Abstract
RhoGTPases are molecular switches that integrate extracellular signals to perform diverse cellular responses. This ability relies on the network of proteins regulating RhoGTPases activity and localization, and on the interaction of RhoGTPases with many different cellular effectors. Myelination is an ideal place for RhoGTPases regulation, as it is the result of fine orchestration of many stimuli from at least two cell types. Recent work has revealed that RhoGTPases are required for Schwann cells to sort, ensheath and myelinate axons. Here we will review recent advances showing the critical roles for RhoGTPases in various aspects of Schwann development and myelination, including the recent discovery of their involvement in Charcot-Marie-Tooth disease. Comparison with potential roles of RhoGTPases in central nervous system myelination will be drawn.
Introduction
RhoGTPAses
The Rho-family of small guanosine triphosphatases (Rho GTPases) comprises a large subgroup of the Ras superfamily of 20-30 kDa GTP-binding proteins that act as molecular switches to control a large variety of cellular processes. They are defined by the presence of a ∼13 aminoacid α-helical domain, the so-called Rho insert domain, which distinguishes them from other small GTPases (Johnson, 1999). Rho GTPases are ubiquitously expressed from yeast to mammals, indicating that these proteins evolved early during evolution. There are 22 different Rho GTPases in mammals sharing over 50% sequence identity. They are divided into eight subfamilies: the RhoA-related subfamily (RhoA, RhoB, RhoC), the Rac1-related subfamily (Rac1, Rac2, Rac3, RhoG), the Cdc42-related subfamily (Cdc42, TC10, TCL, Chp/Wrch-2, Wrch1), the Rnd subfamily (Rnd1, Rnd2, RhoE/Rnd3), the RhoBTB subfamily, the TTF/RhoH subfamily, the RhoD/Rif subfamily and the more recently described Miro subfamily (Miro-1 and Miro-2) (Wennerberg and Der, 2004). Out of these subfamilies Rnd, TTF/RhoH, RhoD/Rif and RhoBTB display novel characteristics that make them atypical as compared to the other family members (Aspenstrom et al., 2007). Among all Rho GTPases, Cdc42 (cell division cycle 42), Rac1 (Ras-related C3 botulinum toxin substrate 1) and RhoA (Ras homologous member A) have been studied extensively and most of our knowledge regarding Rho GTPases derives from the study of these three proteins. Rho GTPases are binary molecular switches that cycle between an inactive GDP-bound and an active GTP-bound state in response to extracellular stimuli. In the active state they can bind to downstream effectors to elicit different biological responses (Luo, 2000; Moon and Zheng, 2003). Each Rho-family protein activates multiple effectors, and different Rho-family proteins can recognize the same effectors. The GDP/GTP cycling is subject to tight control by three different classes of regulatory proteins: (1) GEFs (GTPase exchange factors), which promote the exchange of the bound GDP for GTP and thus activate Rho GTPases (Schmidt and Hall, 2002) to initiate downstream signaling through one of several effector proteins. (2) GAPs (GTPase activating proteins), which catalyze the intrinsic ability of GTPases to hydrolyze the bound GTP to GDP, thereby inactivating them (Moon and Zheng, 2003) and the GDIs (guanine nucleotide dissociation inhibitors), which stabilize the GDP-bound form of the GTPase and inhibit binding of Rho proteins to membranes preventing nucleotide exchange and activation (Olofsson, 1999). The atypical Rho GTPases do not always follow this common scheme of regulation. Their regulation rarely depends on GEFs and/or GAPs, they can associate constitutively to membranes and seem to be highly regulated at the level of their expression (Wennerberg and Der, 2004; Aspenstrom et al., 2007). In addition, they can also be regulated by protein:protein interactions involving domains that are not found in the other members of the Rho GTPase family (Wennerberg and Der, 2004; Aspenstrom et al., 2007).
Rho GTPases were described originally in cytoskeletal regulation in response to extracellular signals. Both growth factor and adhesion receptors are known to activate RhoGTPases. Adhesion receptors of the integrin family activate RhoGTPases at several levels, favoring dissociation of GDIs proteins, translocation to the plasma membrane and association with effectors, and maintenance at the membrane through inhibition of endocytosis (Del Pozo et al., 2002; del Pozo et al., 2004). Over the past few years, Rho GTPases have also been found to participate in many other fundamental processes such as polarization, transcriptional regulation, cell cycle progression and membrane transport pathways (Etienne-Manneville and Hall, 2002). Therefore, Rho GTPases are implicated in a multitude of cellular processes, which might lie in their ability to interact with a number of downstream targets so that they can coordinately activate diverse molecular processes required for a particular cellular response. Although target proteins do not contain many single recognizable sequence motifs useful in database searches, over 60 targets have so far been identified experimentally for Cdc42, Rac, and Rho (Bishop and Hall, 2000; Symons and Settleman, 2000; Riento and Ridley, 2003). It is still unclear which of these are responsible for the diverse biological effects of Rho GTPases. Furthermore, established signaling pathways cannot simply be transferred to every system, but rather seem to be cell type and context dependent. In line with this, recent data from our labs revealed important differences in the manner by which Cdc42 and Rac1 regulate Schwann cell (Benninger et al., 2006; Nodari et al., 2007) and oligodendrocyte cell biology (Thurnherr et al., 2006). Despite being intrinsically different, both these glial cell types proliferate and migrate over long distances before undergoing the remarkable morphological changes associated with ensheathment and myelination of axons. There is mounting evidence that at least some of these processes are regulated by Rho GTPase signaling.
Schwann cell development
Schwann cells precursors originate from the neural crest (reviewed (Jessen and Mirsky, 2005). Precursors colonize nerves directly through the ventro-lateral migratory stream (Le Douarin and Kalcheim, 1999), and spinal roots (dorsal and part of ventral) after becoming boundary cap cells, a transient population that occupies the boundary between central and peripheral nervous system between E10.5 and post-natal (P) day 5) (Maro et al., 2004). Next (E12-13.5 in the mouse) Schwann cells precursors migrate along outgrowing axons, and even precede growth cones on their path to peripheral targets (Wanner et al., 2006). This migratory step likely involves RhoGTPases, and experiments in drosophila described below support this view. Precursors become immature Schwann cells at E14.5-E15.5, which surround large bundles of axons to form families encircled by a common basal lamina (Webster et al., 1973). At this stage Schwann cells initiate radial sorting of axons, by recognizing and segregating large axons destined to be myelinated away from small axons that will remain un-myelinated (Webster et al., 1973). This pre-requisite for peripheral myelination continues until postnatal P10 in the mouse. It involves both matching of Schwann cell number to axons and insertion of Schwann cell processes within axons to segregate and ensheath them. Matching of numbers is orchestrated by survival/proliferation signals sent by neuregulins on axons and laminins in the basal lamina through receptors on Schwann cells (Dong et al., 1995; Grinspan et al., 1996; Yang et al., 2005; Yu et al., 2005). Both of these systems activate phosphatidylinositol 3-kinase (PI3K) to mediate survival and proliferation (Maurel and Salzer, 2000; Yu et al., 2005), but it remains to be established how they are integrated in time and space. Matching of cell numbers and cytoskeletal-mediated protrusions during radial sorting are dependent on RhoGTPases, downstream of neuregulins and laminin receptors, as described below. Thus RhoGTPases are poised as excellent candidates to integrate signals from axons and the extracellular matrix in Schwann cells.
After larger axons have been sorted at the periphery of the bundle, its ensheathing Schwann cell detaches from the family and reorganizes its own basal lamina, a process termed “defasciculation”, to become a promyelinating Schwann cell. The molecular mechanisms controlling defasciculation are poorly understood, but likely involve laminin receptors and focal adhesion kinase (Feltri et al., 2002; Grove et al., 2007).
A promyelinating Schwann cell, in a 1:1 relation with an axon, wraps several layers of membrane around the axon to form myelin. This requires formation of a cytoplasmic extension that have been compared to a giant lamellipodia (Kim et al., 2006a; Nodari et al., 2007), suggesting control by the same molecular machinery that regulate actin polymerization at the leading edge of lamellipodia in cultured cells. Indeed RhoGTPases and their effectors have been found in purified myelin (Bacon et al., 2007).
Rac1
The three founding members of small Rho GTPases (Rac1, Cdc42 and RhoA) are expressed and active in both neurons and Schwann cells (Terashima et al., 2001). Two reports recently addressed the role of Rac1 in nerves, by inactivating the Rac1 gene specifically in Schwann cells starting at E12.5 (using Desert Hedgehog Cre (Joseph et al., 2004) or at E13.5 using mP0TotCre, (Feltri et al., 1999b). Results were similar in both cases and showed a delay in the process of radial axonal sorting and an arrest in myelination (Benninger et al., 2007; Nodari et al., 2007). Rac1 in Schwann cells is activated by β1-integrins, as both GTP loading and translocation of active Rac1 to the Schwann cell membrane was impaired in mice lacking β1 integrins in Schwann cells. β1 integrin mutant mice have a more important arrest in radial sorting of axons (Feltri et al., 2002) that can be partially rescued by expressing dominant active Rac1 in vivo (Nodari et al., 2007). This suggests that Rac1 is a downstream effector of β1 integrins in this process (figure 1).
Figure 1. Proposed roles for RhoGTPases in Schwann cell myelination.
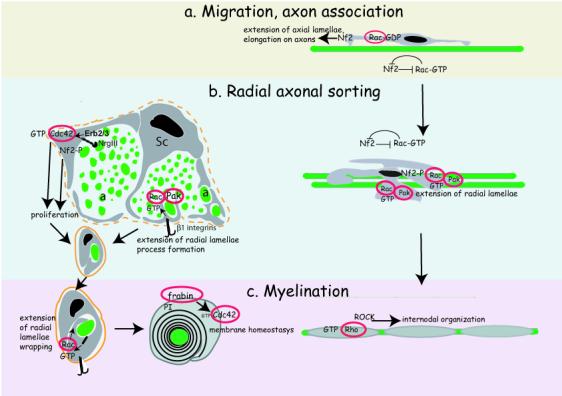
a) Rac1 and Cdc42 may be required during Schwann cell migration on axons. A negative cross-talk between Merlin/Schwannomin/Nf2 and Rac1 is probably involved in maintaining Rac1-GTP levels low and Nf2 active during early development, to allow for directional migration and elongation of Schwann cells on axons. b) Matching of Schwann cell and axon numbers, together with recognition/segregation of large axons destined for myelination by Schwann cell processes are required for radial sorting of axons and defasciculation. At this stage, merlin/Schwannomin/Nf2 become inactive and Rac1 gets activated at the leading edge of Schwann cell process, to allow extension of radial lamellipodia that ensheath and myelinate axons. Concomitantly, Schwann cell proliferation is ensured by activation of Cdc42 and inactivation of merlin/Schwannomin/Nf2. While full Rac1 activation requires a β1-containing integrin, Cdc42 activation appear to be downstream of the neuregulin/ErbB system. c) Frabin, mutated in Charcot-Marie-Tooth 4H, is a Cdc42 GEF that may be involved in myelin membrane homeostasis through interaction with phosphoinositides (PI). Rho(s) and ROCK may be involved in the regulation of internodes.
How do β1 integrin and Rac1 mediate sorting? They may promote formation of radial lamellipodia that are inserted by Schwann cells within axons to sort and segregate them. Both β1 integrin and Rac1 mutant Schwann cells are unable to insert processes within bundles of axons (observed by electron microscopy), or to form lamellipodia when plated on laminins (Benninger et al., 2007; Nodari et al., 2007). It has been shown that the levels of active Rac1 regulate the amount, extension and orientation of lamellipodia: low levels of Rac1 promote migratory behavior by producing axial lamellipodia (at the front and rear of the cells), whereas higher levels of Rac1 induce cells to form peripheral lamellipodia (around the whole perimeter of the cell), become stationary and spread (Pankov et al., 2005). In Schwann cells lacking β1 integrin or treated with Rac1 pharmacological inhibitors, Rac1 levels are low and cells have a specific lack of peripheral lamellipodia, suggesting that this inability precludes wrapping of axons during sorting and ensheathment.
The next question is where activation of Rac1 occurs? As defects of sorting are characteristic of laminin mutants, components of basal lamina, it is likely that a laminin receptor, such as α6β1 integrin, mediates the defective activation of Rac1 in β1 integrin mutants. However Rac1 membrane recruitment and lamellipodia extension likely occurs away from the basal lamina and adjacent to the axon, leaving open the possibility that another β1 integrin interacts with an axonal ligand to mediate Rac1 activation.
Convergence between growth factors and integrins in RhoGTPases regulation is commonly seen, and several growth factors on axons could participate in the process. For example, neuregulins type III are required for determining myelinating Schwann cell fate by engaging ErbB2/3 receptors on Schwann cells (Taveggia et al., 2005), and ErbB receptors interact with β1 integrin in Schwann cells to mediate Rac1 activation (Thaxton et al., 2007a). However engagement of Erb receptors does not seem to activate Rac1 in cultured Schwann cells (Benninger et al., 2007). Alternatively, IGF-1 has been shown to activate Rac1 in Schwann cells (Cheng et al., 2000), upstream of focal adhesion kinase (FAK), another molecule required in radial sorting (Grove et al., 2007) and linked to β1 integrin. Finally axon-derived neurotrophins, such as BDNF, may promote myelination by activating p75NTR receptors on Schwann cells. Recently p75NTR has been show to pair with the polarity protein Par3 that in turn can indirectly modulate Rac1 through the Rac1GEF TIAM (Chan et al., 2006). Thus, much work it still needed to clarify the molecules upstream and downstream of Rac1 in Schwann cells, and the regulation of spatial Rac1 activation during axo-glial interaction.
In this light, an elegant study using FRET on Schwann cells co-cultured with dorsal root ganglia axons, showed that after initial contact, Rac1 activity must be reduced at the tips of Schwann cells that are elongating on axons (Nakai et al., 2006). If Rac1 suppression is not possible, such as in neurofibromatosis 2 Schwann cells, elongation on axons is prevented (Kaempchen et al., 2003). Neurofibromatosis 2 cells are deficient in Merlin/Schwannomin/Nf2, a tumor suppressor that inhibits Rac1 (Shaw et al., 2001; Kissil et al., 2003) by preventing its recruitment to the cell membrane (Okada et al., 2005). While it is clear that merlin/Schwannomin deficiency causes abnormal Rac1 activation and tumorogenesis, its effect of developmental myelination is still unclear. Biallelic inactivation of merlin/Schwannomin in Schwann cells promotes tumorigenesis (Giovannini et al., 2000), but does not grossly prevent myelination, even if a developmental study has not been conducted. Adult mice lacking merlin/Schwannomin show the presence of redundant myelin, possibly due to increased or un-regulated Rac1 activation (Giovannini et al., 2000). These data collectively suggest that Rac1 activation in Schwann cells must be finely regulated in space and time to allow correct axonal contact, sorting and myelination (figure 1).
Cdc42
The role of Cdc42 in PNS myelination was addressed by Schwann cell specific ablation of Cdc42 at E12.5 (Benninger et al., 2007) using a floxed Cdc42 allele and Desert Hedgehog Cre. The loss of Cdc42 in mutant nerves did not affect Schwann cell process extension but resulted in a sharp decrease in Schwann cell numbers due to reduced proliferation. Schwann cell proliferation peaks at the onset of radial sorting and it is believed that a minimum threshold number of these cells is required to initiate this process (Webster, 1971; Martin and Webster, 1973; Webster et al., 1973).
How does Cdc42 regulate Schwann cell proliferation? One possibility is that Cdc42 is required to promote Schwann cell cycle progression. Several studies support a role for Cdc42 in this process (Erickson and Cerione, 2001). While in certain cell types, activated Cdc42 is sufficient to induce S phase entry in the absence of mitogens (Olson et al., 1995; Lamarche et al., 1996), in others it requires the presence of exogenous mitogens to promote proliferation and G1 progression (Chou et al., 2003; Seo et al., 2003) through p70 S6 kinase-mediated induction of cyclin E expression (Chou et al., 2003; Seo et al., 2003). Cdc42 can be activated by different growth factors, including well known Schwann cell mitogens such as hepatocyte growth factor, fibroblast growth factor, platelet derived growth factor and neuregulin 1 (NRG1) (Jessen and Mirsky, 2005). Exposure of Schwann cells to NRG1 induces a strong activation of Cdc42 (Benninger et al., 2007) suggesting that Cdc42 is required for NRG1-mediated Schwann cell proliferation (figure 1).
Cdc42 promotes phosphorylation of Schwannomin/Merlin in primary Schwann cells (Thaxton et al., 2007b). While unphosphorylated Schwannomin restricts cell proliferation acting as a tumor suppressor gene, phosphorylation of Schwannomin on serine 518 by p21-activated kinase (PAK) or protein kinase A following the activation of β1 integrin or ErbB2/ErbB3 receptors inhibits its tumor suppressor function (Thaxton et al., 2007b). Thus Cdc42 may also promote proliferation through inhibition of Schwannomin (figure 1).
Cdc42 and Rac1 have also been linked to the regulation of cell migration in a variety of cell types (Fukata et al., 2003). Indeed co-expression of active or dominant-negative forms of RhoGTPases with actinGFP in drosophila peripheral glia allowed to study the effect of perturbed RhoGTPase activity on glial migration and actin dynamics in vivo. The study revealed that perturbation of RhoA activity inhibited glia migration and defasciculation of sensory axons, whereas Rac1 perturbation interfered with glia migration and axonal ensheathment (Sepp and Auld, 2003). Based on chemotactic Boyden chamber assays, Yamauchi and colleagues postulated that neuregulin-mediated Cdc42 (and Rac1) activation regulates Schwann cell migration (Yamauchi et al., 2003; Yamauchi et al., 2005a; Yamauchi et al., 2005b; Yamauchi et al., 2008). These findings still need to be confirmed in mammals in vivo. The relative late onset of Desert hedgehog Cre expression (E12.5) used to ablate Cdc42 in Schwann cells, in addition to the defect in Schwann cell proliferation caused by the loss of Cdc42, prevented the direct analysis of the role of Cdc42 in Schwann cell migration in peripheral nerves (Benninger et al., 2007). In Rac1 mutant nerves in which proliferation and cell survival were normal using either the Desert hedgehog and the mP0TotCre, the morphological analysis of semithin transverse sections of embryonic mutant nerves (Benninger et al., 2007; Nodari et al., 2007) and the quantification of Schwann cell numbers present in distal regions of early post-natal nerves suggested that the loss of Rac1 did not significantly affect Schwann cell migration, at least after E12.5 (Benninger et al., 2007; Nodari et al., 2007).
Role of Cdc42 and Rac1 in myelination after axonal sorting
It is clear that Rac1 and Cdc42 are required for radial sorting of axons, as this process is blocked in the absence of Cdc42 and delayed in the absence of Rac1. The question remains if these RhoGTPases are also required for the following step, myelination, after the promyelinating 1:1 relationship has been achieved. Although it is difficult to dissect the contribution of these molecules in myelination, when the prerequisite step (sorting) is blocked or delayed, the data collected so far indicate that both RhoGTPases may indeed have a specific role also in wrapping of the myelin sheath. When Rac1 is deleted several Schwann cells achieve with delay the proper pro-myelinating step, but then remain arrested at this stage. Even in older nerves, up to 1 year old (Feltri, unpublished results), a proportion of axons in mutant nerves are devoid of myelin, suggesting that Rac1 is required for myelination. In nerves lacking Cdc42, few pro-myelinating fibers with no myelin are present. These data suggest that both Rac1 and Cdc42 may have a role beyond radial sorting, in wrapping of the myelin sheath.
Rho
RhoA, B and C and their effector, the Rho-associated coiled-coil-containing protein kinase (ROCK, Rok), are expressed in cultured Schwann cells ((Taylor et al., 2003; Melendez-Vasquez et al., 2004), our unpublished data). Little is presently known about the specific functions or the spatio-temporal pattern of expression of RhoB or RhoC. The expression of RhoA and the 2 known ROCK isoforms ROCK1 and 2 (Riento and Ridley, 2003) is developmentally regulated, peaking at the onset of myelination and dropping once myelination is established (Melendez-Vasquez et al., 2004). ROCK phosphorylates myosin light chain phosphatase and myosin light chain, both of which increase the contraction of the actomyosin network (Amano et al., 2000) in a variety of cell types. RhoA activity in primary rat Schwann cells regulates cell morphology (Brancolini et al., 1999) induces actin stress fiber formation, cell clustering and substrate adhesion Increased substrate adhesion might explain why activation of RhoA inhibits Schwann cell migration in response to endogenous BDNF (Yamauchi et al., 2004).
Exposure to the signaling lysophospholipids sphingosine 1-phosphate (S1P) or lysophosphatidic acid (LPA) can also activate RhoA and regulate Schwann cell survival and differentiation (Li et al., 2003; Barber et al., 2004; Birgbauer and Chun, 2006). Receptors for these 2 lipids are abundantly expressed in Schwann cells (Allard et al., 1998) and analysis of sciatic nerves obtained from mice lacking the LPA receptor LPA1 show increased Schwann cell apoptosis (Contos et al., 2000) but nor myelination defects (Birgbauer and Chun, 2006). However, LPA1 is likely to play a role in peripheral nerve demyelination in the (Inoue et al., 2004). Although LPA and S1P receptors are also expressed in CNS white matter tracts and exposure of oligodendrocyte cultures to LPA or S1P induces process retraction in oligodendrocyte progenitors, the genetic ablation of LPA1 or of the S1P receptor, S1P5, do not cause CNS myelination defects (Birgbauer and Chun, 2006).
Experiments in which ROCK was inhibited pharmacologically altered Schwann cell shape and resulted in aberrant myelination in Schwann cell-neuron co-cultures. Schwann cells formed multiple short independent segments along the length of axons, each with associated nodes and paranodes, resembling the myelin formed by oligodendrocytes (Melendez-Vasquez et al., 2004) (figure 1). The inhibition of ROCK in Schwann cell-neuron co-cultures in which myelination was already well established, had little or no effect in myelination (Melendez-Vasquez et al., 2004). This suggests a critical role for Rho/ROCK signaling during ensheathment and/or at the early stages of myelination. In this scenario, Rho/ROCK activity might be required to inhibit premature radial sorting, suppress multibranching during wrapping of axons and to regulate internode length. These effects are likely to be mediated by activation of myosin light chain, which is transiently increased at the onset of myelination and then downregulated (Melendez-Vasquez et al., 2004).
Role of Rac1, Cdc42 and RhoA in central nervous system myelination
RhoGTPases are expressed by oligodendrocytes in the spinal cord (Erschbamer et al., 2005) and, in culture, their expression and activity is developmentally regulated (Liang et al., 2004). In differentiating primary oligodendrocyte cultures, RhoA is expressed and active during the early progenitor stages whereas the expression and activity of Cdc42 and Rac1 increases as differentiation proceeds. Perturbation of the activities of these GTPases in oligodendrocyte cultures by expression of the corresponding dominant-negative or constitutively active mutant molecules, suggests that Cdc42 and Rac1 act as positive regulators of morphological differentiation, inducing process extension and branching, while RhoA acts as a negative regulator inhibiting process extension (Liang et al., 2004). A role for RhoA as a negative regulator of oligodendrocyte differentiation is also supported by in vitro experiments in which ROCK was pharmacologically inhibited (Wolf et al., 2001).
In the context of CNS myelination, pathways known to regulate oligodendrocyte differentiation such as those initiated by the activation of the transmembrane protein LINGO-1, a component of the NgR1/p75 and NgR1/taj (Troy) signaling complex (Mi et al., 2004) or those involving Fyn kinase (Mi et al., 2005), can modulate the activity of RhoA. LINGO-1 negatively regulates CNS myelination through a RhoA-dependent mechanism (Erschbamer et al., 2005). The inhibition of LINGO-1 leads to downregulation of RhoA activity and promotes in vitro OPC differentiation (Zhao et al., 2007). In addition, LINGO-1 null mice show an earlier onset of myelination. LINGO-1 antagonists increased the expression and phosphorylation of Fyn kinase, a molecule whose function is required for CNS myelination (Mi et al., 2005). Fyn phosphorylates p190RhoGAP (Wolf et al., 2001) and p250GAP (Taniguchi et al., 2003) in oligodendrocytes, and both the tyrosine phosphorylated p190RhoGAP and p250GAP downregulate RhoA activity and are thought to enhance oligodendrocyte differentiation. Thus, RhoA may negatively regulate CNS myelination and the onset of myelination. In line with this hypothesis, Kipper and colleagues have recently shown that Rho inactivation is required to trigger plasma membrane specialization in oligodendrocytes (Kippert et al., 2007).
Differently from RhoA, the role of Rac1 and Cdc42 in central nervous system myelination is still unclear. Data from several laboratories have indicated that activation of Rac1 and Cdc42 are required for proper myelination. First, an effector of Rac1, WAVE1, has been linked to myelination in oligodendrocytes in vivo (Kim et al., 2006a). Experiments in cultured oligodendrocytes show that Rac1 and Cdc42 activity increases during oligodendrocytes differentiation, and suggest that integrin engagement stimulate Rac1 and Cdc42 through Fyn kinase and p190RhoGAP, to promote outgrowth of oligodendrocyte processes (Liang et al., 2004). Despite this evidence suggesting a positive link between Rac1, Cdc42 and membrane formation in myelination, tissue-specific conditional ablation of Cdc42 or Rac1 in oligodendrocytes does not affect proliferation, migration or in vitro differentiation, but results in the enlargement of the inner tongue of the oligodendrocyte process and the formation of a particular type of myelin outfoldings (Thurnherr et al., 2006).
Thus, Cdc42 and Rac1 appear to play different roles in peripheral and central nervous system myelination. This supports the view that the signaling role of a given small Rho GTPase in a specific cell type cannot predict its function in another cell type (Wang and Zheng, 2007) even when those cells carry out very unique and similar tasks as myelination in oligodendrocytes and Schwann cells. One can only speculate why Cdc42 and Rac1 play different roles in the biology of these two different types of myelinating glia. One crucial difference is that in contrast to oligodendrocytes, Schwann cells synthesize a basal lamina from early embryonic development (Jessen and Mirsky, 2005). As small Rho GTPases play a prominent role in signal transduction of basal lamina receptors, the absence of an oligodendrocyte basal lamina could explain why Cdc42 (and Rac1) appear to have a more restricted and stage-specific role in CNS compared to PNS myelination. In addition, oligodendrocytes myelinate several axons at once, with no need for radial sorting which is the step critically dependent on Cdc42 and Rac1. This is also in line with the observation that β1 integrin is required for PNS (Feltri et al., 2002) but not for CNS myelination (Benninger et al., 2006).
Rho GTPases Exchance Factors (GEF)s and Rho GTPases Activating Proteins (GAP)s
The current knowledge about the identity of functionally important RhoGEFs and RhoGAPs in Schwann cell biology is generally scarce although some exciting hints have been provided by studies in human genetics indicating that particular RhoGEFs play a crucial role in myelination (see section “RhoGTPases in Schwann cell-related Diseases”). Furthermore, cell culture paradigms revealed that NT3 activation of TrkC stimulates Ras activity and induces the Rac1-GEF TIAM1 to activate Rac1 (Yamauchi et al., 2005b). In parallel, TrkC phosphorylates and activates also the Cdc42-GEF Dbs (Yamauchi et al., 2005b). According to these data, GTP-bound Rac1 and Cdc42 induce the activation of JNK and Schwann cell migration at least in vitro. However, TIAM1 appears not to be required in vivo since the preliminary analysis of nerves from TIAM1-null mice did not reveal abnormalities in peripheral nerve myelination (J. Collard, The Netherlands Cancer Institute and ML. Feltri, unpublished results). Thus, the function of TIAM1 is either redundant or compensated by other Rac1 GEFs.
The RhoGAP oligophrenin-1 (OPHN1) is expressed abundantly by myelinating Schwann cells (Xiao et al., 2004). Patients suffering from X-linked mental retardation associated with OPHN1 mutations show no alterations of nerve conduction velocities have been observed (Bergmann et al., 2003). Similarly, there seems to be no obvious peripheral nerve phenotype in OPHN1 null mice as judged from behavioral analysis (Khelfaoui et al., 2007). Thus, the critical regulatory RhoGAPs in Schwann cells wait to be identified.
Effectors
Active, GTP-bound RhoGTPases bind to a variety of effector proteins to perform their diverse cellular functions. The function performed by a RhoGTPase derives not only from the interaction with specific effectors, but also from duration and subcellular localization of this interaction. Most Cdc42 and Rac1 effectors contain a Cdc42/Rac-interactive binding motif (CRIB domain)(Burbelo et al., 1995) that is part of a intramolecular autoinhibitory domain (Hoffman and Cerione, 2000). The most studied effects are on cytoskeletal dynamics, but adhesion, cellular junctions, polarity, cell cycle and transcription are also targets. For comprehensive reviews on RhoGTPases effectors see (Bishop and Hall, 2000; Zhao and Manser, 2005; Schmandke et al., 2007).
Few effectors with a role in myelinating cells have been described so far. Their role in vivo is yet unexplored or mild, likely due to significant redundancy among effectors. As described above, inhibition of ROCK in myelinating dorsal root ganglia (DRG) Schwann cell co-cultures causes the formation of short internodal segments containing multiple nodes and paranodes, without changes in cell proliferation or differentiation, suggesting that ROCK normally suppresses abnormal branching of the myelin sheath (Melendez-Vasquez et al., 2004).
Wiskott-Aldrich syndrome protein family velprolin-homologues (WAVE) are Rac1 effectors that activate the actin nucleation Arp2/3 complex (reviewed in (Smith and Li, 2004)). WAVEs are negatively regulated by phosphorylation (Kim et al., 2006b; Danson et al., 2007). Oligodendrocytes express predominantly WAVE1 and 2, whereas Schwann cells, at least in culture deprived of axonal contact, express WAVE2 (Kim et al., 2006a; Bacon et al., 2007). WAVEs are located at the leading edge of glia lamellipodia in vitro, and deletion of WAVE1 in mice causes hypomyelination in the corpus callosus and optic nerve (Kim et al., 2006a; Bacon et al., 2007). If this effect is cell autonomous in oligodendrocytes is at present unknown, as WAVE1 also has neuronal intrinsic roles (Kim et al., 2006b; Sung et al., 2008). Finally a role for the Cdc42 effector N-WASP (Wiskott-Aldrich syndrome protein) in Schwann cell and oligodendrocytes process formation and myelination has been proposed, based on the fact that the N-WASP inhibitor wiskostatin causes retraction of filopodia and lamellipodia and impairs myelination in vitro (Kim et al., 2006a; Bacon et al., 2007).
RhoGTPases in Schwann cell-related Diseases
Due to their central role in the biology of Schwann cells, RhoGTPases and associated signaling pathways are predestined to be involved in various disease processes in peripheral nerves. This includes hereditary and acquired neuropathies, notably those entities related to defective laminin signals such as merosin-deficient congenital muscular dystrophy, Charcot-Marie-Tooth Disease (CMT) 4F, neurofibromatosis, and leprosy (Feltri and Wrabetz, 2005), but also other neuropathies due to toxins, inflammation, diabetes, or side-effects of chemotherapies. A direct proof, however, for the crucial functional involvement of RhoGTPases in diseased Schwann cells besides Nf2 has been largely missing. Recently, two reports have described autosomal-recessive mutations in a RhoGEF called Frabin/FGD4 in CMT4H, a specific subtype of hereditary motor and sensory neuropathies (Stendel et al., 2007; Delague et al., 2007). CMT4H patients show slow nerve conduction velocities, a typical feature of demyelinating neuropathies (Suter and Scherer, 2003; Niemann et al., 2006), severe loss of myelinated fibers, thinly myelinated axons occasionally associated with small Schwann cell onion bulbs, and prominent outfoldings of the myelin sheath (De Sandre-Giovannoli et al., 2005; Stendel et al., 2007). Based on this pathology, a major contribution to the disease by the affected Schwann cells appears likely, although axonal (neuronal) contributions of the Frabin/FGD4 mutations cannot be excluded. On the molecular level, Frabin/FGD4 has been described as a GEF for Cdc42 (Umikawa et al., 1999). Consistent with this finding, Frabin/FGD4 contains adjacent Dbl homology (DH) and pleckstrin homology (PH) domains. In addition, the protein contains an N-terminal actin-binding domain, a phosphoinositide binding FYVE domain located C-terminal to the tandem DH/PH domains, and a C-terminal second PH domain. The multiple phosphoinositide-binding domains (PH, FYVE) within Frabin/FGD4 are intriguing and may relate the CMT4H disease mechanism to other CMT subtypes caused by mutant proteins that act within a potential regulatory network of phosphoinositide-binding and phosphoinositide-modifying proteins (figure 1) (Previtali et al., 2007; Suter, 2007). Overexpression of Frabin/FGD4 in Schwann cells and motoneurons in culture leads to altered cell shapes (Delague et al., 2007; Stendel et al., 2007) and putative disease-causing truncated forms of Frabin/FGD4 that miss parts of the DH domain have lost this feature (Delague et al., 2007). These findings are in general agreement with alterations of RhoGTPase signaling in CMT4H nerves but the definitive answer to the question whether the phenotype is specifically due to loss of the Cdc42 GEF activity of Frabin/FGD4 requires additional biochemical and cell biological studies.
Besides Frabin/FGD4, a second putative RhoGEF has been implicated in myelination. This is based on the finding of a dominantly inherited mutation in the ARHGEF10 protein, associated with slow nerve conduction velocity and thinly myelinated axons in a large, not clinically affected family (Verhoeven et al., 2003) ARHGEF10 is unusual within the RhoGEF family since it displays only very weak homology to known PH domains. Nevertheless, ARHGEF10 is able to preferentially activate RhoB and to a lesser extent RhoA and RhoC (Mohl et al., 2006). The mechanism by which this mutation affects peripheral nerves is unknown. Based on the phenotype, one might speculate about the involvement of the neuregulin signaling system (Nave and Salzer, 2006) but numerous alternative hypotheses based on contributions by affected axons (neurons) and/or Schwann cells can also be envisaged.
Concluding Remarks
RhoGTPases have long been suspected to be involved in various biological processes that are critical for proper Schwann cell development and myelination. This includes almost all aspects of cell biology, including cytoskeletal organization, membrane trafficking, cell proliferation, cell migration, cell adhesion, and establishment of cell polarity, only to name a few. The unique bidirectional dialogue between axons and Schwann cells in a myelinated nerve, together with the extreme reorganization and coordinated assembly of membranes that is required during developmental myelination and remyelination after injury, is from a hypothetical point of view perfectly suited to be regulated by RhoGTPases and their regulators based on what we know from other tissues and cell culture experiments. Largely due to the development of novel improved experimental tools, it has now become possible to dissect the precise role of the individual RhoGTPases in early events of Schwann cell biogenesis all the way to myelination and myelin maintenance. It has become clear that these proteins play distinct but also overlapping key roles within a highly regulated network, some aspects common with other tissues, some unique to Schwann cells. We expect that RhoGTPases and their regulators will turn out to have even broader functions in myelinated peripheral nerves since they appear to be major integrators of extracellular signals. Besides pinpointing the precise biochemical pathways involved, it will be particularly exciting, for example, to explore how the RhoGTPase regulated network contributes to the processing of mechanical stress responses in Schwann cells and how the network is affected in diseases of peripheral nerves.
Acknowledgements
work in the laboratories of the authors is supported by the National Institute of Health (NINDS) and Telethon, Italy (MLF) and by the Swiss National Science Foundation, the NCCR “Neural plasticity and repair” and the ETH Zurich (US and JBR). MLF wishes to thank Desirée Zambroni, Alessandro Nodari and Lawrence Wrabetz for useful discussions. US and JBR thank present and former members of their labs for many useful discussions.
References
- Allard J, Barron S, Diaz J, Lubetzki C, Zalc B, Schwartz JC, Sokoloff P. A rat G protein-coupled receptor selectively expressed in myelin-forming cells. Eur J Neurosci. 1998;10:1045–1053. doi: 10.1046/j.1460-9568.1998.00117.x. [DOI] [PubMed] [Google Scholar]
- Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- Aspenstrom P, Ruusala A, Pacholsky D. Taking Rho GTPases to the next level: the cellular functions of atypical Rho GTPases. Exp Cell Res. 2007;313:3673–3679. doi: 10.1016/j.yexcr.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Bacon C, Lakics V, Machesky L, Rumsby M. N-WASP regulates extension of filopodia and processes by oligodendrocyte progenitors, oligodendrocytes, and Schwann cells-implications for axon ensheathment at myelination. Glia. 2007;55:844–858. doi: 10.1002/glia.20505. [DOI] [PubMed] [Google Scholar]
- Barber SC, Mellor H, Gampel A, Scolding NJ. S1P and LPA trigger Schwann cell actin changes and migration. Eur J Neurosci. 2004;19:3142–3150. doi: 10.1111/j.0953-816X.2004.03424.x. [DOI] [PubMed] [Google Scholar]
- Benninger Y, Colognato H, Thurnherr T, Franklin RJ, Leone DP, Atanasoski S, Nave KA, Ffrench-Constant C, Suter U, Relvas JB. Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J Neurosci. 2006;26:7665–7673. doi: 10.1523/JNEUROSCI.0444-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grashoff A, Herzog D, Nave KA, Franklin RJ, Meijer D, Brakebusch C, Suter U, Relvas JB. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J Cell Biol. 2007;177:1051–1061. doi: 10.1083/jcb.200610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann C, Zerres K, Senderek J, Rudnik-Schoneborn S, Eggermann T, Hausler M, Mull M, Ramaekers VT. Oligophrenin 1 (OPHN1) gene mutation causes syndromic X-linked mental retardation with epilepsy, rostral ventricular enlargement and cerebellar hypoplasia. Brain. 2003;126:1537–1544. doi: 10.1093/brain/awg173. [DOI] [PubMed] [Google Scholar]
- Birgbauer E, Chun J. New developments in the biological functions of lysophospholipids. Cell Mol Life Sci. 2006;63:2695–2701. doi: 10.1007/s00018-006-6155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- Brancolini C, Marzinotto S, Edomi P, Agostoni E, Fiorentini C, Muller HW, Schneider C. Rho-dependent regulation of cell spreading by the tetraspan membrane protein Gas3/PMP22. Mol Biol Cell. 1999;10:2441–2459. doi: 10.1091/mbc.10.7.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995;270:29071–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- Chan JR, Jolicoeur C, Yamauchi J, Elliott J, Fawcett JP, Ng BK, Cayouette M. The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science. 2006;314:832–836. doi: 10.1126/science.1134069. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Steinway ML, Russell JW, Feldman EL. GTPases and phosphatidylinositol 3-kinase are critical for insulin-like growth factor-I-mediated Schwann cell motility. J Biol Chem. 2000;275:27197–27204. doi: 10.1074/jbc.M002534200. [DOI] [PubMed] [Google Scholar]
- Chou MM, Masuda-Robens JM, Gupta ML. Cdc42 promotes G1 progression through p70 S6 kinase-mediated induction of cyclin E expression. J Biol Chem. 2003;278:35241–35247. doi: 10.1074/jbc.M305246200. [DOI] [PubMed] [Google Scholar]
- Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci U S A. 2000;97:13384–13389. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danson CM, Pocha SM, Bloomberg GB, Cory GO. Phosphorylation of WAVE2 by MAP kinases regulates persistent cell migration and polarity. J Cell Sci. 2007;120:4144–4154. doi: 10.1242/jcs.013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sandre-Giovannoli A, Delague V, Hamadouche T, Chaouch M, Krahn M, Boccaccio I, Maisonobe T, Chouery E, Jabbour R, Atweh S, Grid D, Megarbane A, Levy N. Homozygosity mapping of autosomal recessive demyelinating Charcot-Marie-Tooth neuropathy (CMT4H) to a novel locus on chromosome 12p11.21-q13.11. J Med Genet. 2005;42:260–265. doi: 10.1136/jmg.2004.024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo MA, Kiosses WB, Alderson NB, Meller N, Hahn KM, Schwartz MA. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat Cell Biol. 2002;4:232–239. doi: 10.1038/ncb759. [DOI] [PubMed] [Google Scholar]
- del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- Delague V, Jacquier A, Hamadouche T, Poitelon Y, Baudot C, Boccaccio I, Chouery E, Chaouch M, Kassouri N, Jabbour R, Grid D, Megarbane A, Haase G, Levy N. Mutations in FGD4 encoding the Rho GDP/GTP exchange factor FRABIN cause autosomal recessive Charcot-Marie-Tooth type 4H. Am J Hum Genet. 2007;81:1–16. doi: 10.1086/518428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen KR. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- Erickson JW, Cerione RA. Multiple roles for Cdc42 in cell regulation. Curr Opin Cell Biol. 2001;13:153–157. doi: 10.1016/s0955-0674(00)00192-7. [DOI] [PubMed] [Google Scholar]
- Erschbamer MK, Hofstetter CP, Olson L. RhoA, RhoB, RhoC, Rac1, Cdc42, and Tc10 mRNA levels in spinal cord, sensory ganglia, and corticospinal tract neurons and long-lasting specific changes following spinal cord injury. J Comp Neurol. 2005;484:224–233. doi: 10.1002/cne.20471. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Feltri ML, Wrabetz L. Laminins and their receptors in Schwann cells and hereditary neuropathies. J Peripher Nerv Syst. 2005;10:128–143. doi: 10.1111/j.1085-9489.2005.0010204.x. [DOI] [PubMed] [Google Scholar]
- Feltri ML, D’Antonio M, Previtali S, Fasolini M, Messing A, Wrabetz L. P0-Cre transgenic mice for inactivation of adhesion molecules in Schwann cells. Ann N Y Acad Sci. 1999b;883:116–123. [PubMed] [Google Scholar]
- Feltri ML, Graus Porta D, Previtali SC, Nodari A, Migliavacca B, Cassetti A, Littlewood-Evans A, Reichardt LF, Messing A, Quattrini A, Mueller U, Wrabetz L. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156:199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L, Woodruff JM, Berns A, Thomas G. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 2000;14:1617–1630. [PMC free article] [PubMed] [Google Scholar]
- Grinspan JB, Marchionni MA, Reeves M, Coulaloglou M, Scherer SS. Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerve: neuregulin receptors and the role of neuregulins. J Neurosci. 1996;16:6107–6118. doi: 10.1523/JNEUROSCI.16-19-06107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove M, Komiyama NH, Nave KA, Grant SG, Sherman DL, Brophy PJ. FAK is required for axonal sorting by Schwann cells. J Cell Biol. 2007;176:277–282. doi: 10.1083/jcb.200609021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GR, Cerione RA. Flipping the switch: the structural basis for signaling through the CRIB motif. Cell. 2000;102:403–406. doi: 10.1016/s0092-8674(00)00045-3. [DOI] [PubMed] [Google Scholar]
- Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2004;10:712–718. doi: 10.1038/nm1060. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Johnson DI. Cdc42: An essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol Mol Biol Rev. 1999;63:54–105. doi: 10.1128/mmbr.63.1.54-105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph NM, Mukouyama YS, Mosher JT, Jaegle M, Crone SA, Dormand EL, Lee KF, Meijer D, Anderson DJ, Morrison SJ. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development. 2004;131:5599–5612. doi: 10.1242/dev.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaempchen K, Mielke K, Utermark T, Langmesser S, Hanemann CO. Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum Mol Genet. 2003;12:1211–1221. doi: 10.1093/hmg/ddg146. [DOI] [PubMed] [Google Scholar]
- Khelfaoui M, Denis C, van Galen E, de Bock F, Schmitt A, Houbron C, Morice E, Giros B, Ramakers G, Fagni L, Chelly J, Nosten Bertrand M, Billuart P. Loss of X-linked mental retardation gene oligophrenin1 in mice impairs spatial memory and leads to ventricular enlargement and dendritic spine immaturity. J Neurosci. 2007;27:9439–9450. doi: 10.1523/JNEUROSCI.2029-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, DiBernardo AB, Sloane JA, Rasband MN, Solomon D, Kosaras B, Kwak SP, Vartanian TK. WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination. J Neurosci. 2006a;26:5849–5859. doi: 10.1523/JNEUROSCI.4921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Sung JY, Ceglia I, Lee KW, Ahn JH, Halford JM, Kim AM, Kwak SP, Park JB, Ho Ryu S, Schenck A, Bardoni B, Scott JD, Nairn AC, Greengard P. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006b;442:814–817. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- Kippert A, Trajkovic K, Rajendran L, Ries J, Simons M. Rho regulates membrane transport in the endocytic pathway to control plasma membrane specialization in oligodendroglial cells. J Neurosci. 2007;27:3560–3570. doi: 10.1523/JNEUROSCI.4926-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell. 2003;12:841–849. doi: 10.1016/s1097-2765(03)00382-4. [DOI] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C. The neural crest. 2 Edition Cambridge University Press; Cambridge, UK: 1999. [Google Scholar]
- Li Y, Gonzalez MI, Meinkoth JL, Field J, Kazanietz MG, Tennekoon GI. Lysophosphatidic acid promotes survival and differentiation of rat Schwann cells. J Biol Chem. 2003;278:9585–9591. doi: 10.1074/jbc.M213244200. [DOI] [PubMed] [Google Scholar]
- Liang X, Draghi NA, Resh MD. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J Neurosci. 2004;24:7140–7149. doi: 10.1523/JNEUROSCI.5319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- Maro GS, Vermeren M, Voiculescu O, Melton L, Cohen J, Charnay P, Topilko P. Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat Neurosci. 2004;7:930–938. doi: 10.1038/nn1299. [DOI] [PubMed] [Google Scholar]
- Martin JR, Webster HD. Mitotic Schwann cells in developing nerve: their changes in shape, fine structure, and axon relationships. Dev Biol. 1973;32:417–431. doi: 10.1016/0012-1606(73)90251-0. [DOI] [PubMed] [Google Scholar]
- Maurel P, Salzer JL. Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J Neurosci. 2000;20:4635–4645. doi: 10.1523/JNEUROSCI.20-12-04635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez-Vasquez CV, Einheber S, Salzer JL. Rho kinase regulates schwann cell myelination and formation of associated axonal domains. J Neurosci. 2004;24:3953–3963. doi: 10.1523/JNEUROSCI.4920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, Crowell T, Cate RL, McCoy JM, Pepinsky RB. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, Chang J, Thill G, Levesque M, Zhang M, Hession C, Sah D, Trapp B, He Z, Jung V, McCoy JM, Pepinsky RB. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- Mohl M, Winkler S, Wieland T, Lutz S. Gef10--the third member of a Rho-specific guanine nucleotide exchange factor subfamily with unusual protein architecture. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:333–341. doi: 10.1007/s00210-006-0083-0. [DOI] [PubMed] [Google Scholar]
- Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13:13–22. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Zheng Y, MacCollin M, Ratner N. Temporal control of Rac in Schwann cell-axon interaction is disrupted in NF2-mutant schwannoma cells. J Neurosci. 2006;26:3390–3395. doi: 10.1523/JNEUROSCI.4865-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Niemann A, Berger P, Suter U. Pathomechanisms of mutant proteins in Charcot-Marie-Tooth disease. Neuromolecular Med. 2006;8:217–242. doi: 10.1385/nmm:8:1-2:217. [DOI] [PubMed] [Google Scholar]
- Nodari A, Zambroni D, Quattrini A, Court FA, D’Urso A, Recchia A, Tybulewicz VL, Wrabetz L, Feltri ML. Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol. 2007;177:1063–1075. doi: 10.1083/jcb.200610014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Lopez-Lago M, Giancotti FG. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J Cell Biol. 2005;171:361–371. doi: 10.1083/jcb.200503165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson B. Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal. 1999;11:545–554. doi: 10.1016/s0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- Pankov R, Endo Y, Even-Ram S, Araki M, Clark K, Cukierman E, Matsumoto K, Yamada KM. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previtali SC, Quattrini A, Bolino A. Charcot-Marie-Tooth type 4B demyelinating neuropathy: deciphering the role of MTMR phosphatases. Expert Rev Mol Med. 2007;9:1–16. doi: 10.1017/S1462399407000439. [DOI] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Schmandke A, Schmandke A, Strittmatter SM. ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist. 2007;13:454–469. doi: 10.1177/1073858407303611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Seo JY, Kim JS, Ghang JH, Kang TC, Suh JG, Kim EG, Kim JI, Kim J, Lee JY, Park JB. Nerve growth factor induces proliferation of PC12 cells through Cdc42. Neuroreport. 2003;14:1277–1281. doi: 10.1097/00001756-200307010-00018. [DOI] [PubMed] [Google Scholar]
- Sepp KJ, Auld VJ. RhoA and Rac1 GTPases mediate the dynamic rearrangement of actin in peripheral glia. Development. 2003;130:1825–1835. doi: 10.1242/dev.00413. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, O’Bryan JP, Gupta V, Ratner N, Der CJ, Jacks T, McClatchey AI. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- Smith LG, Li R. Actin polymerization: riding the wave. Curr Biol. 2004;14:R109–111. [PubMed] [Google Scholar]
- Stendel C, Roos A, Deconinck T, Pereira J, Castagner F, Niemann A, Kirschner J, Korinthenberg R, Ketelsen UP, Battaloglu E, Parman Y, Nicholson G, Ouvrier R, Seeger J, De Jonghe P, Weis J, Kruttgen A, Rudnik-Schoneborn S, Bergmann C, Suter U, Zerres K, Timmerman V, Relvas JB, Senderek J. Peripheral nerve demyelination caused by a mutant Rho GTPase guanine nucleotide exchange factor, frabin/FGD4. Am J Hum Genet. 2007;81:158–164. doi: 10.1086/518770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JY, Engmann O, Teylan MA, Nairn AC, Greengard P, Kim Y. WAVE1 controls neuronal activity-induced mitochondrial distribution in dendritic spines. Proc Natl Acad Sci U S A. 2008;105:3112–3116. doi: 10.1073/pnas.0712180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter U. Phosphoinositides and Charcot-Marie-tooth disease: new keys to old questions. Cell Mol Life Sci. 2007;64:3261–3265. doi: 10.1007/s00018-007-7381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter U, Scherer SS. Disease mechanisms in inherited neuropathies. Nat Rev Neurosci. 2003;4:714–726. doi: 10.1038/nrn1196. [DOI] [PubMed] [Google Scholar]
- Symons M, Settleman J. Rho family GTPases: more than simple switches. Trends Cell Biol. 2000;10:415–419. doi: 10.1016/s0962-8924(00)01832-8. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Liu H, Nakazawa T, Yokoyama K, Tezuka T, Yamamoto T. p250GAP, a neural RhoGAP protein, is associated with and phosphorylated by Fyn. Biochem Biophys Res Commun. 2003;306:151–155. doi: 10.1016/s0006-291x(03)00923-9. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AR, Geden SE, Fernandez-Valle C. Formation of a beta1 integrin signaling complex in Schwann cells is independent of rho. Glia. 2003;41:94–104. doi: 10.1002/glia.10170. [DOI] [PubMed] [Google Scholar]
- Terashima T, Yasuda H, Terada M, Kogawa S, Maeda K, Haneda M, Kashiwagi A, Kikkawa R. Expression of Rho-family GTPases (Rac, cdc42, RhoA) and their association with p-21 activated kinase in adult rat peripheral nerve. J Neurochem. 2001;77:986–993. doi: 10.1046/j.1471-4159.2001.00336.x. [DOI] [PubMed] [Google Scholar]
- Thaxton C, Lopera J, Bott M, Fernandez-Valle C. Neuregulin and laminin stimulate phosphorylation of the NF2 tumor suppressor in Schwann cells by distinct protein kinase A and p21-activated kinase-dependent pathways. Oncogene. 2007a doi: 10.1038/sj.onc.1210923. [DOI] [PubMed] [Google Scholar]
- Thaxton C, Lopera J, Bott M, Baldwin ME, Kalidas P, Fernandez-Valle C. Phosphorylation of the NF2 tumor suppressor in Schwann cells is mediated by Cdc42-Pak and requires paxillin binding. Mol Cell Neurosci. 2007b;34:231–242. doi: 10.1016/j.mcn.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Thurnherr T, Benninger Y, Wu X, Chrostek A, Krause SM, Nave KA, Franklin RJ, Brakebusch C, Suter U, Relvas JB. Cdc42 and Rac1 signaling are both required for and act synergistically in the correct formation of myelin sheaths in the CNS. J Neurosci. 2006;26:10110–10119. doi: 10.1523/JNEUROSCI.2158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umikawa M, Obaishi H, Nakanishi H, Satoh-Horikawa K, Takahashi K, Hotta I, Matsuura Y, Takai Y. Association of frabin with the actin cytoskeleton is essential for microspike formation through activation of Cdc42 small G protein. J Biol Chem. 1999;274:25197–25200. doi: 10.1074/jbc.274.36.25197. [DOI] [PubMed] [Google Scholar]
- Verhoeven K, De Jonghe P, Van de Putte T, Nelis E, Zwijsen A, Verpoorten N, De Vriendt E, Jacobs A, Van Gerwen V, Francis A, Ceuterick C, Huylebroeck D, Timmerman V. Slowed conduction and thin myelination of peripheral nerves associated with mutant rho Guanine-nucleotide exchange factor 10. Am J Hum Genet. 2003;73:926–932. doi: 10.1086/378159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zheng Y. Cell type-specific functions of Rho GTPases revealed by gene targeting in mice. Trends Cell Biol. 2007;17:58–64. doi: 10.1016/j.tcb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Wanner IB, Guerra NK, Mahoney J, Kumar A, Wood PM, Mirsky R, Jessen KR. Role of N-cadherin in Schwann cell precursors of growing nerves. Glia. 2006;54:439–459. doi: 10.1002/glia.20390. [DOI] [PubMed] [Google Scholar]
- Webster HD. The geometry of peripheral myelin sheaths during their formation and growth in rat sciatic nerves. J Cell Biol. 1971;48:348–367. doi: 10.1083/jcb.48.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster HD, Martin R, O’Connell MF. The relationships between interphase Schwann cells and axons before myelination: a quantitative electron microscopic study. Dev Biol. 1973;32:401–416. doi: 10.1016/0012-1606(73)90250-9. [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Der CJ. Rho-family GTPases: it’s not only Rac and Rho (and I like it) J Cell Sci. 2004;117:1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- Wolf RM, Wilkes JJ, Chao MV, Resh MD. Tyrosine phosphorylation of p190 RhoGAP by Fyn regulates oligodendrocyte differentiation. J Neurobiol. 2001;49:62–78. doi: 10.1002/neu.1066. [DOI] [PubMed] [Google Scholar]
- Xiao J, Neylon CB, Nicholson GA, Furness JB. Evidence that a major site of expression of the RHO-GTPASE activating protein, oligophrenin-1, is peripheral myelin. Neuroscience. 2004;124:781–787. doi: 10.1016/j.neuroscience.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Yamauchi J, Chan JR, Shooter EM. Neurotrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc Natl Acad Sci U S A. 2003;100:14421–14426. doi: 10.1073/pnas.2336152100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi J, Chan JR, Shooter EM. Neurotrophins regulate Schwann cell migration by activating divergent signaling pathways dependent on Rho GTPases. Proc Natl Acad Sci U S A. 2004;101:8774–8779. doi: 10.1073/pnas.0402795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi J, Miyamoto Y, Chan JR, Tanoue A. ErbB2 directly activates the exchange factor Dock7 to promote Schwann cell migration. J Cell Biol. 2008;181:351–365. doi: 10.1083/jcb.200709033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi J, Chan JR, Miyamoto Y, Tsujimoto G, Shooter EM. The neurotrophin-3 receptor TrkC directly phosphorylates and activates the nucleotide exchange factor Dbs to enhance Schwann cell migration. Proc Natl Acad Sci U S A. 2005a;102:5198–5203. doi: 10.1073/pnas.0501160102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi J, Miyamoto Y, Tanoue A, Shooter EM, Chan JR. Ras activation of a Rac1 exchange factor, Tiam1, mediates neurotrophin-3-induced Schwann cell migration. Proc Natl Acad Sci U S A. 2005b;102:14889–14894. doi: 10.1073/pnas.0507125102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Bierman J, Tarumi YS, Zhong YP, Rangwala R, Proctor TM, Miyagoe-Suzuki Y, Takeda S, Miner JH, Sherman LS, Gold BG, Patton BL. Coordinate control of axon defasciculation and myelination by laminin-2 and - 8. J Cell Biol. 2005 doi: 10.1083/jcb.200411158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WM, Feltri ML, Wrabetz L, Strickland S, Chen ZL. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. Journal of Neuroscience. 2005;25:4463–4472. doi: 10.1523/JNEUROSCI.5032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XH, Jin WL, Ju G. An in vitro study on the involvement of LINGO-1 and Rho GTPases in Nogo-A regulated differentiation of oligodendrocyte precursor cells. Mol Cell Neurosci. 2007;36:260–269. doi: 10.1016/j.mcn.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Zhao ZS, Manser E. PAK and other Rho-associated kinases--effectors with surprisingly diverse mechanisms of regulation. Biochem J. 2005;386:201–214. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]


