Abstract
During normal pregnancy, the renin-angiotensin system (RAS) plays a vitally important role in salt balance and subsequent well-being of mother and fetus. In this balance, one must consider not only the classical renal RAS but also that of the uteroplacental unit, where both maternal and fetal tissues contribute to the signaling cascade. Many studies have shown that in normal pregnancy there is an increase in almost all of the components of the RAS. In derangements of pregnancy this delicate equilibrium can become unbalanced. Preeclampsia is one such case. It is a disorder of pregnancy characterized by hypertension, proteinuria and placental abnormalities associated with shallow trophoblast invasion and impaired spiral artery remodeling. Despite being a leading cause of maternal death and a major contributor to maternal and perinatal morbidity, the mechanisms responsible for the pathogenesis of preeclampsia are poorly understood. Immunological mechanisms and the RAS have been long considered to be involved in the development of preeclampsia. Numerous recent studies demonstrate the presence of the angiotensin II type I receptor agonistic autoantibody (AT1-AA). This autoantibody can induce many key features of the disorder and upregulate molecules involved in the pathogenesis of preeclampsia. Here we review the functional role of the RAS during pregnancy and the impact of AT1-AA on preeclampsia.
Introduction of the classical RAS pathway
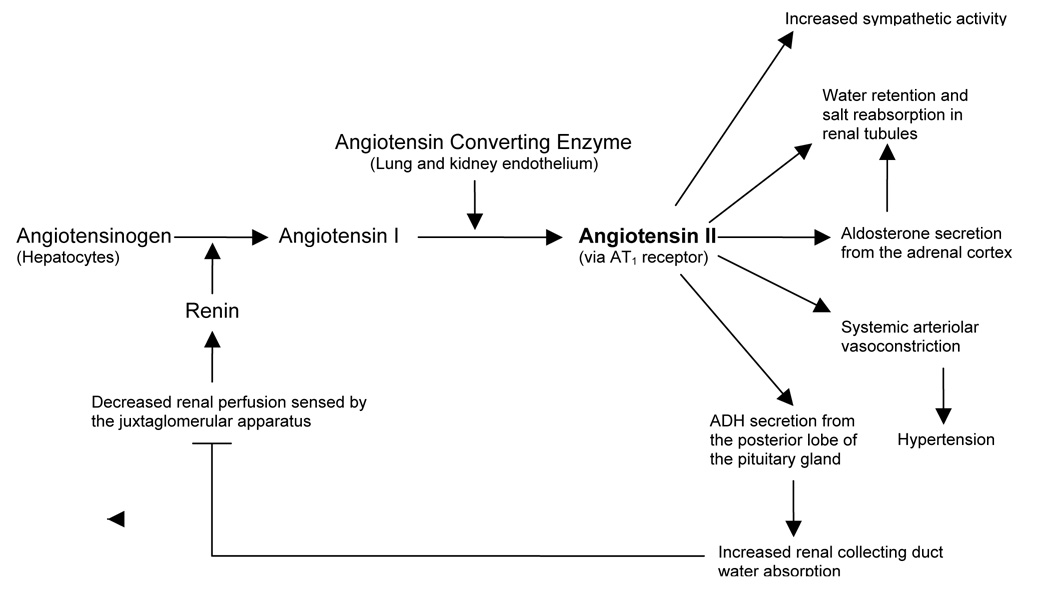
The circulating renin-angiotensin system, herein RAS, is a signaling cascade that plays a key role in regulating blood pressure and electrolyte balance. It is classically described in the kidney. The enzyme renin is synthesized and released by juxtaglomerular cells of the afferent renal arterioles in response to low blood pressure and low circulating sodium chloride. Renin release is mediated in part by prostaglandins produced by cells of the kidney’s macula densa [1]. Renin enzymatically cleaves angiotensinogen, which is made in the liver, to angiotensin-1 (ANG I), a ten amino acid peptide. This is the rate-limiting step of the RAS cascade (Figure 1). ANG I is not biologically functional and is cleaved by angiotensin-converting enzyme (ACE), made primarily in lung endothelium, to the biologically active, eight amino acid effector molecule, angiotensin-II (ANG II).
Figure 1. Classic Renin-Angiotensin System Cascade.
ANG II, the key effector molecule of the RAS and potent vasoconstrictor, acts through AT1 receptors to increase blood pressure. AT1 receptors are found on many cell types. Abbreviations - ADH: Antidiuretic hormone, ANG II: Angiotensin II, AT1 receptor: angiotensin-II type I receptor.
There are two major types of angiotensin receptors: AT1 and AT2. They belong to the seven transmembrane G-protein-coupled receptor family. They have thirty-four percent sequence dentity and have similar affinities for ANG II [2]. Most of the effects of ANG II are mediated through activation of AT1 receptors which are expressed on the surface of vascular smooth muscle cells and adrenal glands, among others. The AT1 receptor is coupled to the Gq protein, that functions in a signaling pathway to increase intracellular calcium. Its activation promotes vasoconstriction, sympathetic activity and aldosterone release. The AT2 receptor is highly expressed in the fetal kidney and its expression decreases during the neonatal period [3]. In the adult kidney AT2 is much less abundant than AT1 [2] AT2 stimulation can inhibit cell growth, increase apoptosis, cause vasodilation and is involved in fetal tissue development [4].
It should be noted that ACE, made by endothelial cells and others such as smooth muscle cells, is not the only enzyme that can generate ANG II from ANG I. Chymase, a chymotrypsin-like serine protease, is a non-ACE angiotensin generating enzyme that is produced by villous syncytiotrophoblasts [5]. Chymase is also found in great quantities in mast cells, as well as in the skin, heart and arteries and is a major contributor to the pool of ANG II found in these tissues [6,7].
A local RAS is present in the placenta
In addition to the classical view of the RAS there is accumulating evidence indicating components of the renin-angiotensin system are synthesized in many tissues, such as the brain, heart, ovary, and placenta [8,9].
One of the major extra-renal RAS during pregnancy is in the placenta. As early as 1967, Hodari et al. described a placental RAS and identified a renin-like substance in human placental tissue [10]. Renin expression in cultured chorionic cells was first reported by Symonds in 1968 [11]. Since then, pro-renin, angiotensinogen, ACE, ANG I and ANG II have all been identified in fetal placental tissues. AT1 receptor expression in fetal placental vasculature has also been shown [12]. Many other experiments using first-trimester human decidua show expression of renin, angiotensinogen, ACE and AT1 receptors [13]. More recent studies using human third trimester decidual cells also indicate the presence of angiotensinogen and renin [14]. Localization studies around the decidual spiral arteries show expression of angiotensinogen, renin, ACE and AT1 receptors [15]. Thus, in the gravid woman, the maternal decidua and the fetal placental tissues each contain all the necessary components for a functional RAS.
Regulation of the RAS during pregnancy
In humans, the RAS undergoes major changes in response to pregnancy. There is an early increase in renin due to extra-renal local release by the ovaries and maternal decidua [16]. Angiotensinogen synthesis by the liver is increased by circulating estrogen produced by the growing placenta. This leads to increased serum ANG II and aldosterone levels [17]. ACE is the only RAS component that decreases during pregnancy [18]. Table 1 compares the levels of serum RAS components between non-pregnant women and normotensive pregnant women.
Table 1. Comparison of serum RAS components in normotensive and preeclamptic pregnancies versus non-pregnant women.
Overall, circulating levels of the RAS are increased in normal pregnancies versus the non-pregnant state. Though there are slight increases in the RAS serum components in preeclamptic women, they are decreased as compared to normotensive pregnant women.
| Serum RAS Component | Normotensive Pregnancy | Preeclamptic Pregnancy | References |
|---|---|---|---|
| Renin | ++ | + | Hsueh [16], Langer [101] |
| Angiotensin I | ++ | + | Merrill [18], Langer [101] |
| ACE | − | + | Merrill [18], Langer [101] |
| Aldosterone | ++ | + | Brown [102], Langer [101] |
| ANG-(1–7) | ++ | − | Merrill [18] |
| Angiotensin II | ++ | + | Langer [101] |
| Angiotensin II sensitivity | −−(system refractory) | ++(system highly sensitive) | Gant [20] |
| AT1-AA | Absent | Present | Wallukat [44] |
| AT1 receptor | Baseline production in maternal decidua | Upregulation in maternal decidua | Herse [35] |
Legend: ++ Greatly increased over non-pregnant
+ Slightly increased over non-pregnant
− Decreased compared to non-pregnant
Although ANG II levels increase during pregnancy, normotensive pregnant women are actually refractory to its vasopressor effects. A historic study performed by Assali et al. showed that the pregnant woman requires twice as much ANG II intravenous infusion over a non-pregnant woman to achieve the same vasomotor response [19]. This is thought to be due to the presence of increased progesterone and prostacyclins which can decrease ANG II sensitivity [20]. Additionally in normal pregnancy, AT1 receptors are monomeric and are inactivated by reactive oxygen species (ROS). This is in comparison to the heterodimeric state seen in ANG II sensitive conditions [21]. These facts help explain why a greater ANG II stimulus is required in order to achieve the appropriate vasomotor response in normotensive pregnancies.
Trophoblasts are rich in AT1 receptors and thus are responsive to the changes in ANG II concentrations that occur during pregnancy. Recent studies demonstrate that multiple genes are regulated by AT1 receptor signaling and include those encoding secreted proteins associated with trophoblast invasion (e.g., plasminogen activator inhibitor-1, PAI-I) and angiogenesis (soluble fms-like tyrosine receptor-1, sFlt-1). ANG II signaling also activates NF-kappa B and stimulates NADPH-oxidase synthesis by trophoblasts [22]. ANG II decreases system A amino acid transporter activity in human placental villous fragments through AT1 receptor activation, a feature believed to contribute to IUGR in some cases [23]. In addition to its regulation of specific gene expression, the RAS is believed to play a critical regulatory role in feto-placental circulation, facilitating adequate placental blood flow for fetal oxygenation and maturation. Recent studies suggest that decidual tissue serves as a source of ANG II production and trophoblasts serve as paracrine targets of ANG II signaling through AT1 receptor activation.
The results obtained with human tissue have been corroborated and extended with studies in mice. Takimoto et al. mated transgenic male mice carrying the human renin gene with female transgenic carrying the human angiotensinogen gene [24]. They showed the activation of renin gene expression in trophoblast cells late in pregnancy and that human renin is released by the placenta into the maternal circulation [24]. Xia et al. used two murine models to study renin gene expression during pregnancy. In ICR mice, high levels of renin gene expression occur at the maternal-fetus interface, first in the maternal decidua and then in the fetal placenta [25]. While ICR mice have two related renin genes, Ren1 and Ren2, C57Bl/6 mice have only one renin gene, Ren1. In these pregnant mice, minimal renin gene expression was observed in placentas but instead was upregulated in kidneys. Though in both ICR and C57Bl/6 mice there is an increase in renin in maternal circulation during pregnancy, they differ with regard to gestation-induced sites of increased renin gene expression.
Taken together, both human and animal studies indicate that the RAS undergoes specific and necessary changes during normal pregnancy.
Preeclampsia is characterized by significant alterations in the RAS
Preeclampsia is a pregnancy-specific syndrome of hypertension and proteinuria resulting in substantial maternal and neonatal morbidity and mortality. The condition is also characterized by placental abnormalities, such as decreased invasion by extravillous trophoblasts into maternal spiral artery endothelium. In advanced stages the clinical symptoms may include cerebral edema, renal failure and the HELLP (Hemolysis, Elevated Liver enzymes and Low Platelets) syndrome. The clinical management of preeclampsia is hampered by the lack of reliable diagnostic tests and effective therapy. Although the underlying pathogenic mechanisms of the disorder are not well understood, preeclampsia is largely believed to be associated with uteroplacental ischemia and the subsequent release of toxic factors from the placenta into the maternal circulation. Roberts and colleagues were among the first to propose that alterations in endothelial cell function by activating agents produced by the placenta initiate the clinical syndrome of preeclampsia [26]. This serious condition affects approximately 7% of pregnancies and is thus a major health concern [27].
Changes in the circulating RAS in preeclampsia
Several features of the RAS in preeclampsia differ from the normal pregnant state. Except for ACE, RAS components in the circulation increase in uncomplicated pregnancy. A study by Merrill et al. demonstrated that this is not the case in preeclamptic women. In preeclamptic women the circulating levels of renin, ANG-1 and aldosterone are lower than their normotensive counterparts (Table 1). There are two exceptions to the decreases observed. The ACE level is approximately the same and ANG-(1–7), a vasodilatory member of the RAS, is significantly reduced in preeclampsia [18]. The exact role ANG-(1–7) plays in uteroplacental blood flow is still uncertain. It is produced throughout the body by many tissues such as kidney, heart, hypothalamus and ovary. This peptide can be derived from ANG I independent of ACE or synthesized from ANG II by removal of the C-terminal phenylalanine by several enzymes, such as ACE-2, prolylendopeptidase and prolylcarboxypeptidase [28]. ANG-(1–7) not only interacts with AT1 and AT2 receptors, but there is growing evidence that it can act through its own specific receptor [29,30].
While normotensive pregnant women demonstrate decreased vascular sensitivity to ANG II, preeclamptic women exhibit increased sensitivity of the adrenal cortex and vascular system to ANG II [20,31]. This phenomenon could be due to heterodimerization of the AT1 receptor. During normal pregnancy monomeric AT1 receptors are inactivated by ROS leading to lower ANG II sensitivity [21]. In preeclampsia, however, the AT1 receptor is found in the heterodimeric form with the bradykinin receptor (B2) [21,32]. These AT1/B2 heterodimers show resistance to ROS-inactivation and remain active and hyper-responsive to ANG II [21,33,34]. Monitoring the presence and activity of these heterodimeric receptors as preeclamptic symptoms subside postpartum will be of future interest.
Changes in the local uteroplacental RAS in preeclampsia
The changes observed in the uteroplacental RAS are different from those in the circulation in preeclampsia. Recent studies by Herse et al. demonstrate that the only change in placental or decidual RAS component expression in preeclampsia is the upregulation of the AT1 receptor in the maternal decidua [35]. They did not observe an increase in renin production in the decidua of preeclamptic over normotensive placentas. This is in contrast to the earlier findings of Shah et al., who demonstrate an increase in renin expression in the decidua vera of preeclamptic women over those of normotensive pregnant women [36]. They believe that the maternal decidua acts as an additional site of RAS activation and that the small amount of ANG II produced locally finds its way into maternal circulation and is sufficient to down-regulate ANG II production in the kidney as seen in preeclampsia. Another recent study by Anton et al. shows an increase in ANG II, but no increase in ANG-(1–7) in the chorionic villi of preeclamptic placentas as compared to placentas of normotensive pregnancies [37]. The varied results of placental RAS studies indicate that further investigation of the RAS during preeclampsia and in placental tissues is necessary.
In addition, chymase, a non-ACE ANG II producing enzyme, is upregulated in trophoblast cells in the placentas of preeclamptic women as compared to those of normotensive women [5]. This serine protease, released by mast cells and smooth muscle cells, has known roles in hypertensive and inflammatory diseases [38,39]. Overexpression of human vascular chymase in transgenic mice leads to increased blood pressure, vasoconstriction and hypertensive arteriopathy, all features of preeclampsia [40]. Chymase also cleaves big endothelin-1 (ET-1) to the 31-amino-acid length endothelin ET-1(1–31) [41], a vasoconstrictor that is increased in the myometrium of preeclamptic women [42]. ET-1-(1–31) is an especially potent vasoconstrictor in the umbilical artery and could play an important role in fetal circulation and the observed intrauterine growth restriction and hypertension observed in preeclampsia [43]. Investigation into the exact role and regulation of chymase in this disease will be of great interest in the near future.
The Angiotensin II type I Agonistic Autoantibody (AT1-AA)
It is a puzzling feature that circulating ANG II levels are decreased in preeclamptic women as compared to normotensive pregnant women [20,31]. Despite this fact, women suffering from preeclampsia exhibit symptoms, such as hypertension and renal damage, which could be attributed to an excess of ANG II or AT1 receptor activation.
A major advance in our understanding of preeclampsia was made in 1999 by Wallukat et al. who reported that women with preeclampsia harbor an autoantibody that stimulates the AT1 receptor [44] This AT1 receptor agonistic autoantibody (AT1-AA) represents a major intrusion into the normal functioning RAS. Using affinity purification and peptide competition experiments they showed that the autoantibody binds to a seven amino acid sequence present on the second extracellular loop of the AT1 receptor.
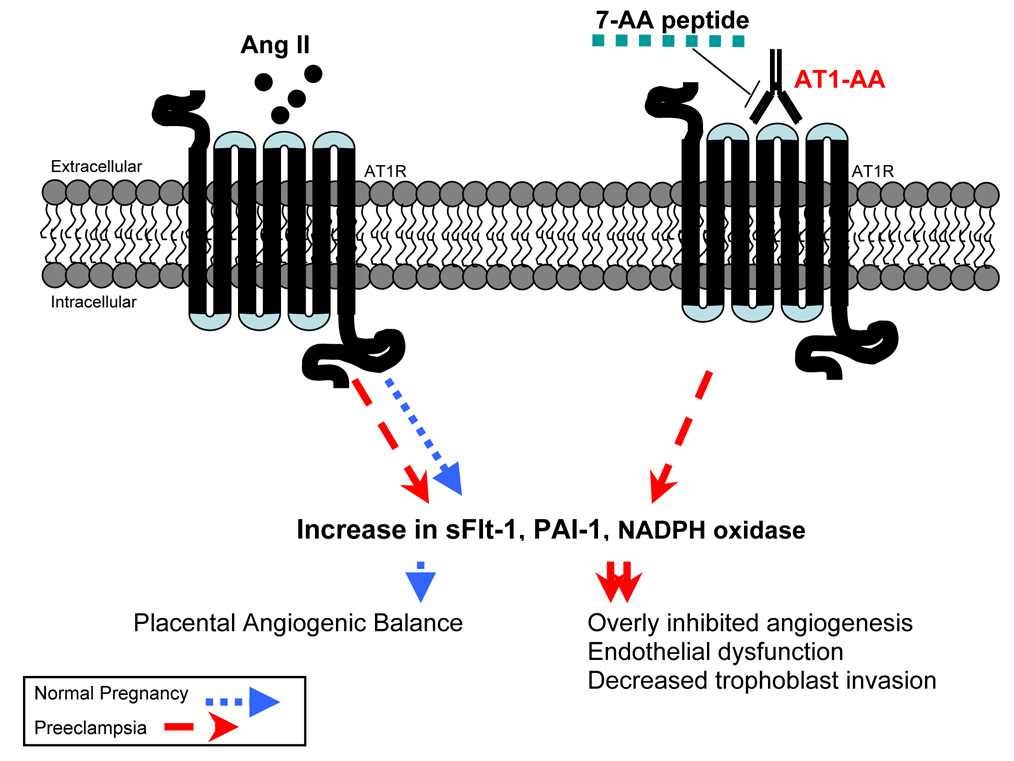
Since its discovery, there has been extensive investigation into the contribution of AT1-AA to the pathogenesis of preeclampsia. Studies by the group above, ours and others show that AT1-AA bind to AT1 receptors on a variety of cells, including trophoblasts, and increase factors attributed to the pathogenesis of preeclampsia. Examples of the regulatory role of AT1-AA in preeclampsia are reviewed below and summarized in Figure 2.
Figure 2. AT1-AA interact with AT1 receptors on trophoblasts which synergistically act with ANG II to impair placentation.
Normal RAS function and AT1 receptor activation is imperative for normal placental development. AT1-AA, found in the serum of preeclamptic women, function as ANG II by activating AT1 receptors to increase production of sFlt-1, PAI-1 and NADPH oxidase in trophoblast cells. The 7-AA peptide corresponds to a sequence on the second extracellular loop of the AT1 receptor. AT1-AA-mediated effects can be neutralized by 7-AA. AT1-AA: Angiotensin II type I receptor agonistic autoantibody.
Pathologic role of AT1-AA in abnormal placental development and the maternal syndrome of preeclampsia
Role of AT1-AA in placental abnormalities
Placentas of preeclamptic women are often small, exhibit shallow trophoblast invasion, aberrant spiral artery remodeling and reduced uteroplacental blood flow. The triggering factor and mechanism of these changes have not been determined. There is a growing body of evidence indicating that AT1-AA and its derangement of the RAS could influence these transformations and contribute to the pathogenesis of preeclampsia.
a) AT1-AA induces excess sFlt-1 secretion and impaired angiogenesis
Recently soluble fms-like tyrosine kinase-1 (sFlt-1) has been brought to the forefront of factors playing a role in placental development and preeclampsia [45,46]. It is a splice variant of VEGFR-1: a secreted soluble form of the VEGFR-1 receptor lacking the transmembrane and cytoplasmic domains. sFlt-1 acts as an antagonist of angiogenesis by binding to free vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) and inhibiting their angiogenic actions [47–49]. In preeclamptic placentas, which are reportedly hypoxic (Roberts 1989, 1992), there is an increase of sFlt-1 secretion two- to five-fold greater than in the placentas of normotensive pregnancies [50–52]. Though both sFlt-1 and VEGF are hypoxia-inducible genes, Nagamatsu et al. have shown that in the hypoxic induction of sFlt-1 in the placenta is cell-specific and that hypoxia/ischemia of cytotrophoblast cells leads to excess sFlt-1 production alone [53]. Khaliq et al. have shown that hypoxia decreases PlGF expression in BeWo choriocarcinoma cells [54]. Ahmed et al. have also shown that there is a diametric expression of VEGF and PlGF during pregnancy [55]. In preeclampsia, Lash et al. report a cytotrophoblastic increase in VEGF and decrease in PlGF [56]. While total VEGF expression may increase during pregnancy, when high sFlt-1 levels are also present, free VEGF decreases, resulting in less angiogenesis [57]. Therefore, the overabundance of sFlt-1 production by the cytotrophoblast leads to a decrease of free VEGF on a background of low PlGF, resulting in an overall anti-angiogenic state in the placenta. This angiogenic imbalance could result in the small, hypoxic placentas described in preeclampsia.
Caniggia and others hypothesize that early decidual hypoxia globally alters placental gene expression and retards trophoblast invasion necessary for a healthy placenta [58]. They suggest that hypoxia-inducible factor-1α (HIF-1α) is upregulated in preeclampsia and this leads to increases in transforming growth factor-β3 (TGF-β3) which limits trophoblast invasion into maternal spiral arteries and decidua thereby generating further hypoxic conditions [58–60]. This mechanism of early placental hypoxia may be one explanation of excessive sFlt-1 production and the alterations in angiogenesis seen in preeclampsia.
Hypoxia, however, is only one plausible hypothesis for the overproduction of sFlt-1 in preeclampsia. The autoantibody, AT1-AA, may also play an important role. During normal pregnancy the placenta produces sFlt-1 through ANG II stimulation of trophoblast cells via the calcineurin-NFAT pathway [61]. Therefore overstimulation of the AT1 receptor by the autoantibody could lead to excessive sFlt-1 production. In this regard, Zhou et al.. have shown that AT1-AA purified from preeclamptic patient serum can not only induce sFlt-1 secretion in both human placental villous explants and human trophoblast cells [61,62], but also in a pregnant mouse model [61]. This leads to the possibility that alongside ANG II and local placental hypoxia, AT1-AA can additively contribute to the excess sFlt-1 secretion reported in preeclamptic patients.
Oversecretion of sFlt-1 secondary to both AT1-AA and placental hypoxia could potentiate a positive feed-forward cycle: increased sFlt-1 could lead to the overly inhibited angiogenesis and further placental hypoxia observed in preeclampsia, with subsequently more placental sFlt-1 production. It will be important in future studies to further clarify the local AT1 receptor-mediated mechanisms of sFlt-1 oversecretion in the placenta.
b) AT1-AA stimulates excess PAI-1 secretion and shallow trophoblast invasion
PAI-1 is another important factor to consider in the pathogenesis of preeclampsia for two reasons. First, in the placenta it plays a role in impaired trophoblast invasion. It does this by inhibiting urokinase-like plasminogen activator (uPA) resulting in decreased conversion of plasminogen to plasmin. This leads to decreased fibrinolysis, less extracellular matrix digestion and shallow trophoblast invasion, a hallmark of the placenta in preeclampsia. AT1-AA activates AT1 receptors on trophoblast cells resulting in elevated PAI-1 levels [63,64]. It has also been shown to decrease trophoblast invasion in vitro using a matrigel assay [63,65]. Thus, activation of AT1 receptors by AT1-AA on human trophoblasts may contribute to increased PAI-1 production and shallow trophoblast invasion.
c) AT1-AA increases ROS production
Free radical species, or reactive oxygen species (ROS) are a normal by-product of aerobic respiration and regulate cellular functions through redox reactions [66]. However, when excess ROS are present, a cell’s natural anti-oxidant defenses are unable to overcome the overload of non-specific damage of cellular DNA, proteins and lipids. In this regard, during pregnancy, oxidative stress could lead directly to placental tissue damage. The teratogenic effects of ROS could be detrimental to the developing fetus, especially during the critical period of organogenesis [67,68]. The generation of reactive oxygen species (ROS) is increased in preeclampsia and could play a role in aberrant placentation [69]. Dechend et al. found that AT1-AA increases intracellular ROS via NADPH oxidase in placental trophoblast cells as well as vascular smooth muscle cells [22]. They also showed that AT1-AA markedly upregulates NFκB as a downstream target and confirmed that there is increased ROS production in preeclamptic placentas in and around the blood vessels. Finally, they demonstrated that NADPH oxidases are elevated in the placentas of preeclamptic women. The authors suggest that AT1-AA could contribute to ROS production in the placenta through activation of NADPH oxidase and in the inflammatory responses associated with preeclampsia [22].
Collectively, AT1-AA-mediated sFlt-1, PAI-1 and NADPH oxidase induction may contribute to the pathological changes observed in the placentas of preeclamptic patients.
The Maternal Syndrome of Preeclampsia
In addition to its potential contribution to placental abnormalities, AT1-AA also plays an important role in the maternal features of preeclampsia and contributes to the endothelial cell dysfunction and vascular damage which also characterize the disease [26,70]. Intuitively, AT1 receptor stimulation would lead to vasoconstriction and subsequent hypertension. Furthermore, AT1-AA has been linked to renal abnormalities and increased hypercoagulation, two other clinical features associated with the disorder.
a) AT1-AA increases PAI-1 production in the kidneys
Plasminogen activator inhibitor-1 (PAI-1) is a serine protease inhibitor which not only decreases the conversion of plasminogen to plasmin leading to decreased fibrinolysis and increased fibrosis, but also indirectly inhibits extracellular matrix breakdown via matrix metalloproteinases [71]. Mesangial cell PAI-1 production is partially controlled by the action of ANG II on AT1 receptors [72,73]. Bobst et al. have shown that AT1 receptors on cultured human mesangial cells can be activated by AT1-AA and increase PAI-1 secretion [63]. This accumulation of PAI-1 and decrease of available plasmin could potentially contribute to the kidney damage via decreased extracellular matrix degradation and sub-endothelial and sub-epithelial fibrin deposition observed in preeclampsia [74,75]. Excess fibrin deposition in the glomeruli decreases the kidney’s filtration capability and could contribute to proteinuria [76,77]. By acting through the RAS, AT1-AA induces increases of PAI-1 in both the placenta and kidney, leading to decreased fibrinolysis and extracellular matrix breakdown that could manifest in the symptoms seen in preeclampsia.
b) AT1-AA and increases calcium mobilization
Preeclampsia is associated with abnormalities in calcium metabolism and increased intracellular calcium levels in erythrocytes, lymphocytes and platelets [78–80]. Haller et al. showed an elevation in basal intracellular free Ca2+ in the platelets of preeclamptic patients in comparison to those of normotensive pregnant women. Similarly, intracellular free Ca2+ concentration is increased in the lymphocytes and erythrocytes of preeclamptic patients [79,81]. Thway et al. tested the possibility that AT1-AA could increase intracellular concentration of free Ca2+ and the downstream activation of Ca2+ signaling pathways via AT1 receptor stimulation [82]. They found that IgG from preeclamptic patients activated AT1 receptors and increased intracellular free Ca2+ whereas IgG from normotensive women was incapable of doing so. They also showed that this increase in intracellular Ca2+ lead to the activation of the NFAT transcription factor [82]. These results suggest that AT1-AA may contribute to the increased intracellular free Ca2+ concentration and downstream signaling associated with the systemic disorder of preeclampsia.
c) AT1-AA induces Tissue Factor production
Tissue factor (TF) is a transmembrane protein that initiates the extrinsic pathway of coagulation and is found in high levels in the placentas of preeclamptic women. TF overexpression could cause additional vascular damage in the placenta and could contribute to the hypercoaguability experienced by some women with severe preeclampsia. Dechend et al. observed that AT1-AA induces TF expression in vascular smooth muscle cells [83] and Dorfell et al. showed similar findings in monocytes [84]. These studies indicate that AT1-AA, by activating AT1 receptor signaling, initiates increased TF expression. Therefore, AT1-AA may contribute to the hypercoaguability associated with preeclampsia by stimulating TF expression in human vascular smooth muscle cells and monocytes.
d) AT1-AA-mediated release of sFlt-1 and renal impairment
A prevailing view is that toxic factors secreted by the placenta contribute to the maternal syndrome of preeclampsia. One such factor is sFlt-1. AT1-AA induces sFlt-1 levels in human placental villous explants, human trophoblast cells [61,62] and in pregnant mice [61]. Maynard et al. have shown that treatment of pregnant rats with sFlt-1 induces a preeclamptic like state: increased blood pressure, proteinuria and histopathologic changes in the renal glomeruli parallel to those lesions observed humans, such as glomerular endotheliosis, a hallmark of endothelial cell injury [52]. Thus, AT1-AA induction of sFlt-1 could lead to the kidney damage observed in preeclampsia.
Overall, multiple studies show that many features of preeclampsia can be explained by the ability of AT1–AA to activate AT1 receptors on a variety of cells and provoke biological responses relevant to the pathophysiology of preeclampsia.
Long Term Consequences of AT1-AA
Though the symptoms of preeclampsia usually abate within 48 hours after the delivery of the baby and placenta, it will be important to evaluate the persistence of AT1-AA in these women. The recent meta-analysis by Bellamy et al. highlights the long-term cardiovascular risks for preeclamptic patients [85]. They report that women who have had preeclampsia are at higher risk for persistent hypertension and future cardiovascular injury. Additionally, it is known that the same AT1-AA found in serum of women with preeclampsia associates with patients who suffer from malignant hypertension [86]. It is therefore reasonable to suggest an association between the persistence of the autoantibody and the long-term derangement of the RAS in women with preeclampsia.
Key animal models used to study the RAS and hypertensive disorders of pregnancy
It has become clear that preeclampsia is a vascular disease involving the interaction of multiple cell types, including trophoblasts, endothelial cells, vascular smooth muscle cells and others. To fully understand the interplay among these cells in response to alterations in the RAS it will be necessary to decipher the intercellular signaling pathways involved. This is a major reason why animal models, in which complex cellular interactions can be studied, will play an especially important role in examining this disorder. Table 2 briefly summarizes the recent animal models concerning the RAS, pregnancy and preeclampsia mentioned in this review.
Table 2. Recent animal models used to investigate the RAS, pregnancy and preeclampsia.
Brief description of the key recent animal models reviewed in this manuscript and their references.
| Description of model | Animal | Findings | Reference |
|---|---|---|---|
| Mated male transgenic mice (overexpressing human renin) with female transgenic mice (overexpressing human angiotensinogen) | Mice | Pregnant female mice demonstrated: increased human rennin production by trophoblasts and renin release in circulation, transient hypertension that resolved upon delivery, proteinuria and placental abnormalities. | Takimoto E, 1996 [24]. |
| Mated male transgenic mice (overexpressing human renin) with female transgenic mice (overexpressing human angiotensinogen) and lacking the AT1a receptor | Mice | Pregnant female mice exhibited no cardiac, renal or placental defects, indicating the important role of the AT1a receptor in a preeclamptic-like state in mice. | Saito T, 2004 [88]. |
| Mated male transgenic rats (overexpressing human renin) with female transgenic rats (overexpressing human angiotensinogen) | Rats | Pregnant female rats demonstrated: transient hypertension that resolved upon delivery, proteinuria, glomerular fibrin deposition and placental vascular defects. | Dechend R, 2005 [89]. |
| Evaluated renin expression in ICR and C57Bl/6 pregnant mice | Mice | Two murine models differ in their gestation-induced sites of renin expression. ICR mice show increased renin the placenta. C57Bl/6 mice show increased renin expression in the maternal kidneys. | Xia Y, 2002 [25]. |
| Overexpression of human vascular chymase in transgenic mice | Mice | Chymase overexpression in mice led to hypertension, vasoconstriction and hypertensive arteriopathy. | Ju H. 2001 [40]. |
| AT1–AA was injected into pregnant mice | Mice | Circulating maternal sFlt-1 increased in AT1-AA injected mice. | Zhou CC, 2008 [61]. |
| sFlt-1 injected directly into pregnant rats | Rats | sFlt-1 injected pregnant rats developed increased blood pressure, proteinuria and glomerular endotheliosis. | Maynard SE, 2003 [52]. |
| Pregnant rats (1) surgically treated to reduce uterine blood flow via RUPP (Reduced Uterine Perfusion Pressure) method or (2) infused with low-dose TNF-alpha | Rats | RUPP- and TNF-alpha treated rats demonstrated similar findings: increased blood pressure, proteinuria, circulating sFlt-1, as well as AT1–AA production. | Granger JP, 2006 [90]. Dechend R, 2006. [91] |
Without genetic manipulation, rodents do not naturally and spontaneously develop preeclampsia. Despite this, many animal models have been used to elucidate the role of RAS in this disorder. Rodent and human placentas share similar forms and vascular structure: discoid and chorioallantoic, respectively [87]. They differ in that the mouse has a labyrinth-type hemotrichorial interdigitation whereas humans have a monochorial villous maternal-fetal interface. The RAS of the rodent and human are remarkably similar. The mouse has two isotypes of the AT1 receptor, AT1a and AT1b, whereas humans only have one form of AT1. In general, there is an upregulation of the components of RAS in normal pregnancy in both rodents and humans [25].
Despite differences between rodent and human placentas, extensive knowledge about the RAS and pregnancy has been gained using both mouse and rat models. Takimoto et al. showed that transient hypertension was induced in pregnant transgenic mice expressing human angiotensinogen that were mated with male transgenic mice expressing the human renin gene [24]. The blood pressure in these females increased late in pregnancy and resolved to normal levels after delivery. They also demonstrated glomerular enlargement coupled with increased proteinuria, myocardial hypertrophy as well as necrotic and edematous changes in their placentas. This group showed an increase in human renin mRNA in chorionic trophoblasts and an increase in placental-derived human renin in the maternal circulation of the pregnant transgenic mice. This implies that secreted placental factors could play a role in the pathogenesis of hypertensive disorders in pregnancy.
The same group went on to investigate the role of the angiotensin receptors in the transgenic mice mentioned above. Female mice expressing the human angiotensinogen gene, but lacking the AT1a receptor, were mated to male mice expressing the human renin gene. During pregnancy, they surprisingly did not have an increase in blood pressure despite having intact AT1b receptors [88]. The other features induced in transgenic mice with human angiotensinogen expression alone were not observed in mice lacking the AT1a receptor. In fact, the AT1a receptor deficient mice demonstrated no renal, cardiac or placental abnormalities. These findings illustrate the importance of AT1a receptors in the development of hypertension and other histopathologic changes in pregnancy in the setting of a dysregulated RAS.
Key animal models involving the AT1-AA and preeclampsia
The above experiments did not highlight the role of AT1-AA in the development of hypertension in pregnancy. To address this issue, Dechend et al. mated female transgenic rats expressing the human angiotensinogen gene with male rats expressing the human renin gene [89]. Much like the mice of Takimoto et al., these rat dams demonstrated hypertension and proteinuria late in pregnancy that resolved upon delivery. They also developed fibrin deposition in their glomeruli and their placentas demonstrated vascular defects, such as atherosis-like lesions in the spiral arteries of the placental beds. AT1-AA, the same autoantibody produced by women with preeclampsia, was found in their serum [89]. The production of AT1-AA and a dysregulated RAS implies a close relationship of these factors to preeclampsia.
Granger et al. used reduction in uterine perfusion pressure (RUPP) in rats to investigate the role of the ischemic placenta during pregnancy. Pregnant rats underwent a surgical procedure wherein small silver clips were placed around the aorta at the iliac bifurcation and on both ovarian arteries which reduced blood supply the uterus [90]. RUPP rats demonstrated a “preeclamptic-like state” with increases in blood pressure, proteinuria, sFlt-1, TNF-α, endothelin production and endothelial dysfunction. Notably, RUPP rats develop AT1-AA, whereas unmanipulated pregnant rats do not [91]. This group also investigated the effect of TNF-α alone. Interestingly, when low-dose TNF-α was infused into pregnant rats, increased blood pressure and the production of AT1-AA followed [91]. These effects were not observed in non-pregnant animals, implying that placental ischemia can lead to an inflammatory response that triggers the production of AT1-AA. The appearance of the AT1-AA in RUPP rats indicates a relationship between reduced placental perfusion and the derangement of RAS.
Recently Zhou et al. have shown that injection of AT1-AA obtained from preeclamptic women into pregnant mice can induce hypertension, proteinuria and increased circulating sFlt-1 [61]. The placentas of these mice are small. The effects of injected AT1-AA are diminished with co-injection of losartan, an AT1-receptor blocker, or a short antibody-neutralizing epitope peptide [61]. These findings indicate that AT1-AA contribute to the pathophysiology of preeclampsia via AT1-receptor activation.
Placental hypoxia and ischemia: a possible explanation for autoantibody production in preeclampsia
Despite the growing body of work described above linking AT1-AA to preeclampsia the cause for this autoantibody generation is unknown. In general, the etiology of autoimmune disease remains unidentified, however many factors have been proposed, including a genetic predisposition, a maladaptive immune response and environmental triggers [92–94]. In the case of preeclampsia, autoantibody generation could be secondary to reduced placental perfusion, leading to vascular injury that exposes the offending antigen, coupled with the increased inflammatory response associated with the disease [95-98]. RUPP-induced hypertension was markedly attenuated by antagonism of the AT1 receptor [99,100], supporting the current finding that it relies on autoantibody stimulation. In addition, this group has shown that low-dose TNF-alpha infusion into pregnant rats induced autoantibody production [91]. In both cases of AT1-AA production, the hallmark features of preeclampsia were also evident: hypertension and proteinuria. Therefore, these experimental models of preeclampsia may provide a valuable system to determine the immunological origin of AT1-AA in the disorder.
Collectively, these studies indicate that placental ischemia, the associated vascular damage and inflammatory response may serve as important stimuli for AT1-AA production during pregnancy. This also implies that during preeclamptic pregnancies, AT1 receptor activation plays an important role in the hypertension produced subsequent to placental ischemia.
It will be important to assess all experimental animal models of preeclampsia for the presence of AT1-AA. In order to do this, a reliable, high throughput assay must be developed to determine both the presence and relative activity of the autoantibody. By determining the exact triggers and environment which give rise to AT1-AA in experimental animal models, insight will be gained on the possible mechanisms of autoantibody generation in the human gravid state.
Conclusions and significance
The renin-angiotensin system has long been thought to play an important role in placentation, normal pregnancy and the pathophysiology of preeclampsia. How all the components of the system interact during normal and abnormal pregnancy has yet to be entirely understood. While there is a general upregulation of the RAS in normal pregnancy, this delicate balance is lost preeclampsia.
The central role of the placenta in preeclampsia is undisputed. Specific factors, such as sFlt-1, liberated by the placenta are now at the forefront of efforts to decipher the pathogenesis of this disease. Therefore, the emerging role of AT1-AA and its induction of these placental factors will be integral to our understanding of preeclampsia. As described, AT1-AA can enhance the cascade of RAS by acting synergistically with ANG II and its biological effects can be blocked by a 7-AA peptide corresponding to a specific epitope on the second extracellular loop of the AT1 receptor. This epitope consistency, i.e. the fact that AT1-AA observed in women with preeclampsia all recognize the same epitope peptide sequence, suggests a common immunological origin of these autoantibodies. This has profound therapeutic implications which could be specifically targeted against AT1-AA. Treatment for preeclampsia is currently limited, and severe cases often require premature delivery of the infant. If maternal circulating AT1-AA play a vital role in preeclampsia, their timely removal from or inhibition in preeclamptic women may provide significant therapeutic benefit. Eventually, it may be possible to block autoantibody-mediated AT1 receptor activation, thereby forestalling or preventing the onset of the symptoms of preeclampsia.
Abbreviations
- ANG II
angiotensin II
- AT1-AA
angiotensin II type I receptor agonistic autoantibody
- NT
normotensive
- PE
preeclampsia
- sFlt-1
soluble fms-like tyrosine kinase-1
- RAS
Renin-Angiotensin System
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jensen BL, Schmid C, Kurtz A. Prostaglandins stimulate renin secretion and renin mRNA in mouse renal juxtaglomerular cells. Am J Physiol. 1996;271(3 Pt 2):F659–F669. doi: 10.1152/ajprenal.1996.271.3.F659. [DOI] [PubMed] [Google Scholar]
- 2.Chung O, Kuhl H, Stoll M, Unger T. Physiological and pharmacological implications of AT1 versus AT2 receptors. Kidney Int. 1998;54(S67):S95–S99. doi: 10.1046/j.1523-1755.1998.06719.x. [DOI] [PubMed] [Google Scholar]
- 3.Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30(5):1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 4.Grishko V, Pastukh V, Solodushko V, Gillespie M, Azuma J, Schaffer S. Apoptotic cascade initiated by angiotensin II in neonatal cardiomyocytes: role of DNA damage. Am J Physiol Heart Circ Physiol. 2003;285(6):H2364–H2372. doi: 10.1152/ajpheart.00408.2003. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Gu Y, Zhang Y, Lewis DF, Alexander JS, Granger DN. Increased chymotrypsin-like protease (chymase) expression and activity in placentas from women with preeclampsia. Placenta. 2007;28(4):263–269. doi: 10.1016/j.placenta.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265(36):22348–22357. [PubMed] [Google Scholar]
- 7.Caughey GH, Schaumberg TH, Zerweck EH, Butterfield JH, Hanson RD, Silverman GA, Ley TJ. The human mast cell chymase gene (CMA1): mapping to the cathepsin G/granzyme gene cluster and lineage-restricted expression. Genomics. 1993;15(3):614–620. doi: 10.1006/geno.1993.1115. [DOI] [PubMed] [Google Scholar]
- 8.Hagemann A, Nielsen AH, Poulsen K. The uteroplacental renin-angiotensin system: a review. Exp Clin Endocrinol. 1994;102(3):252–261. doi: 10.1055/s-0029-1211289. [DOI] [PubMed] [Google Scholar]
- 9.Poisner AM. The human placental renin-angiotensin system. Front Neuroendocrinol. 1998;19(3):232–252. doi: 10.1006/frne.1998.0166. [DOI] [PubMed] [Google Scholar]
- 10.Hodari AA, Smeby R, Bumpus FM. A renin-like substance in the human placenta. Obstet Gynecol. 1967;29(3):313–317. [PubMed] [Google Scholar]
- 11.Symonds EM, Stanley MA, Skinner SL. Production of renin by in vitro cultures of human chorion and uterine muscle. Nature. 1968;217(5134):1152–1153. doi: 10.1038/2171152a0. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Shams M, Zhu J, Khalig A, Wilkes M, Whittle M, Barnes N, Ahmed A. Cellular localization of AT1 receptor mRNA and protein in normal placenta and its reduced expression in intrauterine growth restriction. Angiotensin II stimulates the release of vasorelaxants. J Clin Invest. 1998;101(2):442–454. doi: 10.1172/JCI119881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw KJ, Do YS, Kjos S, Anderson PW, Shinagawa T, Dubeau L, Hsueh WA. Human decidua is a major source of renin. J Clin Invest. 1989;83(6):2085–2092. doi: 10.1172/JCI114121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Ansari R, Yu Z, Shah D. Definitive molecular evidence of renin-angiotensin system in human uterine decidual cells. Hypertension. 2000;36(2):159–164. doi: 10.1161/01.hyp.36.2.159. [DOI] [PubMed] [Google Scholar]
- 15.Morgan T, Craven C, Ward K. Human spiral artery renin-angiotensin system. Hypertension. 1998;32(4):683–687. doi: 10.1161/01.hyp.32.4.683. [DOI] [PubMed] [Google Scholar]
- 16.Hsueh WA, Luetscher JA, Carlson EJ, Grislis G, Fraze E, McHargue A. Changes in active and inactive renin throughout pregnancy. J Clin Endocrinol Metab. 1982;54(5):1010–1016. doi: 10.1210/jcem-54-5-1010. [DOI] [PubMed] [Google Scholar]
- 17.Brown MA, Gallery ED, Ross MR, Esber RP. Sodium excretion in normal and hypertensive pregnancy: a prospective study. Am J Obstet Gynecol. 1988;159(2):297–307. doi: 10.1016/s0002-9378(88)80071-1. [DOI] [PubMed] [Google Scholar]
- 18.Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB. Angiotensin-(1–7) in normal and preeclamptic pregnancy. Endocrine. 2002;18(3):239–245. doi: 10.1385/ENDO:18:3:239. [DOI] [PubMed] [Google Scholar]
- 19.Assali NS, Westersten A. Regional flow-pressure relationship in response to angiotensin in the intact dog and sheep. Circ Res. 1961;9:189–193. doi: 10.1161/01.res.9.1.189. [DOI] [PubMed] [Google Scholar]
- 20.Gant NF, Worley RJ, Everett RB, MacDonald PC. Control of vascular responsiveness during human pregnancy. Kidney Int. 1980;18(2):253–258. doi: 10.1038/ki.1980.133. [DOI] [PubMed] [Google Scholar]
- 21.AbdAlla S, Lother H, el Massiery A, Quitterer U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat Med. 2001;7(9):1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- 22.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107(12):1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 23.Shibata E, Powers RW, Rajakumar A, von Versen-Hoynck F, Gallaher MJ, Lykins DL, Roberts JM, Hubel CA. Angiotensin II decreases system A amino acid transporter activity in human placental villous fragments through AT1 receptor activation. Am J Physiol Endocrinol Metab. 2006;291(5):E1009–E1016. doi: 10.1152/ajpendo.00134.2006. [DOI] [PubMed] [Google Scholar]
- 24.Takimoto E, Ishida J, Sugiyama F, Horiguchi H, Murakami K, Fukamizu A. Hypertension induced in pregnant mice by placental renin and maternal angiotensinogen. Science. 1996;274(5289):995–998. doi: 10.1126/science.274.5289.995. [DOI] [PubMed] [Google Scholar]
- 25.Xia Y, Wen H, Prashner HR, Chen R, Inagami T, Catanzaro DF, Kellems RE. Pregnancy-induced changes in renin gene expression in mice. Biol Reprod. 2002;66(1):135–143. doi: 10.1095/biolreprod66.1.135. [DOI] [PubMed] [Google Scholar]
- 26.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 27.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 28.Santos RA, Haibara AS, Campagnole-Santos MJ, Simoes e Silva AC, Paula RD, Pinheiro SV, Leite MF, Lemos VS, Silva DM, Guerra MT, Khosla MC. Characterization of a new selective antagonist for angiotensin-(1–7), D-pro7-angiotensin-(1–7) Hypertension. 2003;41(3 Pt 2):737–743. doi: 10.1161/01.HYP.0000052947.60363.24. [DOI] [PubMed] [Google Scholar]
- 29.Handa RK. Angiotensin-(1–7) can interact with the rat proximal tubule AT(4) receptor system. Am J Physiol. 1999;277(1 Pt 2):F75–F83. doi: 10.1152/ajprenal.1999.277.1.F75. [DOI] [PubMed] [Google Scholar]
- 30.Handa RK. Metabolism alters the selectivity of angiotensin-(1–7) receptor ligands for angiotensin receptors. J Am Soc Nephrol. 2000;11(8):1377–1386. doi: 10.1681/ASN.V1181377. [DOI] [PubMed] [Google Scholar]
- 31.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52(11):2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quitterer U, Lother H, Abdalla S. AT1 receptor heterodimers and angiotensin II responsiveness in preeclampsia. Semin Nephrol. 2004;24(2):115–119. doi: 10.1016/j.semnephrol.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 33.AbdAlla S, Abdel-Baset A, Lother H, el Massiery A, Quitterer U. Mesangial AT1/B2 receptor heterodimers contribute to angiotensin II hyperresponsiveness in experimental hypertension. J Mol Neurosci. 2005;26(2–3):185–192. doi: 10.1385/JMN:26:2-3:185. [DOI] [PubMed] [Google Scholar]
- 34.Ariza AC, Bobadilla NA, Halhali A. Endothelin 1 and angiotensin II in preeeclampsia. Rev Invest Clin. 2007;59(1):48–56. [PubMed] [Google Scholar]
- 35.Herse F, Dechend R, Harsem NK, Wallukat G, Janke J, Qadri F, Hering L, Muller DN, Luft FC, Staff AC. Dysregulation of the circulating and tissue-based renin-angiotensin system in preeclampsia. Hypertension. 2007;49(3):604–611. doi: 10.1161/01.HYP.0000257797.49289.71. [DOI] [PubMed] [Google Scholar]
- 36.Shah DM, Banu JM, Chirgwin JM, Tekmal RR. Reproductive tissue renin gene expression in preeclampsia. Hypertens Pregnancy. 2000;19(3):341–351. doi: 10.1081/prg-100101996. [DOI] [PubMed] [Google Scholar]
- 37.Anton L, Merrill DC, Neves LA, Stovall K, Gallagher PE, Diz DI, Moorefield C, Gruver C, Ferrario CM, Brosnihan KB. Activation of Local Chorionic Villi Angiotensin II Levels But Not Angiotensin (1–7) in Preeclampsia. Hypertension. 2008 doi: 10.1161/HYPERTENSIONAHA.107.103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schechter NM, Wang ZM, Blacher RW, Lessin SR, Lazarus GS, Rubin H. Determination of the primary structures of human skin chymase and cathepsin G from cutaneous mast cells of urticaria pigmentosa lesions. J Immunol. 1994;152(8):4062–4069. [PubMed] [Google Scholar]
- 39.Muto T, Fukami H. Recent chymase inhibitors and their effects in in vivo models. IDrugs. 2002;5(12):1141–1150. [PubMed] [Google Scholar]
- 40.Ju H, Gros R, You X, Tsang S, Husain M, Rabinovitch M. Conditional and targeted overexpression of vascular chymase causes hypertension in transgenic mice. Proc Natl Acad Sci U S A. 2001;98(13):7469–7474. doi: 10.1073/pnas.131147598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakano A, Kishi F, Minami K, Wakabayashi H, Nakaya Y, Kido H. Selective conversion of big endothelins to tracheal smooth muscle-constricting 31-amino acid-length endothelins by chymase from human mast cells. J Immunol. 1997;159(4):1987–1992. [PubMed] [Google Scholar]
- 42.Mitani R, Maeda K, Fukui R, Endo S, Saijo Y, Shinohara K, Kamada M, Irahara M, Yamano S, Nakaya Y, Aono T. Production of human mast cell chymase in human myometrium and placenta in cases of normal pregnancy and preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2002;101(2):155–160. doi: 10.1016/s0301-2115(01)00546-2. [DOI] [PubMed] [Google Scholar]
- 43.Takeji T, Nakaya Y, Kamada M, Maeda K, Saijo Y, Mitani R, Irahara M, Aono T. Effect of a novel vasoconstrictor endothelin-1 (1–31) on human umbilical artery. Biochem Biophys Res Commun. 2000;270(2):622–624. doi: 10.1006/bbrc.2000.2476. [DOI] [PubMed] [Google Scholar]
- 44.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karumanchi SA, Epstein FH. Placental ischemia and soluble fms-like tyrosine kinase 1: cause or consequence of preeclampsia? Kidney Int. 2007;71(10):959–961. doi: 10.1038/sj.ki.5002281. [DOI] [PubMed] [Google Scholar]
- 46.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 47.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996;226(2):324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 49.Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001;26(1):25–35. doi: 10.1247/csf.26.25. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160:1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsatsaris V, Goffin F, Munaut C, Brichant J-F, Pignon M-R, Noel A, Schaaps J-P, Cabrol D, Frankenne F, Foidart J-M. Overexpression of the Soluble Vascular Endothelial Growth Factor Receptor in Preeclamptic Patients: Pathophysiological Consequences. J Clin Endocrinol Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 52.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction,hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, Kozuma S, Taketani Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838–4845. doi: 10.1210/en.2004-0533. [DOI] [PubMed] [Google Scholar]
- 54.Khaliq A, Dunk C, Jiang J, Shams M, Li XF, Acevedo C, Weich H, Whittle M, Ahmed A. Hypoxia down-regulates placenta growth factor, whereas fetal growth restriction up-regulates placenta growth factor expression: molecular evidence for "placental hyperoxia" in intrauterine growth restriction. Lab Invest. 1999;79(2):151–170. [PubMed] [Google Scholar]
- 55.Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen--a review. Placenta. 2000;21 Suppl A:S16–S24. doi: 10.1053/plac.1999.0524. [DOI] [PubMed] [Google Scholar]
- 56.Lash GE, Taylor CM, Trew AJ, Cooper S, Anthony FW, Wheeler T, Baker PN. Vascular endothelial growth factor and placental growth factor release in cultured trophoblast cells under different oxygen tensions. Growth Factors. 2002;20(4):189–196. doi: 10.1080/0897719021000069560. [DOI] [PubMed] [Google Scholar]
- 57.Karumanchi SA, Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the "chicken-and-egg" question. Endocrinology. 2004;145:4835–4837. doi: 10.1210/en.2004-1028. [DOI] [PubMed] [Google Scholar]
- 58.Caniggia I, Grisaru-Gravnosky S, Kuliszewsky M, Post M, Lye SJ. Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J Clin Invest. 1999;103(12):1641–1650. doi: 10.1172/JCI6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Invest. 2000;105(5):577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90(7):4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou CC, Ahmad S, Mi T, Abbasi S, Xia L, Day MC, Ramin SM, Ahmed A, Kellems RE, Xia Y. Autoantibody From Women With Preeclampsia Induces Soluble Fms-Like Tyrosine Kinase-1 Production via Angiotensin Type 1 Receptor and Calcineurin/Nuclear Factor of Activated T-Cells Signaling. Hypertension. 2008 doi: 10.1161/HYPERTENSIONAHA.107.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou CC, Ahmad S, Mi T, Xia L, Abbasi S, Hewett PW, Sun C, Ahmed A, Kellems RE, Xia Y. Angiotensin II induces soluble fms-Like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ Res. 2007;100(1):88–95. doi: 10.1161/01.RES.0000254703.11154.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bobst SM, Day MC, Gilstrap LC, 3rd, Xia Y, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human mesangial cells and induce interleukin-6 and plasminogen activator inhibitor-1 secretion. Am J Hypertens. 2005;18(3):330–336. doi: 10.1016/j.amjhyper.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Xia Y, Wen H, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Soc Gynecol Investig. 2003;10(2):82–93. doi: 10.1016/s1071-5576(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 65.Xia Y, Wen HY, Kellems RE. Angiotensin II inhibits human trophoblast invasion through AT1 receptor activation. J Biol Chem. 2002;277(27):24601–24608. doi: 10.1074/jbc.M201369200. [DOI] [PubMed] [Google Scholar]
- 66.Gutteridge JM, Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci. 2000;899:136–147. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 67.Burton GJ, Hempstock J, Jauniaux E. Oxygen, early embryonic metabolism and free radical-mediated embryopathies. Reprod Biomed Online. 2003;6(1):84–96. doi: 10.1016/s1472-6483(10)62060-3. [DOI] [PubMed] [Google Scholar]
- 68.van Tuyl M, Liu J, Wang J, Kuliszewski M, Tibboel D, Post M. Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am J Physiol Lung Cell Mol Physiol. 2005;288(1):L167–L178. doi: 10.1152/ajplung.00185.2004. [DOI] [PubMed] [Google Scholar]
- 69.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222(3):222–235. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- 70.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161(5):1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 71.Fay WP. Plasminogen activator inhibitor 1, fibrin, and the vascular response to injury. Trends Cardiovasc Med. 2004;14(5):196–202. doi: 10.1016/j.tcm.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 72.Nakamura S, Nakamura I, Ma L, Vaughan DE, Fogo AB. Plasminogen activator inhibitor-1 expression is regulated by the angiotensin type 1 receptor in vivo. Kidney Int. 2000;58(1):251–259. doi: 10.1046/j.1523-1755.2000.00160.x. [DOI] [PubMed] [Google Scholar]
- 73.Fogo AB. The role of angiotensin II and plasminogen activator inhibitor-1 in progressive glomerulosclerosis. Am J Kidney Dis. 2000;35(2):179–188. doi: 10.1016/s0272-6386(00)70324-6. [DOI] [PubMed] [Google Scholar]
- 74.Petrucco OM, Thomson NM, Lawrence JR, Weldon MW. Immunofluorescent studies in renal biopsies in pre-eclampsia. Br Med J. 1974;1(5906):473–476. doi: 10.1136/bmj.1.5906.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Packham DK, Mathews DC, Fairley KF, Whitworth JA, Kincaid-Smith PS. Morphometric analysis of pre-eclampsia in women biopsied in pregnancy and post-partum. Kidney Int. 1988;34(5):704–711. doi: 10.1038/ki.1988.236. [DOI] [PubMed] [Google Scholar]
- 76.Erlich JH, Holdsworth SR, Tipping PG. Tissue factor initiates glomerular fibrin deposition and promotes major histocompatibility complex class II expression in crescentic glomerulonephritis. Am J Pathol. 1997;150(3):873–880. [PMC free article] [PubMed] [Google Scholar]
- 77.Xu Y, Berrou J, Chen X, Fouqueray B, Callard P, Sraer JD, Rondeau E. Induction of urokinase receptor expression in nephrotoxic nephritis. Exp Nephrol. 2001;9(6):397–404. doi: 10.1159/000052638. [DOI] [PubMed] [Google Scholar]
- 78.Haller HOT, Hauck U, Distler A, Philipp T. Increased intracellular free calcium and sensitivity to angiotensin II in platelets of preeclamptic women. Am J Hypertens. 1989;2(4):238–243. doi: 10.1093/ajh/2.4.238. [DOI] [PubMed] [Google Scholar]
- 79.Hojo M, Suthanthiran M, Helseth G, August P. Lymphocyte intracellular free calcium concentration is increased in preeclampsia. Am J Obstet Gynecol. 1999;180(5):1209–1214. doi: 10.1016/s0002-9378(99)70618-6. [DOI] [PubMed] [Google Scholar]
- 80.Ray J, Vasishta K, Kaur S, Majumdar S, Sawhney H. Calcium metabolism in preeclampsia. Int J Gynaecol Obstet. 1999;66(3):245–250. doi: 10.1016/s0020-7292(99)00096-x. [DOI] [PubMed] [Google Scholar]
- 81.Sowers JR, Zemel MB, Bronsteen RA, Zemel PC, Walsh MF, Standley PR, Sokol RJ. Erythrocyte cation metabolism in preeclampsia. Am J Obstet Gynecol. 1989;161(2):441–445. doi: 10.1016/0002-9378(89)90539-5. [DOI] [PubMed] [Google Scholar]
- 82.Thway TM, Shlykov SG, Day MC, Sanborn BM, Gilstrap LC, 3rd, Xia Y, Kellems RE. Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation. 2004;110(12):1612–1619. doi: 10.1161/01.CIR.0000142855.68398.3A. [DOI] [PubMed] [Google Scholar]
- 83.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101(20):2382–2387. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- 84.Dorffel Y, Wallukat G, Bochnig N, Homuth V, Herberg M, Dorffel W, Pruss A, Chaoui R, Scholze J. Agonistic AT(1) receptor autoantibodies and monocyte stimulation in hypertensive patients. Am J Hypertens. 2003;16(10):827–833. doi: 10.1016/s0895-7061(03)00982-8. [DOI] [PubMed] [Google Scholar]
- 85.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Bmj. 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fu ML, Herlitz H, Schulze W, Wallukat G, Micke P, Eftekhari P, Sjogren KG, Hjalmarson A, Muller-Esterl W, Hoebeke J. Autoantibodies against the angiotensin receptor (AT1) in patients with hypertension. J Hypertens. 2000;18(7):945–953. doi: 10.1097/00004872-200018070-00017. [DOI] [PubMed] [Google Scholar]
- 87.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23(1):3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- 88.Saito T, Ishida J, Takimoto-Ohnishi E, Takamine S, Shimizu T, Sugaya T, Kato H, Matsuoka T, Nangaku M, Kon Y, Sugiyama F, Yagami K, Fukamizu A. An essential role for angiotensin II type 1a receptor in pregnancy-associated hypertension with intrauterine growth retardation. Faseb J. 2004;18(2):388–390. doi: 10.1096/fj.03-0321fje. [DOI] [PubMed] [Google Scholar]
- 89.Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brasen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Muller DN. Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension. 2005;45(4):742–746. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- 90.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods in Molecular Medicine. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 91.Dechend R, Llinas M, Caluwaerts S, Herse F, Lamarca B, Mueller DN, Luft FC, Pijnenborg R, Wallukat G, Granger JP. Agonistic autoantibodies to the AT1 receptor in rat models of preeclampsia: induced by chronic reduction in uterine perfusion pressure (RUPP) and low dose TNF-a infusion. Hypertension in pregnancy. 2006;25:70. (abstract). [Google Scholar]
- 92.Pearce SH, Merriman TR. Genetic progress towards the molecular basis of autoimmunity. Trends Mol Med. 2006;12:90–98. doi: 10.1016/j.molmed.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 93.Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev. 2006;19:80–94. doi: 10.1128/CMR.19.1.80-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barak Y. The immune system and happiness. Autoimmun Rev. 2006;5:523–527. doi: 10.1016/j.autrev.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 95.Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 96.Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2001;86(6):2505–2512. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 97.Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11:309–316. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Schiessl B. Inflammatory response in preeclampsia. Mol Aspects Med. 2007;28(2):210–219. doi: 10.1016/j.mam.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 99.Alexander BT, Cockrell K, Cline FD, Llinas MT, Sedeek M, Granger JP. Effect of angiotensin II synthesis blockade on the hypertensive response to chronic reductions in uterine perfusion pressure in pregnant rats. Hypertension. 2001;38(3 Pt 2):742–745. doi: 10.1161/01.hyp.38.3.742. [DOI] [PubMed] [Google Scholar]
- 100.Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006;47(3):615–618. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- 101.Langer B, Grima M, Coquard C, Bader AM, Schlaeder G, Imbs JL. Plasma active renin, angiotensin I, and angiotensin II during pregnancy and in preeclampsia. Obstet Gynecol. 1998;91(2):196–202. doi: 10.1016/s0029-7844(97)00660-1. [DOI] [PubMed] [Google Scholar]
- 102.Brown MA, Zammit VC, Mitar DA, Whitworth JA. Renin-aldosterone relationships in pregnancy-induced hypertension. Am J Hypertens. 1992;5(6 Pt 1):366–371. doi: 10.1093/ajh/5.6.366. [DOI] [PubMed] [Google Scholar]