Abstract
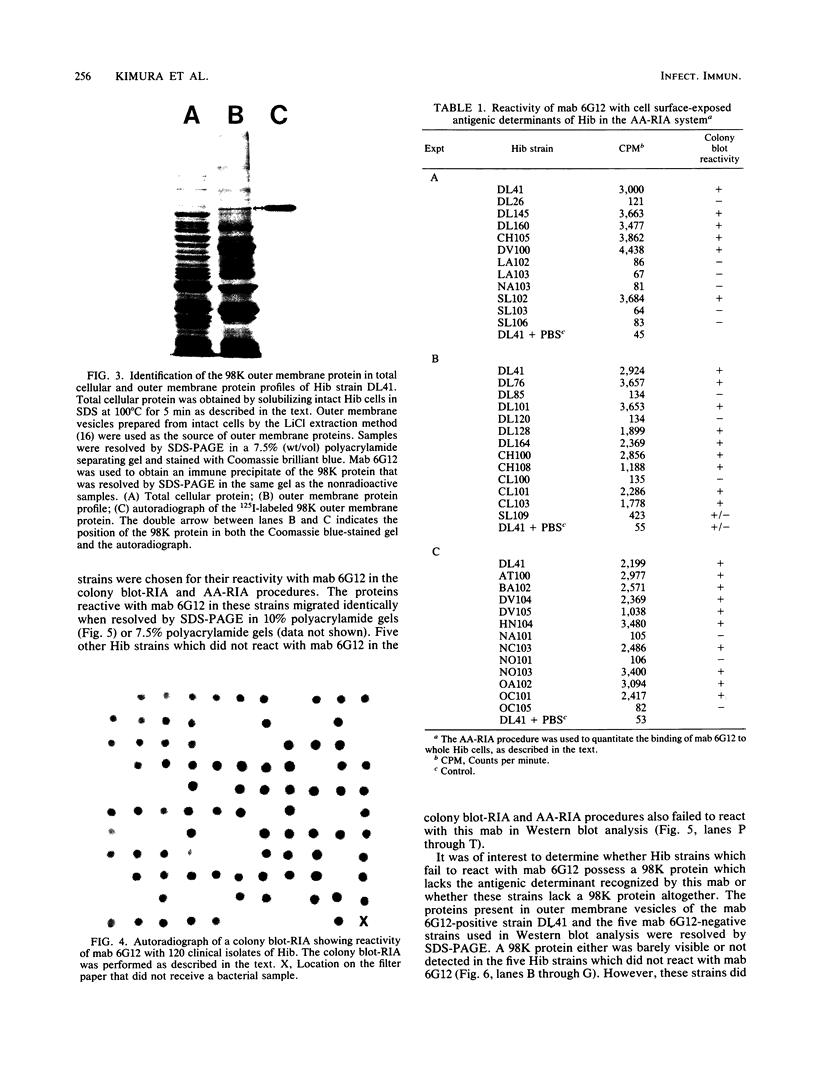
Cell surface-exposed antigenic determinants of several high-molecular-weight outer membrane proteins of Haemophilus influenzae type b (Hib) have been shown to be consistently immunogenic in human infants convalescing from Hib meningitis. A monoclonal antibody (mab), 6G12, directed against one of these cell surface-exposed outer membrane proteins that has an apparent molecular weight of 98,000 (98K) was identified by radioimmunoprecipitation analysis. Of 120 clinical isolates of Hib, 83 were found to possess antigenic determinants which reacted with mab 6G12 in a colony blot-radioimmunoassay procedure, indicating that the antigenic determinant recognized by mab 6G12 is present in the majority of Hib strains. A different radioimmunoassay, which uses whole Hib cells as antigen, confirmed that strains reactive with mab 6G12 in the colony blot-radioimmunoassay procedure possessed a cell surface-exposed and antibody-accessible antigenic determinant recognized by this mab. Hib strains which did not react with mab 6G12 were found to lack a 98K protein. Passive immunization with mab 6G12 reduced the level of bacteremia that developed in infant rats challenged with the homologous Hib strain against which this mab was raised. In contrast, no protection was observed when the challenge strain was one which lacks the antigenic determinant recognized by mab 6G12. Radioimmunoprecipitation analysis of sera from human infants convalescing from Hib meningitis detected an antibody response directed against the 98K protein. The protection against experimental Hib disease provided by antibody to the 98K protein, the immunogenicity of this protein in human infants, and its presence in a majority of Hib strains indicate that the 98K outer membrane protein may have potential for vaccine development.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. Antibody responses to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of the type b capsule with the nontoxic protein CRM197. Infect Immun. 1983 Jan;39(1):233–238. doi: 10.1128/iai.39.1.233-238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Schneerson R., Robbins J. B., Rastogi S. C. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun. 1983 Apr;40(1):245–256. doi: 10.1128/iai.40.1.245-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Insel R. A. Protection from infection with Haemophilus influenzae type b by monoclonal antibody to the capsule. J Infect Dis. 1982 Aug;146(2):249–254. doi: 10.1093/infdis/146.2.249. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Frisch C. F., Hansen E. J. A set of two monoclonal antibodies specific for the cell surface-exposed 39K major outer membrane protein of Haemophilus influenzae type b defines all strains of this pathogen. Infect Immun. 1983 Nov;42(2):516–524. doi: 10.1128/iai.42.2.516-524.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Holmans P. L., Hansen E. J. Immunogenic proteins in cell-free culture supernatants of Haemophilus influenzae type b. Infect Immun. 1984 Apr;44(1):41–48. doi: 10.1128/iai.44.1.41-48.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Haemophilus influenzae type b: disease and immunity in humans. Ann Intern Med. 1973 Feb;78(2):259–269. doi: 10.7326/0003-4819-78-2-259. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., Johnston K. H. Detection of antibody-accessible proteins on the cell surface of Haemophilus influenzae type b. Infect Immun. 1981 Sep;33(3):950–953. doi: 10.1128/iai.33.3.950-953.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Robertson S. M., Gulig P. A., Frisch C. F., Haanes E. J. Immunoprotection of rats against Haemophilus influenzae type B disease mediated by monoclonal antibody against a haemophilus outer-membrane protein. Lancet. 1982 Feb 13;1(8268):366–368. doi: 10.1016/s0140-6736(82)91394-0. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Human antibody response to individual outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1982 Sep;37(3):1032–1036. doi: 10.1128/iai.37.3.1032-1036.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon E. R., Murphy P. A. Haemophilus influenzae bacteremia and meningitis resulting from survival of a single organism. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1534–1536. doi: 10.1073/pnas.75.3.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Peltola H., Käyhty H., Jousimies H., Pettay O., Ruoslahti E., Sivonen A., Renkonen O. V. Polysaccharide vaccines of group A Neisseria meningtitidis and Haemophilus influenzae type b: a field trial in Finland. J Infect Dis. 1977 Aug;136 (Suppl):S43–S50. doi: 10.1093/infdis/136.supplement.s43. [DOI] [PubMed] [Google Scholar]
- Robertson S. M., Frisch C. F., Gulig P. A., Kettman J. R., Johnston K. H., Hansen E. J. Monoclonal antibodies directed against a cell surface-exposed outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1982 Apr;36(1):80–88. doi: 10.1128/iai.36.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P. F., Smith A. L., Smith D. H., Anderson P. Role of immunity in the clearance of bacteremia due to Haemophilus influenzae. J Infect Dis. 1978 Oct;138(4):427–436. doi: 10.1093/infdis/138.4.427. [DOI] [PubMed] [Google Scholar]