Abstract
17β-Estradiol (E2) may influence cognitive and/or affective behavior in part via the β isoform of the estrogen receptor (ERβ). Endocrine status and behavior in cognitive (object recognition, T-maze), anxiety (open field, elevated plus maze, mirror maze, emergence), and motor/coordination (rotarod, activity chamber) tasks of proestrous and diestrous wildtype (WT) and ERβ knockout (βERKO) mice was examined. Proestrous (WT or βERKO), versus diestrous, mice had higher E2 and progestin levels in plasma, hippocampus, and cortex. The only effect of genotype on hormone levels was for corticosterone, such that βERKO mice had higher concentrations of corticosterone than did WT mice. Proestrous WT, but not βERKO, mice had improved performance in the object recognition (greater percentage of time with novel object) and T-maze tasks (greater percentage of spontaneous alternations) and less anxiety-like behavior in the plus maze (increased duration on open arms) and mirror chamber task (increased duration in mirror) than did diestrous mice. This pattern was not seen in the rotarod, open field, or activity monitor, suggesting effects may be specific to affective and cognitive behavior, rather than motor behavior/coordination. Thus, enhanced performance in cognitive tasks and anti-anxiety-like behavior of proestrous mice may require actions of ERβ in the hippocampus and/or cortex.
Keywords: estradiol, estrous cycle, prefrontal cortex, hippocampus, anxiety, learning
1. Introduction
17β-estradiol (E2) alters cognitive and affective processes. Proestrous, versus diestrous, rodents have higher E2 levels and demonstrate better cognitive performance in the inhibitory avoidance, object recognition, object placement, and classical eye-blink conditioning tasks [10,48,49]. Proestrous, versus diestrous, rats have greater anti-anxiety-like behavior in the open field and elevated plus maze [11]. Ovariectomy (ovx) can decrease cognitive performance and anti-anxiety behavior, and E2 administration reverses this [4,23,43,45]. Thus, E2 can alter cognitive and affective processes of rodents, but these patterns of results are not observed in all studies (e.g. [24]).
Actions at intracellular E2 receptors (ERs) may underlie some of these functional effects and differences in the pattern of E2's effects. ERs function as transcription factors that can be modulated by E2. E2 binds to intracellular ERs in a ligand-dependent manner, resulting in synthesis of proteins that carry out the cell's functional response [7]. The original ER identified [21] is now referred to as ERα, after a second form of ER, called ERβ was identified in rat prostate and uterine tissue [27,39]. The expression of ERα and ERβ differs between and within different tissues in the body and brain [18,36]. In the brain, there are some regional similarities and differences in expression of ERα and ERβ, which support both convergent and divergent actions of E2 and these substrates. ERα and ERβ are both co-expressed in the preoptic area, bed nucleus of the stria terminalis, and medial amygdala [33,36]. However, expression of ERα is greater than ERβ in the ventromedial hypothalamus and pituitary. Moreover, ERβ expression is more predominant than ERα in the cerebral cortex, hippocampus, anterior olfactory nucleus, dorsal raphe, substantia nigra, midbrain ventral tegmental area, and cerebellum [36,37]. This distribution of ERα and ERβ, which is overlapping but also shows distinct differences in expression, has substantiated investigation of whether there are functional effects associated with actions of ERβ for cortical and hippocampal processes.
ERβ may play a more predominant role in E2-enhanced cognitive and affective processes. Selective ER modulators (SERMs) with greater affinity for ERβ produce mnemonic effects in the water maze and inhibitory avoidance tasks and anti-anxiety effects in the open field and elevated plus maze of ovx rats [34,42]. Knockdown of ERα or ERβ in the hypothalamus and hippocampus, respectively, attenuates acute effects of E2 to facilitate sexual behavior or produce anti-anxiety effects among ovx rats [41]. ERβ knockout mice (βERKO) demonstrate poorer performance in the water maze and object recognition tasks, and more anxiety-like behavior in the elevated plus maze, that is not reversed by E2 or ERβ-SERM treatment [20,26,35,43,46,47]. Whether βERKO mice are sensitive, and respond normatively, to endogenous fluctuations in E2 for effects on cognitive and/or affective behavior is of interest. To this end, cycling mice were tested in a variety of cognitive, affective, and motor/coordination tasks. Given the putative roles of the cortex and hippocampus for these functional effects, plasma, cortical, and hippocampal concentrations of E2 and progestins (which fluctuate over the cycle) were measured. We hypothesized that if E2 has effects to improve cognitive and anti-anxiety effects in part through actions at ERβ, then: (1) WT mice in proestrus will demonstrate better performance in the cognitive tasks (T-Maze, object recognition) and more anti-anxiety-like behavior, compared to diestrous WT mice, and (2) these estrous cycle effects would be abrogated in βERKO mice.
2. Materials and Methods
These methods were pre-approved by the Institutional Animal Care and Use Committee at The University at Albany-SUNY. Adequate measures were taken to minimize pain or discomfort, and all procedures were carried out in accordance with the guidelines laid down by the National Institute of Health (NIH) regarding the care and use of animals for experimental procedures
2.1. Mice Husbandry
Subjects were adult (8-10 weeks old), gonadally-intact, female mice. Mice were group-housed (4-5 per cage) in polycarbonate cages (26 × 16 × 12.5 cm) in a temperature-controlled room (21 ± 1 °C) in the Laboratory Animal Care Facility. Mice were maintained on a 12/12-hour reversed light cycle (lights off at 8:00 am) with continuous access to Purina Mice Chow and tap water in their home cages.
2.2. Strain and Genotyping
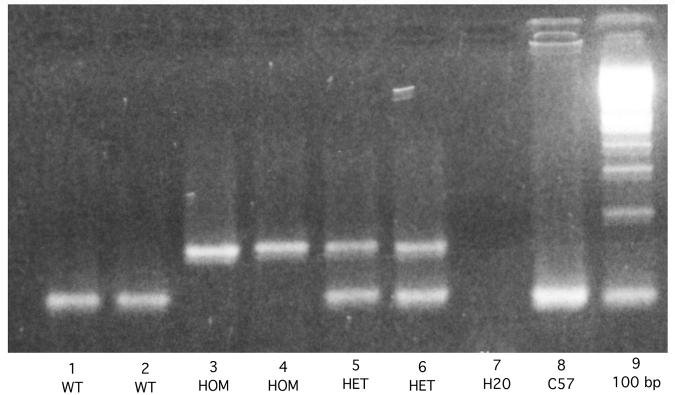
WT (n=26) and homozygous βERKO (n=30) mice were on a C57BL/6 background and derived from breeder pairs purchased from Jackson Laboratories (Bar Harbor, ME). Genotype of these mice cannot be determined based on phenotypic characteristics. As such, genomic DNA was isolated from tails and analyzed by PCR. PCR was conducted in the laboratory of Dr. Anne Messer at The Wadsworth Center by K. Manley, in the Molecular Core Facility at SUNY Albany, or in our laboratory. Briefly, DNA was denatured at 94°C for 3 min, followed by 35 cycles of amplification: 94°C for 30 secs, 60°C for 30 secs, 72°C for 30 secs and a final primer extension step at 72°C for 2 min. Specific primers used: ESR2-1, which lies upstream of insertion site in exon 2 (5-GTTGTGCCAGCCCTGTTACT-3), ESR2-2, which lies downstream of the insertion site in exon 2 (5′-TCACAGGACCAGACACCGTA-3), and ESR2-3, a neo gene-specific primer (5′-GCAGCCTCTGTTCCACATACAC-3). Primers were obtained from Integrated DNA Technologies (Coralville, IL). Bands of approximately 106 and 160 base pairs were amplified for wild-type and βERKO mice, respectively. Figure 1 shows characteristics bands for WT, heterozygous, and homozygous βERKO mice, as well as appropriate control lanes. For this study, only WT and homozygous βERKO mice were included.
Figure 1.
Picture of PCR. Lanes 1 and 2 show represent bands for wildtype (WT) mice. Representative bands for homozygous (hom; Lanes 3 and 4) and heterozygous (het; Lanes 5 and 6) βERKO mice are shown. Lanes 7 and 8 show bands for H20 loaded control lane and lane with template from a C57Bl/6 mouse (c57), respectively. Lane 9 depicts 100 base pair DNA ladder.
2.3. Estrous cycle
One cohort of mice was utilized in the present study. Mice were removed from their housing room and transported on a cart in their home cages to the adjacent core behavioral testing facility. Vaginal epithelium of experimental mice was obtained by lavage and examined under a light microscope daily between 0700 and 0900. After two weeks of regular, 4-5 day cycles, mice were tested when in proestrus or diestrus. Mice were considered in proestrus when their vaginal epithelium had characteristic nucleated cells, 4-5 days following the previous smear of this type. Mice in diestrus had heterogeneous cell types in their vaginal smears for two consecutive days and nucleated cell were absent. Mice that were to be tested on a given day were then individually housed (from 0900 to 1400) and the remaining mice were returned to their home cages to the animal facility.
2.4. Handling/habituation
Before behavioral testing commenced, all mice were subjected to 5-days of handling/habituation [46]. In brief, this procedure involved mice being picked up from their homecage, handled for 15 secs, and returned to their homecage on Day 1. On Day 2, mice were transferred from their homecage to a novel cage. On Day 3, mice were weighed and then returned to their homecage. Day 4 involved mice in their homecage being transferred to another room on a cart. On Day 5, mice were transferred on a cart to another room and then were placed in a novel environment (i.e. the open field utilized for object recognition testing) for 5 minutes.
2.5. Behavioral Testing
Given that differences in ER isoform action and distribution may underlie behavioral effects of E2, it was important to characterize estrous cycle differences in WT and βERKO mice across a variety of tasks. Object recognition and T-maze were utilized to assess cognitive behavior, that is spontaneous alternation and novelty detection, respectively, because cycling mice could be retested in these tasks in both proestrus and diestrus. On the contrary, repeated-testing is generally avoided for anxiety tasks because the tasks typically assess the mouse's response to a novel situation. As such, we utilized four different anxiety tasks that assess the propensity of mice to spend time in, or have a reduced latency to enter, a bright, open space (i.e. open field, elevated plus maze, mirror maze, emergence). By doing this, mice were tested in either proestrus or diestrus, but four assessments were done, and mice showed similar patterns of behavior across tasks when they were in the same condition. A similar protocol has been used with comparable results in ovx WT and βERKO mice administered E2 [47]. Motor/coordination indices were determined using the rotarod task and activity monitor. In these tasks, mice were repeatedly tested in different endogenous hormonal milieu. The order in which mice were tested through behavioral tasks was the same for all animals and was as indicated below. Mice were tested once or twice (in proestrus and/or diestrus) in tasks that are not sensitive to test decay (object recognition, T maze), but were tested only once in anxiety measures. Most mice were tested in all tasks; however, data from some mice on some measures could not be collected and/or considered valid because of acyclicity. Obvious behavioral differences were not noted in mice depending on which tasks they were exposed to. Behavioral data were collected by trained observers and video-recorded with the aide of a video-camera and/or video-tracking system (Any-maze- Stoelting, Inc., Wood Dale, IL).
2.5.1. Object Recognition
Object recognition can be considered a novelty detection task. Object recognition was assessed in an open field (46 × 57 × 30 cm) with small, plastic washable toys that have distinct circular (oranges, lemons, apples) or cone-like shapes (buoys, soda bottles), as stimuli (as per previous reports [29,46]). Mice were placed in an open field (one in which mice were placed in during habituation procedure, described above) that contained 2 identical objects during training for three mins. If mice did not explore (i.e. be in close proximity and facing objects, sniffing, touching) objects during training, they were excluded from testing. Four hours later, mice were placed in the open field with the familiar and a novel object for three minutes [46]. Increased percentage of time spent with the novel object is an index of improved performance in this task. Chance levels of performing in this task are typically 50% in our laboratory, and diestrous control mice performed close to chance in the present study, suggesting that diestrous mice can perform in this task, but may not have a preference for investigating novel objects.
2.5.2. T-Maze
Spontaneous alternation was assessed in the T-Maze, which has a clear Plexiglas start box connected to a start arm (30.5 × 9 × 7 cm) and two goal arms (17.8 × 9 × 7 cm; [38]). Mice were placed in the start box, the door was opened, and following one forced trial (where either the left or right side was blocked in a random fashion), the number of spontaneous alternations made to each goal was assessed for 13 consecutive trials (max latency = 900 secs). Each of the 13 trials consisted of the mouse fully returning to the start arm and then entering the right or left goal arm. Spontaneous alternation relies on the innate desire of the mouse to avoid revisiting a recently-visited location. Although it does require an intact memory, changes in preferred strategy can also account for differences in performance in this task. Chance levels of performing in this task are 50%, and we typically see that mice in control conditions have 40-60% alternations, as was observed in diestrous control mice in the present study (see [13]).
2.5.3. Rotarod
Motor behaviors of mice were assessed on a 3 cm diameter rod, elevated 35 cm above the floor using the Accurotor Roto-Rod Apparatus (AccuScan Instruments, Inc., Columbus, OH; [12,31]). One hour after three, 30-sec habituation trials, mice were placed on a horizontal rod for two consecutive sessions with a one-hour interval between each session. For the first session, the rod was rotating at a constant speed. For the second session, the speed of the rod accelerated (0 to 20 rpm over a 60-s period). There were 2 trials in each session and the latency to fall from the rod was determined (max latency = 180 secs).
2.5.4. Open Field/Activity Monitor
As per previously reported methods [14], motor behavior of mice was assessed in a 39 × 39 × 30 cm activity monitor (AccuScan Instruments, Inc., Columbus, OH) that had a grid floor with a total of 16 equal squares delineated. An observer recorded the number of entries into the 12 peripheral or 4 central squares for five min, whilst interruptions in light beams in a horizontal plane were automatically recorded. Increased central entries are indicative of anti-anxiety-like behavior [14].
2.5.5. Elevated Plus Maze
The elevated plus maze is made of stainless steel, painted a matte black, and was obtained from Columbus Instruments, Inc., Columbus, OH. It has 2 open arms, which are 30 cm in length and 5 cm in width, and 2 closed arms, which are the same size but enclosed by 14.5 cm high walls. The arms are 47.5 cm off the ground. Mice are placed at the juncture of the open and closed arms, facing an open arm. The time spent, and number of entries to, the open and closed arms was recorded during a five-minute test [44]. Increases in open arm time are considered an index of anti-anxiety-like behavior [44]. Total entries are indicative or general motor behavior.
2.5.6. Mirror Maze
The Mirror Maze consists of an open field (30 × 35 × 30 cm) with mirrors on all four walls and an adjacent alleyway (30 cm × 5 cm × 30 cm) that does not have mirrored walls. Mice were placed in the center of the mirrored-chamber and the time spent in, and entries made to, the mirrored chamber vs. the alleyway were recorded for five minutes [12,22]. Anti-anxiety-like behavior in this task is characterized by an increase in time and/or entries to the mirrored-chamber.
2.5.7. Emergence
For the emergence task, mice were placed in a closed opaque cylinder (20 × 4 × 4 cm), secured in a corner of a brightly-lit open field (39 × 39 × 30 cm) to prevent rolling. Mice were placed in this cylinder and their latency to emerge was recorded (max latency = 900 secs). A shorter latency indicates anti-anxiety-like behavior [11].
2.6. Hormone Measurement
2.6.1. Tissue Collection and Dissection
Mice were euthanized by cervical dislocation. Mice were then rapidly decapitated, and brains were quickly removed from the skull, and placed on weighboats on dry ice. Whole brains were stored at −80° C until radioimmunoassay. Blood was collected via cardiac puncture and/or from the trunk following decapitation. Blood was collected in chilled eppendorf tubes containing 10 μl saturated EDTA solution and spun at 4 °C at a speed of 3000 × g for 20 minutes and then stored at −20 °C until radioimmunoassay of plasma.
Immediately before radioimmunoassay, blood was spun at 4°C at a speed of 3000 × g for 10 minutes and whole brains were gently thawed in weighboats placed on ice. Brain sections were dissected out (cortex, hippocampus) from the whole brain samples and stored temporarily on ice until steroids were extracted from samples (as described below).
2.6.2. Radioimmunoassay for Steroid Hormones
To address whether mice exhibited normal estrous cycle variations in E2, as well as stress and other ovarian hormones, corticosterone, E2, P4 and its metabolite allopregnanolone were measured by radioimmunoassay. Detailed methods are not included because standard steroid extraction and radioimmunoassay techniques were used by our laboratory and have been described in detail elsewhere [13,46,47]. The inter-and intra-assay reliability co-efficients were: 0.05 and 0.06 for corticosterone, 0.06 and 0.08 for E2, 0.08 and 0.10 for P4, and 0.09 and 0.10 for allopregnanolone.
2.7. Statistical analyses
Two-way analyses of variance (ANOVA) were used to examine effects of genotype and cycle phase on dependent measures described above. The α level for statistical significance was p < 0.05 and a trend was considered p < 0.10. Where appropriate, Fisher's post hoc tests were used to determine group differences.
3. Results
3.1. Hormone Measures
Cycle phase, but not genotype, influenced concentrations of estradiol and progestins in plasma (E2: F(1,46) = 21.62, p < 0.01; progesterone: F(1,46) = 9.24, p < 0.05; allopregnanolone: F(1,46) = 56.68, p < 0.05), prefrontal cortex (E2: F(1,46) = 23.66, p < 0.01; progesterone: F(1,46) = 37.38, P < 0.05; allopregnanolone: F(1,46) = 5.67, p < 0.05), and hippocampus (E2: F(1,46) = 40.59, P < 0.01; progesterone: F(1,46) = 39.96, p < 0.05; allopregnanolone: F(1,46) = 13.837, p < 0.05). Proestrous mice, irrespective of genotype, had significantly higher levels of E2, progesterone, and allopregnanolone in plasma, prefrontal cortex, and hippocampus, compared to their diestrous counterparts (Table 1). The only main effect of genotype on hormone levels was for corticosterone, F(1, 46) = 5.82, p < 0.05, such that βERKO mice had higher plasma concentrations of corticosterone than did WT mice.
Table 1.
Mean (± standard error of the mean) plasma concentrations of corticosterone (B), estradiol (E2), progesterone (P4) and allopregnanolone (3α,5α-THP), or prefrontal cortex or hippocampus levels of E2, P4, and 3α,5α-THP, of diestrous wildtype (WT; far left) or estrogen receptor b knockout (βERKO; center left) and proestrous WT (middle right) or βERKO (far right) mice.
| Condition | ||||
|---|---|---|---|---|
| Diestrous | Proestrous | |||
| WT | βERKO | WT | βERKO | |
| n= | 12 | 9 | 11 | 18 |
| levels in plasma | ||||
| B (μg/dl) |
0.9 ± 0.2 |
#2.4 ± 1.42 |
0.9 ± 0.2 |
#4.6 ± 1.3 |
| E2 (pg/ml) |
10.0 ± 3.0 |
11.0 ± 2.9 |
*40.5 ± 8.0 |
*32.9 ± 5.2 |
| P4 (ng/ml) |
15.3 ± 7.6 |
10.8 ± 3.8 |
*38.1 ± 9.2 |
*34.9 ± 6.8 |
| 3α,5α- THP(ng/ml) |
0.7 ± 0.3 |
0.4 ± 0.2 |
*19.4 ± 4.6 |
*22.5 ± 2.7 |
| levels in the prefrontal cortex | ||||
| E2 (pg/mg) |
8.5 ± 1.2 |
7.3 ± 1.7 |
*13.9 ± 1.1 |
*14.2 ± 1.0 |
| P4 (ng/mg) |
1.8 ± 0.7 |
2.2 ± 1.1 |
*17.4 ± 3.9 |
*16.4 ± 2.2 |
| 3α,5α- THP(ng/mg) |
7.6 ± 2.8 |
8.5 ± 2.5 |
*14.2 ± 2.5 |
*12.3 ± 1.0 |
| levels in the lippocampus | ||||
| E2 (pg/mg) |
6.4 ± 1.3 |
6.7 ± 1.7 |
*14.7 ± 2.1 |
*16.5 ± 0.8 |
| P4 (ng/mg) |
0.8 ± 0.3 |
1.7 ± 1.2 |
*18.6 ± 4.6 |
*21.3 ± 2.8 |
| 3α,5α-THP- THP(ng/mg) |
9.4 ± 2.8 |
8.3 ± 2.7 |
*20.4 ± 2.4 |
*14.8 ± 1.5 |
indicates a significant (P ≤ 0.05) effect of genotype, due to higher levels among the βERKO mice.
indicates a significant (P ≤ 0.05) effect of cycle phase, which is attributable to higher levels among proestrous mice.
3.2. Behavioral Measures
3.2.1. Object Recognition
There was a main effect of genotype, F(1, 97) = 16.57, p< 0.01, no significant main effect of cycle phase, and a significant interaction of cycle phase and genotype, F(1, 97) = 3.78, p< 0.05, for the percentage of time spent exploring a novel object (Figure 2). Proestrous WT, but not βERKO, mice spent more time investigating the novel object during testing than did diestrous mice.
Figure 2.
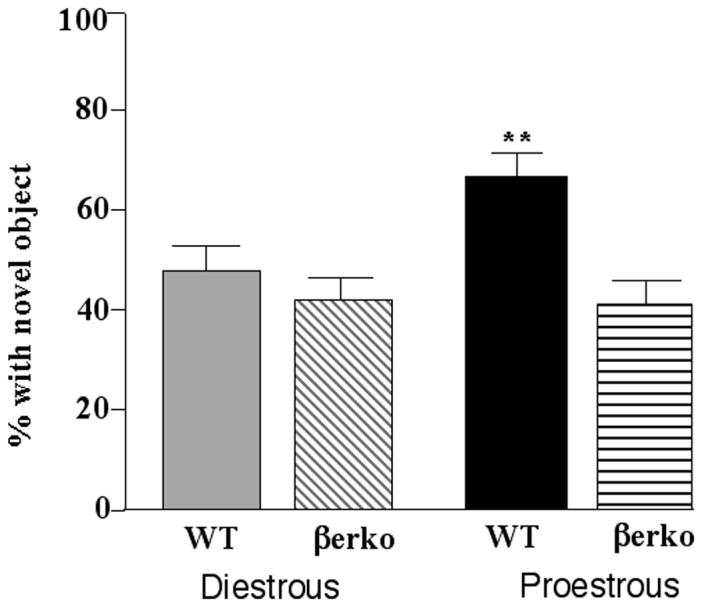
Mean (± standard error of the mean) percentage of time spent exploring the novel object made by diestrous wildtype (WT; n=27) or estrogen receptor β knockout (βERKO; n=28) mice or proestrous WT (n=20) or βERKO (n=26) mice. ** indicates a significant (P ≤ 0.05) interaction due to proestrous WT, but not βERKO, mice being greater than diestrous mice.
3.2.2. T-Maze
There was a main effect of genotype, F(1, 82) = 13.42, p < 0.01, no main effect of cycle phase, and a significant interaction of cycle phase and genotype, F(1, 82) = 7.81, p < 0.05, for the percentage of spontaneous alternations made in the T-maze (Figure 3). WT, but not βERKO, proestrous mice made more spontaneous alterations than did their diestrous counterparts.
Figure 3.
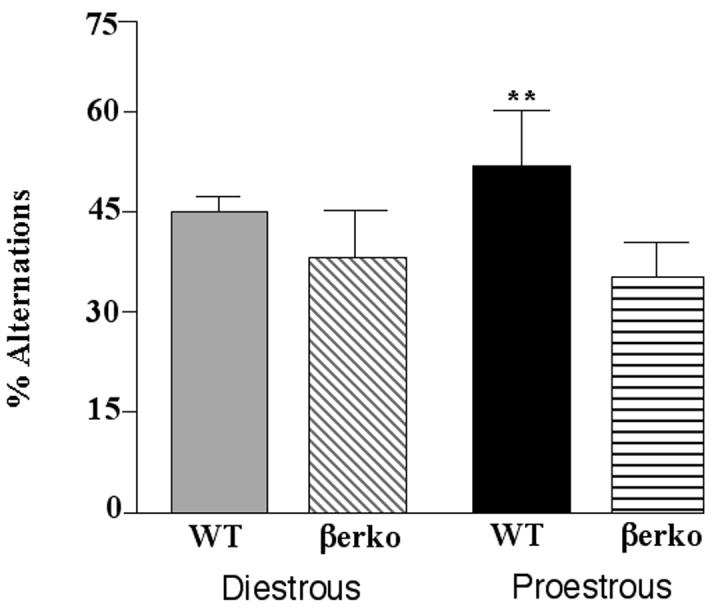
Mean (± standard error of the mean) percentage of alternations in the T-maze made by diestrous wildtype (WT; n=23) or estrogen receptor β knockout (βERKO; n=22) mice or proestrous WT (n=16) or βERKO (n=25) mice. **indicates a significant (P ≤ 0.05) interaction due to proestrous WT, but not βERKO, mice being greater than diestrous mice.
3.2.3. Rotarod
Cycle phase, F(1,108) =6.05, p< 0.05, but neither genotype, nor an interaction of cycle phase and genotype, influenced the latency to fall from the rod, when rotating at a fixed speed (Table 4). Proestrous mice had longer latencies to fall from the rotarod during the fixed speed trials than did diestrous mice. Cycle phase and genotype did not alter performance during the accelerated speed trials.
3.2.4. Open Field/Activity Monitor
Genotype, F(1,46) = 23.42, p < 0.05, and cycle phase, F(1,46) = 5.96, p < 0.05, influenced the number of entries to central squares in the open field, but there were no significant interaction between these variables (Table 2). WT mice made more central entries than did βERKO mice and proestrous mice made more central entries than did diestrous mice. Genotype, F(1,46) = 6.67, p < 0.01, but not cycle phase, or an interaction between these variables, altered the total entries made in the open field (Table 2). WT mice made more total entries than did βERKO mice. Cycle phase and genotype did not alter general motor behavior (i.e. number of beam breaks made) in the activity monitor.
Table 2.
Behavior (mean ± standard error of the mean) of diestrous wildtype (WT; far left) or estrogen receptor β knockout (βERKO; center left) and proestrous WT (middle right) or βERKO (far right) mice.
| Condition | ||||
|---|---|---|---|---|
| Diestrous | Proestrous | |||
| WT | βERKO | WT | βERKO | |
| Fixed Rotorod: Latency to fall (secs) |
139.8 ± 11.2 |
151.0 ± 9.1 |
*161.7 ± 6.9 |
*171.0 ± 6.2 |
| Accelerated Rotorod: Latency to fall (secs) |
108.6 ± 7.0 |
111.3 ± 6.7 |
111.3 ± 5.9 |
102.7 ± 6.4 |
| Open Field- Central entries |
19.2 ± 4.4 |
#7.2 ± 2.0 |
*29.9 ± 4.1 |
*#11.7 ± 1.7 |
| Open Field- Total entries |
122.0 ± 15.4 |
#102.6 ± 10.2 |
150.9 ± 16.9 |
#100.4 ± 12.2 |
| Activity Monitor-# of beam breaks |
150.0 ± 54.9 |
71.6 ± 11.6 |
122.4 ± 24.3 |
137.8 ± 31.4 |
| Latency to emerge (secs) |
68.9 ± 19.6 |
#136.9 ± 39.4 |
51.1 ± 20.8 |
#109.7 ± 28.9 |
indicates a significant (P < 0.05) effect of genotype due to differences among βERKO and WT mice.
indicates a significant (P < 0.05) effect of cycle phase, due to increases among proestrous compared to diestrous mice.
3.2.5. Elevated Plus Maze
There was a main effect of cycle phase, F(1,35) = 14.03, p < 0.01, and genotype, F(1,35) = 6.18, p < 0.02, and a trend for an interaction between cycle phase and genotype, F(1,35) = 3.68, p < 0.06, for duration on open arms in the elevated plus maze. Proestrous WT, but not βERKO, mice spent more time on the open arms of the elevated plus maze than did their diestrous counterparts (Figure 4). Cycle phase, F(1,35) = 6.66, p < 0.05, but not genotype, influenced the number of total arm entries in the elevated plus maze. Proestrous mice (9.8 ± 1.1) made more entries than did diestrous mice (6.7 ± 1.1).
Figure 4.
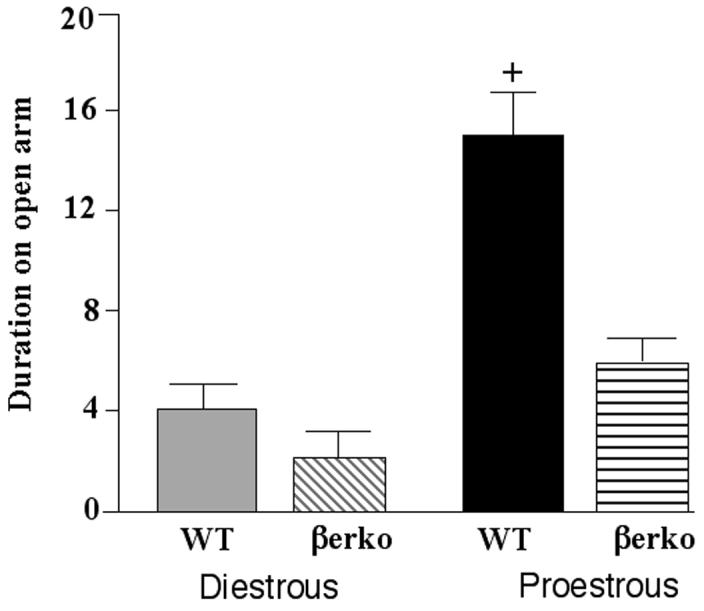
Mean (± standard error of the mean) duration of time spent on the open arms of the elevated plus maze by diestrous wildtype (WT; n=10) or estrogen receptor β knockout (βERKO; n=11) or proestrous WT (n=10) or βERKO (n=8) mice. + indicates a trend (P ≤ 0.06) for an interaction due to proestrous WT, but not βERKO, mice being greater than diestrous mice.
3.2.6. Mirror Chamber
There was a trend for a main effect of genotype, F(1,29) = 3.22, p < 0.08, no effect of cycle phase, and an interaction between these variables, F(1,29) = 5.27, p < 0.03, to influence time spent in the mirror chamber (Figure 5). Proestrous WT, but not βERKO, mice spent significantly more time in the mirror chamber than did their diestrous counterparts.
Figure 5.
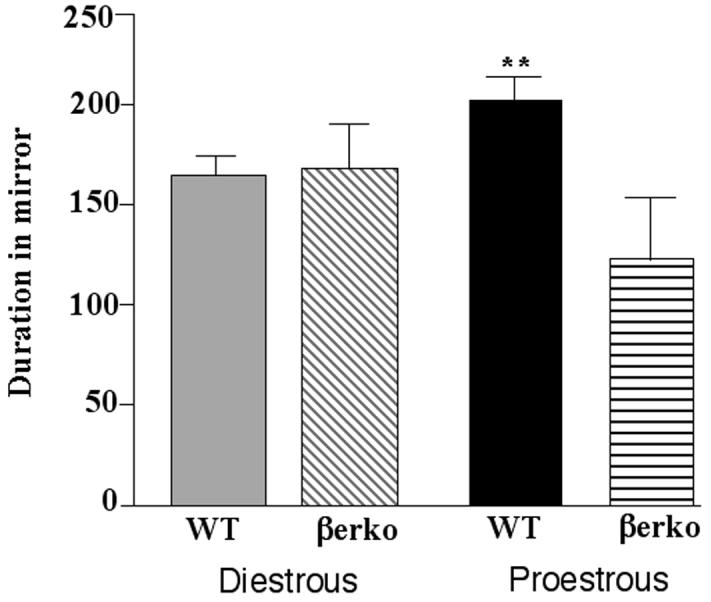
Mean (± standard error of the mean) duration of time spent in the mirror chamber made by diestrous wildtype (WT; n=7) or estrogen receptor β knockout (βERKO; n=10) mice or proestrous WT (n=8) or βERKO (n=8) mice. **indicates a significant (P ≤ 0.05) interaction due to proestrous WT, but not βERKO, mice being greater than diestrous mice.
3.2.7. Emergence
Genotype, F(1,37) = 4.15, p < 0 .05, but not cycle phase, nor an interaction between these variables, influenced the latency to emerge (Table 2). WT mice had decreased latencies as compared to βERKO mice.
4. Discussion
Our hypothesis that endogenous E2 in mice may alter performance in cognitive and anxiety tasks in part through actions at ERβ was partially supported. E2 levels were similarly increased among proestrous βERKO and WT mice. WT, but not βERKO, mice in proestrus made more alternations in the T-maze task, spent more time investigating novel objects in the object recognition task, spent more time in the mirror chamber, and tended to have increased time spent in the open arm in the elevated plus maze, than did their diestrous counterparts. However, these interactions were not observed in all behavioral tasks. For example, irrespective of cycle phase, βERKO, compared to WT, mice made fewer central and total entries in the open field and had longer latencies to emerge from a dark container. Both WT and βERKO mice, when in proestrus, made more central entries in the open field, more total arm entries in the plus maze, and had longer latencies to fall from the fixed speed rotating rotarod, compared to diestrous mice. Thus, enhancements in some measures of cognitive performance and anti-anxiety like behavior of mice in the proestrous phase of the estrous cycle may require actions at ERβ.
The results of this study confirm and extend previous findings from our laboratory and others on the potential role of ERβ for performance in cognitive tasks. Although βERKO compared to WT mice show deficits in water maze performance [35], in the object recognition and T-maze task, we found similar baseline performance of WT and βERKO mice (i.e. close to chance levels typically observed in mice in our laboratory). WT and βERKO mice perform in a comparable manner in the inhibitory avoidance task, which is mediated by the hippocampus and amygdala, but ERα knockout mice show deficits [15]. These data suggest that some effects of E2 for cognitive processes may involve ERβ in the hippocampus, and extends these results to suggest that the prefrontal cortex may also be a target. Proestrous, compared to diestrous, mice performed better in the T-maze and object recognition tasks. There are similarities in these tasks in that they both take advantage of animals' natural tendency to explore novel stimuli and performance in these tasks is not dependent upon presentation of rewarding or aversive stimuli. Although there is little evidence for direct comparisons of specific cortical and hippocampal circuitry for spontaneous alternation and object recognition memory, and lesion studies have yet to demonstrate irrefutable evidence of the role of these regions, there is some indication from the literature that performance in both tasks rely to some degree on proper functioning of the hippocampus and cortex [1,6,19,28,32]. Given that we found similar effects in both tasks, further investigations of the relative role of the cortex and hippocampus as targets for performance in this task are warranted. Indeed, few studies have assessed the role of E2 for spontaneous alternation; however, it has been demonstrated that chronic oral administration of E2 to middle-aged mice enhances performance in the object recognition task [8]. Although we, and others, have shown that ERβ may be involved in cognitive tasks, such as the water maze, inhibitory avoidance, and object recognition/placement, by using SERMs or βERKO mice [15, 34, 35, 45, 46], this is the first report showing that endogenous variations in steroids may alter performance similarly in the T-maze and object recognition tasks of young female mice. Thus, it may be that natural elevations in E2 have effects on cortical-hippocampal circuitry to enhance non-spatial, working memory of young mice, but this needs to be investigated further.
The present results that proestrous WT, but not βERKO, mice demonstrated more anti-anxiety-like behavior than did diestrous mice, confirms and extend previous findings. Proestrous, compared to diestrous, mice spend twice as long on the open arms of the elevated plus maze [16]. Moreover, proestrous rats spend more time on the open arms of the elevated plus maze, more time in social interaction with a conspecific, and less time freezing in response to shock than do diestrous or male rats [9,11,43]. Ovx typically increases anxiety-like behavior, and systemic or intrahippocampal E2 reverses this effect, but this depends upon regimen of E2 administered [43]. We, and others, have demonstrated that cognitive-enhancing and anti-anxiety-like effects may occur through actions at ERβ using SERMs, knockout mice, or targeted knock down strategies [20,26,41,43,45]. Thus, the present results confirm previous reports that E2 can alter affective behaviors of female rodents, and suggest that the hippocampus, and actions through ERβ, are important for these effects.
WT and βERKO mice generally showed normative patterns of steroid hormone levels. Plasma, cortical, and hippocampal levels of E2 were greater among proestrous versus diestrous mice, irrespective of genotype. These findings are analogous to previous reports that βERKO mice have normal levels of gonadal steroids [3]. In addition, βERKO mice can respond to ovarian steroid stimulation and demonstrate reproductive behavior, albeit, with deficits in ovulation [25]. Here, we see that βERKO mice have E2 and progestin levels commensurate with those previously reported over the estrous cycle [2]. Although functional effects we observed may be related to E2, progestins and androgens also are elevated during proestrus and can alter cognitive and affective performance [5,10,48]. Furthermore, we found differences in circulating corticosterone levels between WT and βERKO mice, such that βERKO mice had higher levels than did WT mice. These results are supported by previous findings that show that subcutaneous administration of E2 or an ERβ agonist reduces plasma corticosterone levels of rats [30] or WT, but not βERKO, mice [47]. Thus, we cannot rule out the contributory role of other hormones that are elevated in proestrus for performance in the tasks utilized in the present study.
The central targets for E2's actions at ERβ for the behavioral effects observed require further investigation. It is likely that there are diverse targets for actions of E2 for these behaviors. For instance, in vivo work has demonstrated that membrane ER-mediated actions of E2 can potentiate intracellular ER-mediated actions [40]. Despite these limitations, the present findings do provide insight into behaviors that are sensitive and responsive to normal fluctuations in E2, such that we can further investigate their functions and the underlying mechanisms in the future.
Acknowledgement
This research was supported in part by funding from the Department of Defense (BC051001), National Science Foundation (IBN03-16083), and National Institute of Mental Health (MH0676980). The technical assistance of Theresa Blakesley, Judy Horan, and Allicia Ryan is greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER. The role of the hippocampus in object recognition in rats: examination of the influence of task parameters and lesion size. Behav Brain Res. 2006;167:183–95. doi: 10.1016/j.bbr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Corpechot C, Collinss BE, Carey MP, Tsouros A, Robel P, Fry JP. Brain neurosteroids during the mouse estrous cycle. Brain Res. 1997;766:276–278. doi: 10.1016/s0006-8993(97)00749-x. [DOI] [PubMed] [Google Scholar]
- 3.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–317. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 4.Daniel J, Fader A, Spencer A, Dohanich G. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- 5.Edinger KL, Frye CA. Androgens' effects to enhance learning may be mediated in part through actions at estrogen receptor-β in the hippocampus. Neurobiol Learn Mem. 2007;87:78–8. doi: 10.1016/j.nlm.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- 7.Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones--a focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–56. [PubMed] [Google Scholar]
- 8.Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behav Neurosci. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Guasti A, Martinez-Mota L, Estrada-Camarena E, Contreras CM, Lopez-Rubalcava C. Chronic treatment with desipramine induces an estrous cycle-dependent anxiolytic-like action in the burying behavior, but not in the elevated plus-maze test. Pharmacol Biochem Behav. 1999;63:13–20. doi: 10.1016/s0091-3057(98)00231-7. [DOI] [PubMed] [Google Scholar]
- 10.Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–16. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α -THP. Pharmacol Biochem Behav. 2000;67:587–96. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 12.Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O'Malley BW, Pfaff DW, Rhodes ME. Progesterone's effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology. 2006;186:312–322. doi: 10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- 13.Frye CA, Walf AA. Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alternation, object recognition, but not placement, water maze, and contextual and cued conditioned fear tasks. Neurobiol Learn Mem. 2008;90:171–7. doi: 10.1016/j.nlm.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5α-reductase. Brain Res. 2004;1004:116–24. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor α and β on cognitive function. Brain Res. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- 16.Galeeva AY, Tuohimaa P. Analysis of mouse plus-maze behavior modulated by ovarian steroids. Behav Brain Res. 2001;119:41–47. doi: 10.1016/s0166-4328(00)00341-7. [DOI] [PubMed] [Google Scholar]
- 17.Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;6:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson JA. What pharmacologists can learn from recent advances in estrogen signaling. Trends Pharmacol Sci. 2003;9:479–85. doi: 10.1016/S0165-6147(03)00229-3. [DOI] [PubMed] [Google Scholar]
- 19.Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor β influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84:157–63. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Jensen EV, Jacobson HI. Basic guides to the mechanism of estrogen action. Recent Prog Horm Res. 1962;18:387–414. [Google Scholar]
- 22.Kliethermes CL, Finn DA, Crabbe JC. Validation of a modified mirrored chamber sensitive to anxiolytics and anxiogenics in mice. Psychopharmacology. 2003;2:190–197. doi: 10.1007/s00213-003-1493-z. [DOI] [PubMed] [Google Scholar]
- 23.Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Korol DL, Kolo L. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- 25.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. PNAS. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor-b deficient mice. PNAS. 2001;98:12278–82. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson, Gustafson JA. Cloning of a novel receptor expressed in rat prostate and ovary. PNAS. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 29.Luine TD, Jacome LF, Maclusky NY. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 30.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology. 2005;146:97–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 31.McFadyen MP, Kusek G, Bolivar VJ, Flaherty L. Differences among eight inbred strains of mice in motor ability and motor learning on a rotorod. Genes Brain Behav. 2003;2:214–9. doi: 10.1034/j.1601-183x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 32.Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behav Brain Res. 2001;127:159–81. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- 33.Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-α and -β mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54:175–80. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- 34.Rhodes ME, Frye CA. ERβ-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–91. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor β gene impairs spatial learning in mice. PNAS. 2002;99:3996–01. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor β immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- 38.Toubas PL, Abla KA, Cao W, Logan LG, Seale TW. Latency to enter a mirrored chamber: a novel behavioral assay for anxiolytic agents. Pharmacol Biochem Behav. 1990;35:121–6. doi: 10.1016/0091-3057(90)90215-4. [DOI] [PubMed] [Google Scholar]
- 39.Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor β. Mol Endocrinol. 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- 40.Vasudevan N, Kow LM, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. PNAS. 2001;98:12267–71. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walf AA, Ciriza I, Garcia-Segura LM, Frye CA. Antisense oligodeoxynucleotides for estrogen receptor-β and α attenuate estradiol's modulation of affective and sexual behavior, respectively. Neuropsychopharmacology. 2008;33:431–40. doi: 10.1038/sj.npp.1301416. [DOI] [PubMed] [Google Scholar]
- 42.Walf AA, Frye CA. ERβ-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- 43.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walf AA, Frye CA. Rapid and estrogen receptor beta mediated actions in the hippocampus mediate some functional effects of estrogen. Steroids. 2008;73:997–1007. doi: 10.1016/j.steroids.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–21. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav Neurosci. doi: 10.1037/a0012749. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci. 2001;115:175–87. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]