease (FasL−/−) mice were cotransferred with Qa-1 R72A 2D2 CD4 T cells. Expansion of R72A 2D2 CD4 T cells was inhibited by FasL−/− CD8 cells but not by Prf1−/− CD8 cells.
The requirement for perforin (but not Fas ligand) for CD8+ Treg cell activity also was noted in vitro. CD8 T cells from immunized Prf1−/− mice were unable to inhibit the response of R72A 2D2+ CD4 cells, in contrast to CD8 T cells from FasL−/− mice (Fig. 5B). Although Qa-1 R72A CD4 cells may be lysed by CD8 cells from immunized mice (Fig. 5C), these observations do not rule out a nonlytic mechanism of suppression. Indeed, a cardinal feature of “suppressed” CD4 cells is their failure to express effector (Th1) cytokines, including IFN-γ, on stimulation with antigen.
Fig. 5.
(A) Suppression of Qa-1 R72A 2D2 cell expansion by CD8 Treg cells requires perforin. First, 2D2 CD4+ T cells (106) from Qa-1 R72A mice were transferred into syngeneic Rag2−/−Prf1−/− hosts (n = 3) with CD8 suppressor cells generated in B6 mice or mice deficient in perforin and Fas ligand. Hosts were then immunized with 10 μg of MOG/CFA. 2D2 CD4 T cells in draining lymph nodes and spleen were enumerated after 14 days. Data are shown as mean ± SD (n = 3). (B) In vitro suppression of Qa-1 R72A 2D2 CD4 cell response by CD8 Treg cells. CD8 cells were purified from the draining lymph nodes of Qa-1 R72A 2D2 and CD8 suppressor cells cotransferred into mice. 2D2 CD4 T cells were isolated from Qa-1 R72A mice and stimulated in vitro with different doses of MOG peptide in the presence of irradiated splenocytes as antigen-presenting cells with or without different purified CD8 suppressor cells, as indicated. Proliferation of CD4 T cells was measured 60 h later by 3H-thymidine incorporation. Data shown represent two independent experiments. (C) In vitro lysis of Qa-1 R72A CD4 targets by CD8 Treg cells from 2D2-immunized mice. Qa-1 WT and Qa-1 R72A 2D2 CD4 T cells were activated by MOG peptide for 48 h and used as target cells in a lysis assay by CD8 T cells purified from OT2-immunized mice. CD8 cells from naïve B6 mice were used as controls. Percent lysis is shown at the indicated E:T ratios.
Although the regulatory lineage of CD4+ T cells has been the object of intense study, regulatory cells within the CD8+ lineage have received much less attention. Analyses of normal and Qa-1-deficient mice have defined a subpopulation of CD8 cells that mediate Treg activity through recognition of the class Ib MHC molecule Qa-1 expressed on activated CD4 cells. The interactions between CD8+ Treg cells and target CD4 cells that regulate this process are not well understood, however. The present study establishes that this inhibitory interaction requires CD8 corecognition of peptides complexed to the Qa-1 MHC molecule expressed by activated target CD4 cells. These findings also provide new insight into the molecular constraints that dampen CD8+ Treg cell activity.
We find that Qa-1/Qdm engagement of CD94/NKG2A on CD8+ Treg cells transmits an inhibitory signal that attenuates CD8 Treg suppressive activity. The CD94/NKG2A receptors belong to the inhibitory NK receptor family (iNKRs) that inhibits cellular activation through dephosphorylation of signaling molecules. Although the CD94 chain can pair with other members of the NKG2 family, including NKG2C and NKG2E (9), the high levels of expression and strong binding affinity of CD94/NKG2A for Qa-1/Qdm dominate binding. As a result, engagement of CD94/NKG2A receptors expressed by activated CD8 cells during viral infection down-regulates cytotoxicity and production of proinflammatory cytokines (6, 10). Although only a small proportion of CD8 cells express NKG2A according to immunofluorescence with available antibodies, most CD8 cells specifically bind Qa-1/Qdm tetramers and this may account for the substantial affect of NKG2A ligation (10).
Autoreactive CD4 cells that express the Qa-1 R72A point mutation fail to transmit an inhibitory signal through NKG2A to CD8+ Treg cells. As a result, these CD4 cells received maximal inhibitory signals from CD8 Treg cells and failed to induce EAE. These findings suggest that releasing the brakes on CD8 Treg activity by blockade of NKG2A with Ab may represent a new CD8+ Treg-based approach to ameliorating autoimmune disease.
The timing of CD8+-dependent inhibitory responses can be contrasted with that of naturally occurring CD4+CD25+ Treg cells, which interrupt expansion of self-reactive T cells during the initial stages of primary responses. Regulatory CD8+ T cells arise later in the immune response and become apparent experimentally only after restimulation by antigen (11). The development of Qa-1-restricted Treg activity more closely resembles the kinetics of CD8+-dependent memory/effector activity rather than CD4+CD25+ regulatory activity. CD8+-dependent suppressive and memory/effector responses both require primary immunization to generate TCR-dependent lysis or Qa-1-restricted inhibition of target cells (4, 12).
These data also suggest that the relative ratio of the two classes of Qa-1 peptide ligand— Qdm peptide versus non-Qdm (e.g., HSP60) peptide—on the surface of activated CD4 cells may determine the susceptibility of these CD4+ T cells to suppression by CD8+ Treg cells. According to this view, engagement of NKG2A on CD8+ Treg cells by the Qdm/Qa-1 ligand on target CD4 cells inhibits Treg activity, whereas engagement of the TCR expressed by CD8+ Treg cells by Qa-1 peptide ligands, such as HSP60 peptides (13), activates suppressive activity. Because the majority of murine CD8+ T cells bind to Qa-1/Qdm tetramer, differential expression of Qa-1/Qdm and Qa-1/HSP60 peptide ligands on activated Qa-1 CD4 target cells may determine the cell's fate.
Although MS is characterized by a clinical sequence of exacerbations followed by remissions, the clinical course is generally progressive. Analysis of disease in a murine model of MS (i.e., EAE) has begun to define the immunologic mechanisms responsible for this process. Immunologic susceptibility to disease and disease remission depends in part on inhibition of pathogenic autoreactive CD4 T cells by CD8+ Treg cells, resulting in decreased tissue destruction and inhibition of disease progression (4, 7, 14, 15). These findings provide the basis for development of new therapeutic approaches to autoimmune diseases, including MS. Although current approaches have generated CD8+ Treg cell activity before disease induction (16, 17), these cells are not potent enough to shut down disease progression.
Recent analysis of T cells from MS patients also indicates that CD94/NKG2A inhibitory receptors expressed by CD8 Treg cells restrain their HLA-E-restricted suppressive activity, and that these HLA-E-restricted CD8 Treg cells cloned from patients during disease exacerbation express higher levels of CD94/NKG2A compared with CD8 cells cloned from MS patients in remission or CD8 cells obtained from healthy controls (18). Moreover, Ab-dependent blockade of the HLA-E–CD94/NKG2A interaction enhances lysis of autoreactive CD4 T cell clones by HLA-E-restricted CD8 cells from MS patients (18), consistent with our findings using a murine model of MS. Additional studies are currently underway using combination therapy of CD8+ Treg cells with blocking anti-NKG2A Ab to treat ongoing MOG-induced EAE in B6 mice, in an effort to increase the therapeutic potential of this approach to MS.
Methods
Animals and Reagents.
C57BL/6 (B6) and Rag2−/−Prf1−/− (perforin) mice were purchased from Taconic. Qa-1 D227K and Qa-1 R72A mice were generated as described previously and backcrossed onto B6 for eight generations. OT-2 TCR-transgenic mice (provided by H. Ploegh, MIT) and 2D2 TCR-transgenic mice that recognize the MOG autoantigen (19) were crossed with Qa-1 D227K and Qa-1 R72A mice. Mice were housed in a specific pathogen-free, viral Ab-free animal facility at the Dana-Farber Cancer Institute. All experiments were done in compliance with federal laws and institutional guidelines and were approved by the DFCI Animal Care and Use Committee.
Peptides MOG 35–55 (MEVGWYRSPFSRVVHLYRNGK), OVA 323–339 (ISQAVHAAHAEINEAGR), and PLP 172–183 (PVYIYFNTWTTC) were synthesized by New England Peptide. Mouse CD4 and CD8 T lymphocyte enrichment kits, ELISA kits for cytokines, and other purified antibodies were purchased from BD Biosciences.
Generation of Qa-1 Mutant Knock-In Mice.
A BAC clone containing a 11-kb DNA fragment including the Qa-1 gene was identified and fully mapped. A 4-kb fragment containing exons 1–5 of Qa-1 (from NdeI to AvrII) was cloned into a cloning vector, and the mutation of D227 to K was introduced by site-directed mutagenesis. After confirmation by sequencing, the mutated DNA fragment was cloned into the SalI site of pLNTK (20). The 2.3-kb short arm (AvrII to NdeI) was cloned into the XhoI site of pLNTK to complete the replacement vector. The targeting vector was linearized by NotI and used for ES cell electroporation. After TC1 ES cells were transfected with targeting vector, positively selected recombinants were identified by long-range PCR screening and Southern blot analysis. The Neor gene was deleted by crossing germline-transmitted litters to EIIα-CRE mice, followed by backcrossing to B6 mice for eight generations. Homozygous Qa-1-D227K mutant mice were obtained by intercrossing heterozygous littermates.
NK Cell Purification and NK Cytotoxic Assay.
To purify NKG2A+ NK cells, splenocytes from Rag2−/− mice were incubated with biotinylated NKG2AB6 mAb (eBioscience), followed by incubation with antibiotin Ab-coated magnetic microbeads (Mytech) before cell separation using magnetic-activated cell sorting. Purified NKG2A+ NK cells were cultured in RPMI 1640 complete medium with 10% FCS and 1000 U/ml of human recombinant IL-2 (BD Biosciences) for 5 days. Then 106 target cells were labeled with 100 μCi of Na2(51Cr)O4 for 1 h at 37 °C. After three washes with PBS, 104 target cells were mixed with NK cells at different E:T ratios and incubated for 4 h. Cell-free supernatants were collected, and their radioactivity was measured with a Wallac Microbeta counter. The percentage of lysis was calculated by the following formula: (sample release − spontaneous release)/(maximum release − spontaneous release) × 100%.
Preimmunization with Peptides and Induction of EAE.
To analyze EAE resistance, mice were injected s.c. on the back with 10–25 μg of PLP peptide in CFA without pertussis toxin. Fourteen days later, the mice were immunized s.c. with 150 μg of PLP peptide and 200 μg of killed Mycobacterium tuberculosis (H37ev) in CFA on the flanks. In addition, 200 ng of pertussis toxin was injected i.p. on days 0 and 2. Mice were monitored daily and scored from 0–5: 0, normal mouse with no sign of disease; 1, limp tail; 2, limp tail and partial hind limb weakness; 3, complete hind limb paralysis; 4, complete hind limb and partial front limb paralysis; or 5, moribund state or death.
Induction of EAE by MOG Peptide Immunization.
For active EAE induction, WT and Qa-1 D227K mice were immunized s.c. at two sites on the flank with a total of 100 μg of MOG35–55 peptide emulsified in an equal volume of CFA containing 1 mg/ml of M. tuberculosis on days 0 and 7. The mice also received i.p. injections of 200 ng of pertussis toxin on days 0 and 2 after immunization.
Generation of CD8 Suppressor Cells and In Vivo Suppression Assay.
CD4 T cells were purified and activated in vitro by ConA (1 μg/ml) or peptide antigen for 2 days before irradiation (3,000 rads) and injection into B6 mice (2 × 106 cells i.v.). After 14 days, CD8 T cells purified from the spleen were adoptively transferred into Rag2−/−Prf1−/− mice along with purified target CD4 T cells. Adoptive hosts were challenged with antigen peptide (50 μg) in CFA, and the expansion of CD4 T cells and CD8 cells was analyzed 14 days later. CD8+ Treg cells also were generated after injection of 106 2D2+ TCR-transgenic CD4 cells i.v. into B6 mice, followed by immunization with 50 μg of MOG peptide to activate and expand 2D2 cells. CD8 cells were purified 14 days later from both draining lymph nodes and spleen and tested for suppressive activity against 2D2 CD4 T cells in Rag2−/−Prf1−/− hosts, as described above. CD8 cells expressing suppressive activity against polyclonal MOG-reactive CD4 T cells were purified from draining lymph nodes of MOG-induced EAE mice.
Adoptive CD4 T Cell Transfer and Induction and Assessment of EAE.
CD4 T cells (106) from either MOG-immunized mice or 2D2 TCR-transgenic mice were transferred i.v. into Rag2−/−Prf1−/− hosts with or without CD8 suppressor cells (1.5 × 106). Because incomplete removal of NK cells from donor T cell populations obtained after CD4 or CD8 enrichment can affect expansion of Qa-1−/− CD4 T cells in adoptive hosts (7), we depleted NK cells from donors of CD4 or CD8 cells by injecting anti-NK1.1 Ab (200 μg/mouse, i.v.) 48 h before harvesting draining lymph nodes and spleen and treated again with anti-NK1.1-coated beads in vitro to ensure depletion of NK cells. EAE was induced by s.c. immunization with MOG peptide emulsified in CFA supplemented with 4 mg/ml of M. tuberculosis in the flanks. A total of 150 μg of peptide was used for polyclonal MOG-immunized CD4 transfer, and only 10 μg of peptide was used for 2D2 CD4 transfer. Mice were injected i.p. on days 0 and 2 with 200 ng of pertussis toxin. Clinical assessment of EAE was performed daily and scored as described above.
In Vitro T Cell Stimulation and Suppression Assay.
CD4 T cells were purified from draining lymph nodes of immunized mice or TCR-transgenic mice (2 × 104 to 1 × 105 cells/well) and cultured with Ag and irradiated splenocytes from B6 mice (4 × 105 cells/well) in RPMI 1640 complete medium, 10% FCS, and 50 μM β-mercaptoethanol. Proliferation was measured by [3H]thymidine incorporation (1 μCi/well) during the last 18 h of culture. To test the suppressive activity of CD8 Treg cells, CD8 T cells were titrated into cultures at different CD4:CD8 ratios before supernatants were collected at 48 h and cytokine concentrations determined by ELISA (BD PharMingen).
Generation of CTLs and In Vitro Lysis.
To generate anti Qa-1 alloreactive CTLs, CD4 T cells were purified from B6 mice and activated in vitro by ConA (1 μg/ml) for 2 days before irradiation (3,000 rads) and injection into B6.Tla mice (2 ×106 cells i.v.). Fourteen days later, 5 × 106 purified CD8 T cells from spleen were restimulated in vitro with 107 irradiated ConA-activated B6 lymphocytes with 20 U/ml of IL-2 in RPMI 1640 complete medium, 10% FCS, and 50 μM β-mercaptoethanol. Cytotoxic activity was measured after 5 days of culture. First, 106 target cells in 100 μl of RPMI 1640 complete medium were labeled with 100 μCi of Na2(51Cr)O4 for 1 h at 37 °C and washed three times with PBS. After resuspension in RPMI 1640 complete medium, 104 labeled target cells were mixed with effector cells at different E:T ratios (5:1, 10:1, and 20:1) in triplicate and then incubated for 4 h at 37 °C. Cell-free supernatants were collected, and their radioactivity was measured with a Wallac Microbeta counter. The percentage of lysis was calculated by the following formula: (sample release − spontaneous release)/(maximum release − spontaneous release) ×100%.
Acknowledgments.
This work was supported by grants from the National Institutes of Health (AI 37562) and the National Multiple Sclerosis Society and a gift from the Schecter Family Research Foundation (to H.C.). L. L. is a Claudia Adams Barr Investigator and NRSA Fellow (T32 AI07386). We thank V. K. Kuchroo for providing the 2D2 TCR transgenic mice, D. Laznik and X. Sun for providing technical assistance, and A. Angel for assisting with manuscript and graphics preparation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Sakaguchi S, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 2.Noble A, Zhao Z-S, Cantor H. Suppression of immune responses by CD8 cells, II: Qa-1 on activated B-cells stimulates CD8 cell suppression of Th2-dependent antibody responses. J Immunol. 1998;160:566–571. [PubMed] [Google Scholar]
- 3.Jiang H, Chess L. An integrated view of suppressor T cell subsets in immunoregulation. J Clin Invest. 2004;114:1198–1208. doi: 10.1172/JCI23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu D, et al. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 5.Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b) J Exp Med. 1998;188:1841–1848. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8(+) T cell responses. Nat Immunol. 2002;3:189–195. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- 7.Lu L, et al. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luescher IF, et al. CD8 modulation of T-cell antigen receptor-ligand interactions on living cytotoxic T lymphocytes. Nature. 1995;373:353–356. doi: 10.1038/373353a0. [DOI] [PubMed] [Google Scholar]
- 9.Vivier E, Anfossi N. Inhibitory NK-cell receptors on T cells: Witness of the past, actors of the future. Nat Rev Immunol. 2004;4:190–198. doi: 10.1038/nri1306. [DOI] [PubMed] [Google Scholar]
- 10.Wang R, Ramaswamy S, Hu D, Cantor H. Definition of a novel binding site on CD8 cells for a conserved region of the MHC class Ib molecule Qa-1 that regulates IFN-gamma expression. Eur J Immunol. 2001;31:87–93. doi: 10.1002/1521-4141(200101)31:1<87::aid-immu87>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+ suppressor T cells. J Clin Invest. 2004;114:1218–1221. doi: 10.1172/JCI23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang H, Chess L. An integrated model of immunoregulation mediated by regulatory T cell subsets. Adv Immunol. 2004;83:253–288. doi: 10.1016/S0065-2776(04)83008-6. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, et al. Perceiving the avidity of T cell activation can be translated into peripheral T cell regulation. Proc Natl Acad Sci U S A. 2007;104:20472–20477. doi: 10.1073/pnas.0709878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H, et al. Regulatory CD8+ T cells fine-tune the myelin basic protein-reactive T cell receptor V beta repertoire during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2003;100:8378–8383. doi: 10.1073/pnas.1432871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu L, Werneck MB, Cantor H. The immunoregulatory effects of Qa-1. Immunol Rev. 2006;212:51–59. doi: 10.1111/j.0105-2896.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, et al. T cell vaccination induces TCR Vβ-specific, Qa-1-restricted regulatory CD8+ T cells. Proc Natl Acad Sci U S A. 1998;95:4533–4537. doi: 10.1073/pnas.95.8.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Correale J, et al. T cell vaccination in secondary progressive multiple sclerosis. J Neuroimmunol. 2000;107:130–139. doi: 10.1016/s0165-5728(00)00235-6. [DOI] [PubMed] [Google Scholar]
- 18.Correale J, Villa A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol. 2008;195:121–134. doi: 10.1016/j.jneuroim.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorman JR, et al. The Ig(kappa) enhancer influences the ratio of Ig(kappa) versus Ig(lambda) B lymphocytes. Immunity. 1996;5:241–252. doi: 10.1016/s1074-7613(00)80319-2. [DOI] [PubMed] [Google Scholar]