Abstract
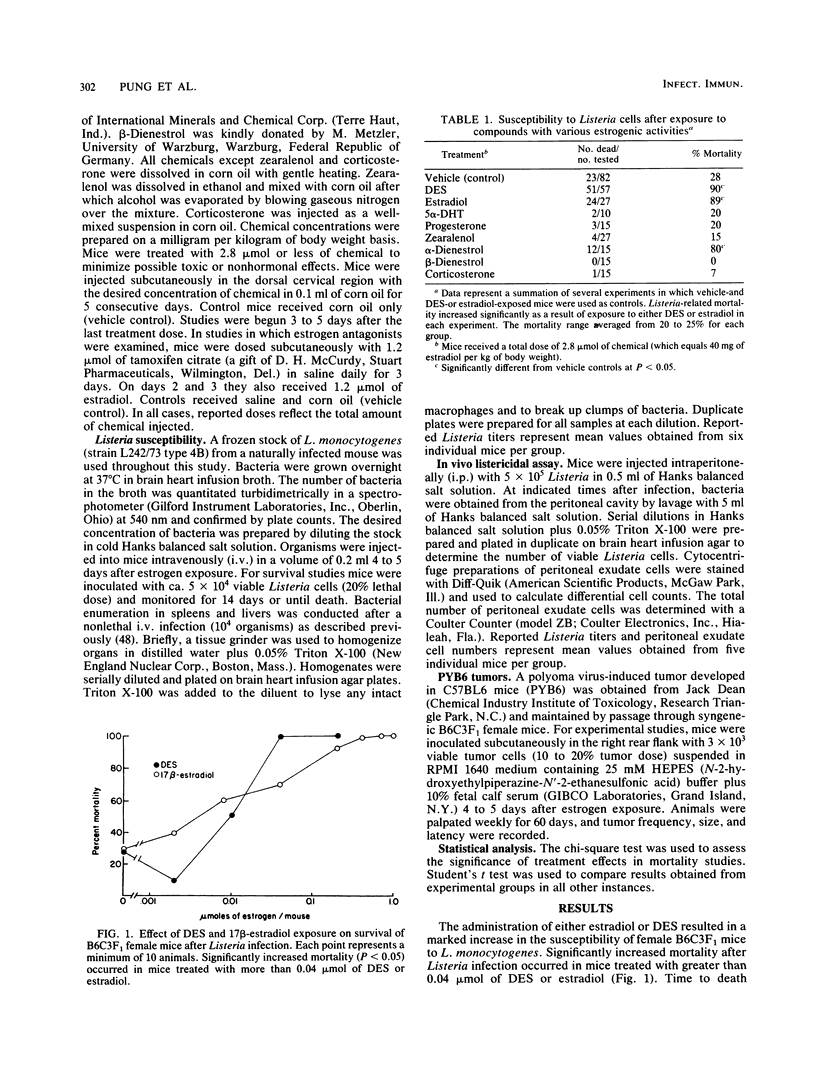
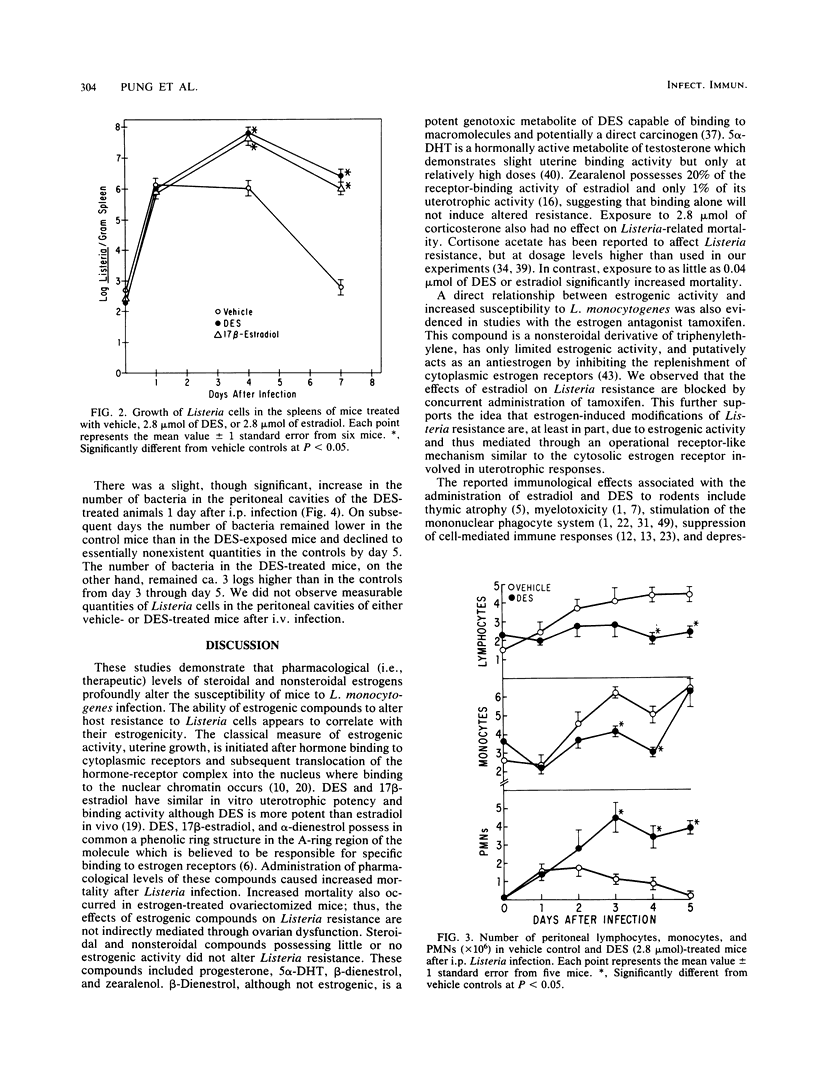
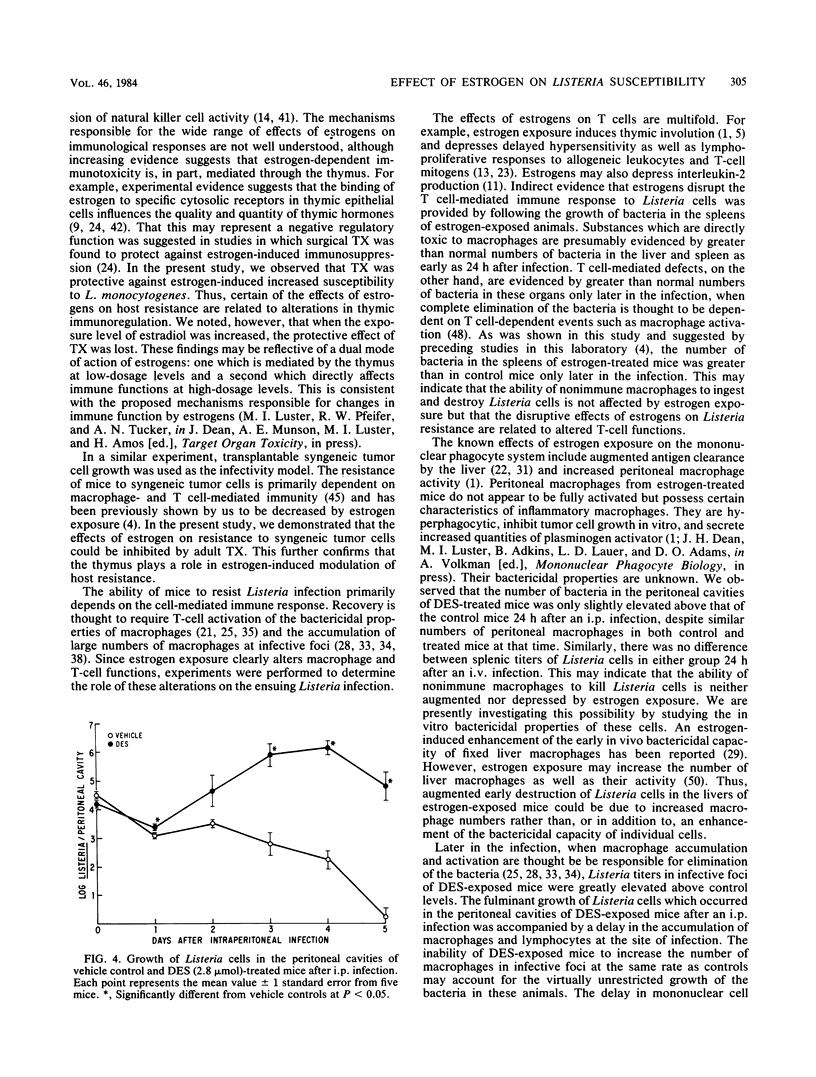
Subchronic exposure to pharmacological levels of estrogenic compounds, including 17 beta-estradiol, diethylstilbestrol, and alpha-dienestrol, significantly increased the mortality of B6C3F1 female mice after Listeria infection. Compounds with little estrogenic activity, including 5 alpha-dihydrotestosterone, progesterone, zearalenol, and corticosterone, did not alter Listeria-related mortality. Estrogen-induced alterations in resistance were inhibited by both adult thymectomy and the estrogen antagonist tamoxifen. Estrogen exposure depressed the accumulation of monocytes and lymphocytes at infective foci. Significantly elevated numbers of bacteria were observed in infective foci of estrogen-treated mice later in the infection when bacteria were nearly eliminated from untreated animals. These results indicate that estrogen-induced suppression of Listeria immunity is partially mediated by the thymus, probably through receptor events which may ultimately suppress the activation of T cell-dependent defense mechanisms. This may be partially reflected by the inability of estrogen-exposed mice to eliminate Listeria cells or to accumulate mononuclear effector cells at infective foci at the same rate as controls.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boorman G. A., Luster M. I., Dean J. H., Wilson R. E. The effect of adult exposure to diethylstilbestrol in the mouse on macrophage function and numbers. J Reticuloendothel Soc. 1980 Dec;28(6):547–560. [PubMed] [Google Scholar]
- Cottrell B. J., Playfair J. H., de Sousa B. Plasmodium yoelii and Plasmodium vinckei: the effects of nonspecific immunostimulation on murine malaria. Exp Parasitol. 1977 Oct;43(1):45–53. doi: 10.1016/0014-4894(77)90006-6. [DOI] [PubMed] [Google Scholar]
- Czuprynski C. J., Henson P. M., Campbell P. A. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J Leukoc Biol. 1984 Feb;35(2):193–208. doi: 10.1002/jlb.35.2.193. [DOI] [PubMed] [Google Scholar]
- DOUGHERTY T. F. Effect of hormones on lympatic tissue. Physiol Rev. 1952 Oct;32(4):379–401. doi: 10.1152/physrev.1952.32.4.379. [DOI] [PubMed] [Google Scholar]
- Dean J. H., Luster M. I., Boorman G. A., Luebke R. W., Lauer L. D. The effect of adult exposure to diethylstilbestrol in the mouse: alterations in tumor susceptibility and host resistance parameters. J Reticuloendothel Soc. 1980 Dec;28(6):571–583. [PubMed] [Google Scholar]
- Fried W., Tichler T., Dennenberg I., Barone J., Wang F. Effects of estrogens on hematopoietic stem cells and on hematopoiesis of mice. J Lab Clin Med. 1974 May;83(5):807–815. [PubMed] [Google Scholar]
- Fugmann R. A., Aranyi C., Barbera P. W., Bradof J. N., Gibbons R. D., Fenters J. D. The effect of diethylstilbestrol as measured by host resistance and tumor susceptibility assays in mice. J Toxicol Environ Health. 1983 Apr-Jun;11(4-6):827–841. doi: 10.1080/15287398309530387. [DOI] [PubMed] [Google Scholar]
- Grossman C. J., Roselle G. A. The interrelationship of the HPG-thymic axis and immune system regulation. J Steroid Biochem. 1983 Jul;19(1B):461–467. doi: 10.1016/0022-4731(83)90204-2. [DOI] [PubMed] [Google Scholar]
- Henriksen O., Frey J. R. Control of the expression of interleukin-2 activity. Cell Immunol. 1982 Oct;73(1):106–114. doi: 10.1016/0008-8749(82)90439-7. [DOI] [PubMed] [Google Scholar]
- Holsapple M. P., Munson A. E., Munson J. A., Bick P. H. Suppression of cell-mediated immunocompetence after subchronic exposure to diethylstilbestrol in female B6C3F1 mice. J Pharmacol Exp Ther. 1983 Oct;227(1):130–138. [PubMed] [Google Scholar]
- Jensen E. V., DeSombre E. R. Mechanism of action of the female sex hormones. Annu Rev Biochem. 1972;41:203–230. doi: 10.1146/annurev.bi.41.070172.001223. [DOI] [PubMed] [Google Scholar]
- Kalland T. Alterations of antibody response in female mice after neonatal exposure to diethylstilbestrol. J Immunol. 1980 Jan;124(1):194–198. [PubMed] [Google Scholar]
- Kalland T. Decreased and disproportionate T-cell population in adult mice after neonatal exposure to diethylstilbestrol. Cell Immunol. 1980 Apr;51(1):55–63. doi: 10.1016/0008-8749(80)90237-3. [DOI] [PubMed] [Google Scholar]
- Kalland T., Forsberg J. G. Natural killer cell activity and tumor susceptibility in female mice treated neonatally with diethylstilbestrol. Cancer Res. 1981 Dec;41(12 Pt 1):5134–5140. [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Katzenellenbogen J. A., Mordecai D. Zearalenones: characterization of the estrogenic potencies and receptor interactions of a series of fungal beta-resorcylic acid lactones. Endocrinology. 1979 Jul;105(1):33–40. doi: 10.1210/endo-105-1-33. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F., Knecht E., Budzko D. B., Pizzimenti M. C. Phagocytosis: a defense mechanism against infection with Trypanosoma cruzi. J Immunol. 1974 May;112(5):1839–1844. [PubMed] [Google Scholar]
- Kittas C., Henry L. Effect of sex hormones on the response of mice to infection with Toxoplasma gondii. Br J Exp Pathol. 1980 Dec;61(6):590–600. [PMC free article] [PubMed] [Google Scholar]
- Korach K. S. Estrogen action in the mouse uterus: characterization of the cytosol and nuclear receptor systems. Endocrinology. 1979 May;104(5):1324–1332. doi: 10.1210/endo-104-5-1324. [DOI] [PubMed] [Google Scholar]
- Korach K. S., Metzler M., McLachlan J. A. Estrogenic activity in vivo and in vitro of some diethylstilbestrol metabolites and analogs. Proc Natl Acad Sci U S A. 1978 Jan;75(1):468–471. doi: 10.1073/pnas.75.1.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose L. D., DiLuzio N. R. Dose-related reticuloendothelial system stimulation by diethylstilbestrol. J Reticuloendothel Soc. 1976 Dec;20(6):457–460. [PubMed] [Google Scholar]
- Luster M. I., Boorman G. A., Dean J. H., Luebke R. W., Lawson L. D. The effect of adult exposure to diethylstilbestrol in the mouse: alterations in immunological functions. J Reticuloendothel Soc. 1980 Dec;28(6):561–569. [PubMed] [Google Scholar]
- Luster M. I., Hayes H. T., Korach K., Tucker A. N., Dean J. H., Greenlee W. F., Boorman G. A. Estrogen immunosuppression is regulated through estrogenic responses in the thymus. J Immunol. 1984 Jul;133(1):110–116. [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMartin K. E., Kennedy K. A., Greenspan P., Alam S. N., Greiner P., Yam J. Diethylstilbestrol: a review of its toxicity and use as a growth promotant in food-producing animals. J Environ Pathol Toxicol. 1978 Jan-Feb;1(3):279–313. [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Morahan P. S., Bradley S. G., Munson A. E., Duke S., Fromtling R. A., Marciano-Cabral F. Immunotoxic effects of diethylstilbestrol on host resistance: comparison with cyclophosphamide. J Leukoc Biol. 1984 Mar;35(3):329–341. doi: 10.1002/jlb.35.3.329. [DOI] [PubMed] [Google Scholar]
- NICOL T., BILBEY D. L., CHARLES L. M., CORDINGLEY J. L., VERNON-ROBERTS B. OESTROGEN: THE NATURAL STIMULANT OF BODY DEFENCE. J Endocrinol. 1964 Oct;30:277–291. doi: 10.1677/joe.0.0300277. [DOI] [PubMed] [Google Scholar]
- Nicol T., Vernon-Roberts B., Quantock D. C. The influence of various hormones on the reticulo-endothelial system: endocrine control of body defense. J Endocrinol. 1965 Nov;33(3):365–383. doi: 10.1677/joe.0.0330365. [DOI] [PubMed] [Google Scholar]
- Noller K. L., Kurland L. T. Study of prenatal DES exposure and its consequences in Rochester, Minnesota. J Toxicol Environ Health Suppl. 1976;1:1–11. [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The action of cortisone acetate on cell-mediated immunity to infection. Suppression of host cell proliferation and alteration of cellular composition of infective foci. J Exp Med. 1971 Dec 1;134(6):1485–1500. doi: 10.1084/jem.134.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., White H. J., Hough A. J., Jr, Pasley J. N., Barron A. L. Effect of estradiol on chlamydial genital infection of female guinea pigs. Infect Immun. 1982 Nov;38(2):699–705. doi: 10.1128/iai.38.2.699-705.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdiger H. W., Haenisch F., Metzler M., Oesch F., Glatt H. R. Metabolites of diethylstilboestrol induce sister chromatid exchange in human cultured fibroblasts. Nature. 1979 Oct 4;281(5730):392–394. doi: 10.1038/281392a0. [DOI] [PubMed] [Google Scholar]
- Sadarangani C., Skamene E., Kongshavn P. A. Cellular basis for genetically determined enhanced resistance of certain mouse strains to listeriosis. Infect Immun. 1980 May;28(2):381–386. doi: 10.1128/iai.28.2.381-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Davis C. E. Models of T cell deficiency in listeriosis: the effects of cortisone and cyclosporin A on normal and nude BALB/c mice. J Immunol. 1983 Jul;131(1):450–453. [PubMed] [Google Scholar]
- Schmidt W. N., Sadler M. A., Katzenellenbogen B. S. Androgen-uterine interaction: nuclear translocation of the estrogen receptor and induction of the synthesis of the uterine-induced protein (IP) by high concentrations of androgens in vitro but not in vivo. Endocrinology. 1976 Mar;98(3):702–716. doi: 10.1210/endo-98-3-702. [DOI] [PubMed] [Google Scholar]
- Seaman W. E., Blackman M. A., Gindhart T. D., Roubinian J. R., Loeb J. M., Talal N. beta-Estradiol reduces natural killer cells in mice. J Immunol. 1978 Dec;121(6):2193–2198. [PubMed] [Google Scholar]
- Stimson W. H., Crilly P. J. Effects of steroids on the secretion of immunoregulatory factors by thymic epithelial cell cultures. Immunology. 1981 Oct;44(2):401–407. [PMC free article] [PubMed] [Google Scholar]
- Sutherland R. L., Murphy L. C. Mechanisms of oestrogen antagonism by nonsteroidal antioestrogens. Mol Cell Endocrinol. 1982 Jan;25(1):5–23. doi: 10.1016/0303-7207(82)90165-4. [DOI] [PubMed] [Google Scholar]
- Tatsukawa K., Mitsuyama M., Takeya K., Nomoto K. Differing contribution of polymorphonuclear cells and macrophages to protection of mice against Listeria monocytogenes and Pseudomonas aeruginosa. J Gen Microbiol. 1979 Nov;115(1):161–166. doi: 10.1099/00221287-115-1-161. [DOI] [PubMed] [Google Scholar]
- Thieme T. R., Mirkovich A., Maloney P., Goodwin D. A. In vivo and in vitro effects of dexamethasone on leukocyte migration in the rat adjuvant arthritis model. Inflammation. 1982 Dec;6(4):371–386. doi: 10.1007/BF00917308. [DOI] [PubMed] [Google Scholar]
- Toivanen P. Enhancement of staphylococcal infection in mice by estrogens. I. Effect of the timing, quantity and quality of the hormone. Ann Med Exp Biol Fenn. 1967;45(2):138–146. [PubMed] [Google Scholar]
- Tripathy S. P., Mackaness G. B. The effect of cytotoxic agents on the primary immune response to Listeria monocytogenes. J Exp Med. 1969 Jul 1;130(1):1–16. doi: 10.1084/jem.130.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon-Roberts B. The effects of steroid hormones on macrophage activity. Int Rev Cytol. 1969;25:131–159. doi: 10.1016/s0074-7696(08)60202-8. [DOI] [PubMed] [Google Scholar]
- Warr G. W., Sljivić V. S. Activity of the reticuloendothelial system and the antibody response. I. Effect of stilboestrol on RES activity and localization of sheep erythrocytes in the mouse. Br J Exp Pathol. 1973 Feb;54(1):56–68. [PMC free article] [PubMed] [Google Scholar]


