Abstract
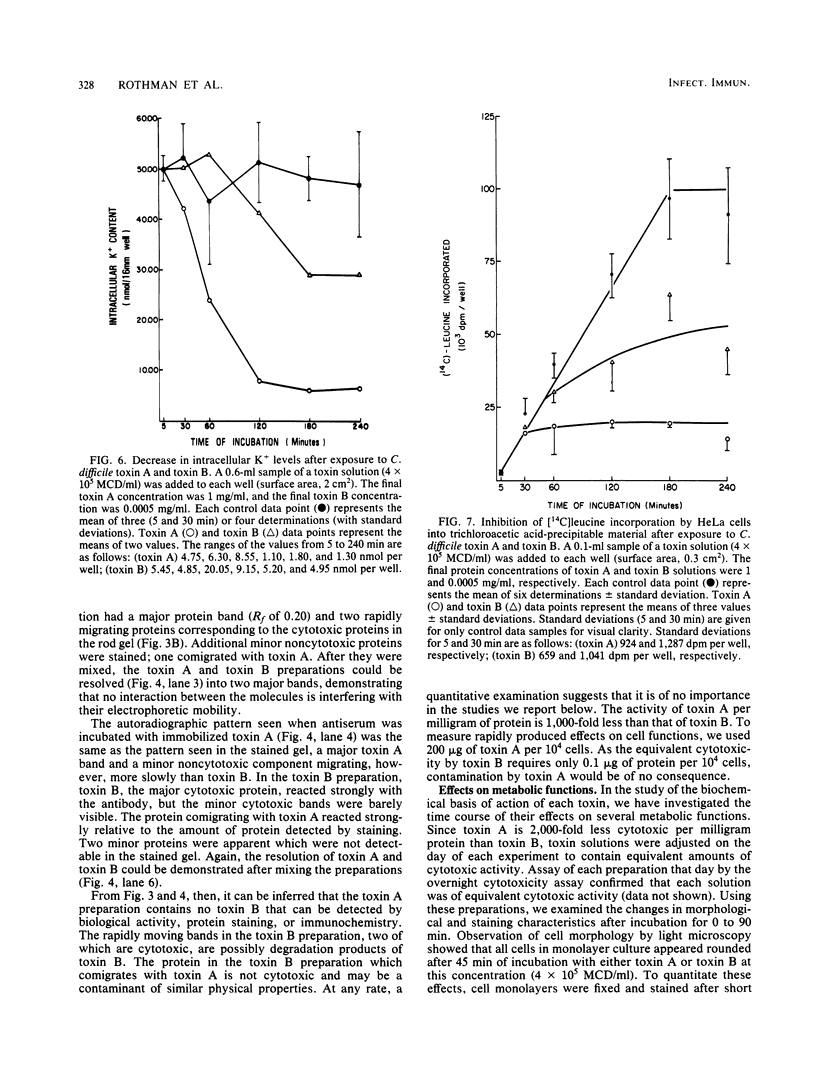
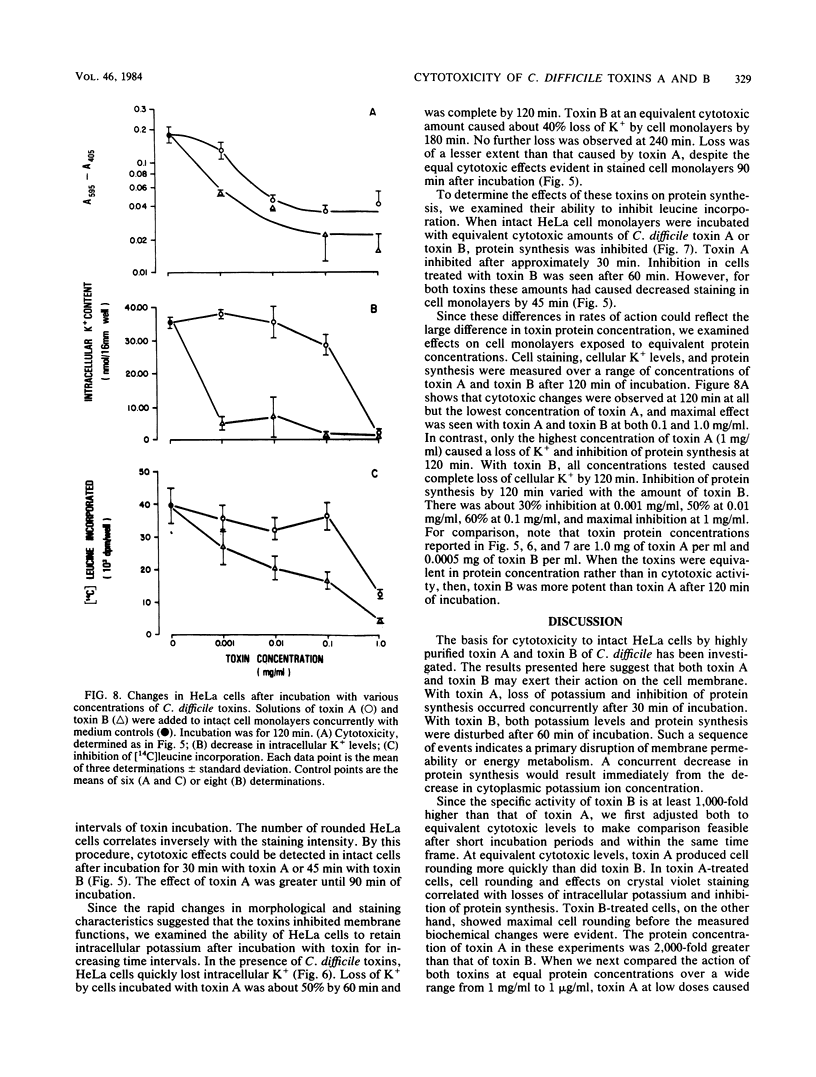
Toxin A and toxin B preparations of Clostridium difficile have been shown to affect metabolic functions of intact HeLa cells with different kinetics. The cytotoxins were purified from dialyzed filtrates of C. difficile strain VPI 10463 by hydrophobic interaction chromatography and ion-exchange chromatography and were concentrated by dialysis or by ultrafiltration. The toxins, which are immunologically unrelated, were analyzed by polyacrylamide gel electrophoresis and by immunochemistry with the Western blot technique. Toxin A was resolved into one major cytotoxic protein and a minor, rapidly migrating species that did not comigrate with toxin B. Toxin B was resolved into one major and three minor cytotoxic proteins. One protein comigrating with toxin A had no cytotoxic activity. The highly purified toxin A at 1.0 mg/ml caused loss of intracellular K+ and inhibition of protein synthesis in HeLa cells within 1 h. These effects correlated with morphological changes indicating cytotoxicity. At lower protein concentrations of toxin A (10- to 100-fold less), however, cytotoxic effects were seen at 120 min, whereas no changes in K+ levels or protein synthesis were yet evident. The toxin B preparation, 1,000-fold more toxic than toxin A, was diluted to equivalent cytotoxicity as measured in the overnight assay. Toxin B caused loss of K+ and inhibition of protein synthesis well after cytotoxic morphological changes were complete. In contrast, at higher protein concentrations (2- to 2,000-fold more), intracellular K+ was lost completely by 120 min. The effects on cell rounding and protein synthesis were incomplete at 120 min, but increased with the toxin B concentration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlgren T., Florin I., Jarstrand C., Thelestam M. Loss of surface fibronectin from human lung fibroblasts exposed to cytotoxin from Clostridium difficile. Infect Immun. 1983 Mar;39(3):1470–1472. doi: 10.1128/iai.39.3.1470-1472.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J. G., Onderdonk A. B., Cisneros R. L., Kasper D. L. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J Infect Dis. 1977 Nov;136(5):701–705. doi: 10.1093/infdis/136.5.701. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Griffin D. E., Rothman S. W., Doctor B. P. Purification and biological characterization of shiga toxin from Shigella dysenteriae 1. Infect Immun. 1982 Jun;36(3):996–1005. doi: 10.1128/iai.36.3.996-1005.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Rothman S. W., Doctor B. P. Inhibition of protein synthesis in intact HeLa cells by Shigella dysenteriae 1 toxin. Infect Immun. 1980 Jul;29(1):98–107. doi: 10.1128/iai.29.1.98-107.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Lauermann M., Bartlett J. G. Cytotoxicity assay in antibiotic-associated colitis. J Infect Dis. 1979 Nov;140(5):765–770. doi: 10.1093/infdis/140.5.765. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Sullivan N., Wilkins T. D. Differential effects of Clostridium difficile toxins on tissue-cultured cells. J Clin Microbiol. 1982 Jun;15(6):1157–1158. doi: 10.1128/jcm.15.6.1157-1158.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin I., Thelestam M. Intoxication of cultured human lung fibroblasts with Clostridium difficile toxin. Infect Immun. 1981 Jul;33(1):67–74. doi: 10.1128/iai.33.1.67-74.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwith M. J., Langston C., Dunsmore B. Morphologic and functional effects of Clostridium difficile enterotoxin in tissue culture. Can J Microbiol. 1982 Jan;28(1):100–105. doi: 10.1139/m82-009. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson H. E., Price A. B. Pseudomembranous colitis: Presence of clostridial toxin. Lancet. 1977 Dec 24;2(8052-8053):1312–1314. doi: 10.1016/s0140-6736(77)90363-4. [DOI] [PubMed] [Google Scholar]
- Rothman S. W. Presence of Clostridium difficile toxin in guinea pigs with penicillin-associated colitis. Med Microbiol Immunol. 1981;169(3):187–196. doi: 10.1007/BF02123592. [DOI] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Thorne G. M., Bartlett J. G. Comparison of two toxins produced by Clostridium difficile. Infect Immun. 1981 Dec;34(3):1036–1043. doi: 10.1128/iai.34.3.1036-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Brönnegård M. Interaction of cytopathogenic toxin from Clostridium difficile with cells in tissue culture. Scand J Infect Dis Suppl. 1980;(Suppl 22):16–29. [PubMed] [Google Scholar]
- Vesely D. L., Straub K. D., Nolan C. M., Rolfe R. D., Finegold S. M., Monson T. P. Purified Clostridium difficile cytotoxin stimulates guanylate cyclase activity and inhibits adenylate cyclase activity. Infect Immun. 1981 Jul;33(1):285–291. doi: 10.1128/iai.33.1.285-291.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedel N., Toselli P., Pothoulakis C., Faris B., Oliver P., Franzblau C., LaMont T. Ultrastructural effects of Clostridium difficile toxin B on smooth muscle cells and fibroblasts. Exp Cell Res. 1983 Oct 15;148(2):413–422. doi: 10.1016/0014-4827(83)90163-5. [DOI] [PubMed] [Google Scholar]