Abstract
A vast portion of human disease results when the process of apoptosis is defective. Disorders resulting from inappropriate cell death range from autoimmune and neurodegenerative conditions to heart disease. Conversely, prevention of apoptosis is the hallmark of cancer and confounds the efficacy of cancer therapeutics. In the search for optimal targets that would enable the control of apoptosis, members of the BCL-2 family of anti- and pro-apoptotic factors have figured prominently. Development of BCL-2 antisense approaches, small molecules, and BH3 peptidomimetics has met with both success and failure. Success-because BCL-2 proteins play essential roles in apoptosis. Failure-because single targets for drug development have limited scope. By examining the activity of the BCL-2 proteins in relation to the mitochondrial landscape and drawing attention to the significant mitochondrial membrane alterations that ensue during apoptosis, we demonstrate the need for a broader based multi-disciplinary approach for the design of novel apoptosis-modulating compounds in the treatment of human disease.
Keywords: Apoptosis, BAX, BH3, drug design, peptide therapy
FUNDAMENTALS OF APOPTOSIS
Apoptosis or programmed cell death is a fundamental process that is essential for embryonic development and the maintenance of adult tissue homeostasis. Apoptosis is also the most common mechanism by which damaged, infected or unneeded cells are eliminated without producing an inflammatory response, as results when damage induces necrosis. It is therefore not surprising that human disease frequently result from dysregulation of the apoptotic process, making this cell suicide mechanism an effective therapeutic target.
Our understanding of the apoptotic process and its complex levels of regulation are founded in the pioneering studies of Horvitz and colleagues who dissected the cell death pathway in the nematode Caenorhabditis elegans (C. elgans).1 Their discovery of two genes, ced-3 and ced-4 (cell death abnormal), revealed the core machinery required for executing apoptosis in somatic cells. Ced-3 encodes a gene product that is a member of the caspase (cysteine-dependent, aspartate-specific) family of proteases that are responsible for proteolysis and destruction of key cell components.2 Ced-4 encodes a gene product that is a scaffolding/adaptor protein needed for activation of Ced-3.3 Its mammalian counterpart is Apaf-1 (apoptotic protease-activating factor-1). The action of Ced-3/Ced-4 is regulated by a third gene called ced-9. Ced-9 prevents cell death in C-elegans.4 Lastly, the discovery of the egl-1 gene (egg, laying defective) revealed the final regulatory component of the death machinery in C-elegan.5 The mammalian counterparts of Ced-9 and Egl-1 belong to the long-studied, but still poorly understood, BCL-2 (B cell lymphoma) family of apoptotic modulators. An anti-apoptotic member of the family is the mammalian counterpart of Ced-9, while a pro-apoptotic member of the Bcl-2 family is the mammalian counterpart of Egl-1.
THE BCL-2 FAMILY
BCL-2 was the first human proto-oncogene discovered that promoted neoplastic expansion by inhibiting death.6,7 BCL-2 became the founding member of a family of anti-apoptotic and pro-apoptotic proteins that share one to four homology domains (BCL-2 homology regions BH1-BH4).8-10 Other anti-apoptotic BCL-2 homologues have been identified, such as BCL-XL11 and MCL-1.12 Two classes of BCL-2 pro-apoptotic members are known. Of the class of multi-domain proteins, BAX was the first death-inducing protein identified as part of the BCL-2 family.13 Other multi-domain proteins that share structural similarities with BAX are BAK14,15 and BOK.16 The second class of BCL-2 pro-apoptotic proteins share a single homology domain - the BH3 domain - which include BAD,17 BID,18 BIM,19 NOXA and PUMA.20 To date, over twenty different BCL-2 family members have been identified.
Ablation, in mice, of the genes encoding members of the BCL-2 family revealed that despite functional overlap, these proteins are essential for normal tissue development and homeostasis.21-23 Over time, a certain progress was made revealing the mechanisms that regulate the apoptotic activity of these proteins. Homo- and hetero-dimerization among BCL-2 family is one means by which their activity is regulated. The BH3 domain, common to many BCL2 family members, is integral in this regard.24 Thus BH3-only proteins can act as sensitizers or activators of anti- and pro-apoptotic BCL-2 proteins,25 and their specialization is evident in their regulation. A truncated and active form of BID is produced by caspase 8-mediated cleavage.26 In contrast, BAD is phosphorylated by Akt (Protein Kinase B) and then sequestered by 14-3-3.27 Alternative splicing generates multiple isoforms of BIM19,28 that are sequestered by LC8, a component of the dynein motor complex,29 while Forkhead (FKHR) transcription factors may regulate its synthesis.30 NOXA and PUMA are transcriptionally regulated by p53.20
Regulation of the apoptotic activity of the multi-domain pro-apoptotic BCL-2 family members is less well understood. BAK is a mitochondrial-resident protein whose intramembreanous oligomerization is inhibited by association with the voltage-dependent anion channel 2 (VDAC2).31 BAX is found as a cytosolic monomer in non-apoptotic cells that inserts into the outer mitochondrial membrane (OMM) upon induction of cell death and leads to the release of cytochrome c.32,33 Multiple conformation-specific binding partners have been identified for BAX,34 but their role in the translocation, oligomerization, or membrane-insertion of BAX remains poorly defined. Our own studies contributed by demonstrating that cytokine withdrawal in lymphocytes leads to the mitochondrial translocation of BAX in parallel with intracellular alkalinization35 mediated by the sodium hydrogen exchanger 1 (NHE1),36,37 and that BAX deficiency could partially restore lymphocyte development in cytokine-deficient mice, demonstrating the importance of BAX in the homeostasis of immune cells.38 These studies showed that BAX is indispensable for the induction of apoptosis, specifically in the immune system, and suggest that disease could be the outcome when the activity of this death factor is deregulated.
APOPTOSIS AND HUMAN DISEASE
A balance between cell death and cell proliferation must be maintained to ensure the health of every human being. Recent findings indicate that approximately one-half of all major human diseases are a consequence of abnormal apoptosis.39 Inappropriate apoptosis can cause autoimmune and neurodegenerative disorders as well as heart disease, while resistance to apoptosis can promote cancer and impede the effectiveness of cancer therapeutics.
Autoimmune diseases may arise from defective clearance of autoreactive lymphocytes and problems with the elimination of apoptotic bodies. The resulting tissue destruction can be devastating. The BH3-only protein, BIM, may be an essential mediator in the death of autoreactive lymphocytes, since loss of BIM prevented the deletion of these cells.40 Myocardial ischemia and cerebral ischemia are leading causes of death in the developed world. In mouse models of these diseases, interference with the activation of BAX or overexpression of BCL-2 prevented apoptosis and reduced infarct size,41,42 implicating these proteins in tissue destruction. BAX itself plays a principal role in the death of neurons that cause Parkinson's disease43 and may contribute to multiple sclerosis44 and autoimmunity.45
Cancer is the result of a succession of genetic changes that ultimately confer some form of growth advantage. Resistance to apoptosis is a fundamental part of carcinogenesis. Almost half of human cancers contain mutations in the gene for the tumor suppressor, p53. Multiple BCL-2 family members, such as BAX, BID, NOXA and PUMA are upregulated by and responsive to p53.20,46,47 Deregulation of numerous BCL-2 family members have been detected in diverse cancers, starting with its founding member BCL-2, discovered as chromosomal translocation with the immunoglobulin heavy chain locus in non-Hodgkin's lymphoma, follicular B-cell lymphoma, and diffuse large cell lymphomas.48 ,49 Gastrointestinal tumors and leukemias can have abnormalities in BAX.50,51 Moreover, mouse models have revealed that deficiency in BAD,52 inactivation of BIM,53 loss of BAX54 or overexpression of BCL-XL can underlie myc-induced leukemias or lymphomas, suggesting that these apoptotic modulators are significant contributors to the process of carcinogenesis.
Dysregulation of BCL-2 family members also confer resistance to the cytotoxic effects of cancer therapeutic agents. For example, in human epithelial cancers the absence of BAX completely eliminated death in response to certain chemo-preventive strategies.55 Therapeutics targeting BCL-2 proteins must therefore be engineered to not only inhibit or modify the function of these apoptotic factors but must also work in concert with the current modes of treatment to maximize effectiveness.
BH3 MIMETICS: THE GOOD, THE BAD AND THE UGLY
Of the myriad of signals being transmitted along the apoptotic network, we have decoded only the most obvious. It is known that the anti-apoptotic proteins, BCL-2 and BCL-XL, inhibit apoptosis when over-expressed,56,57 that when cleaved, the BH3-only protein, BID, enhances the binding of the pro-apoptotic, multidomain protein, BAX,58 that the oligomerization of BAX and its counterpart, BAK, are necessary for permeabilization of the OMM,59 that the BH3-only protein, BIM, enhances but is not required for BAX to destabilize an engineered membrane60 What is not known, however, is precisely how these proteins interact with the membranes of organelles like mitochondria to inhibit or induce apoptosis, making the design of modulating compounds a challenge.
Recent advances in attempting to harness apoptosis further elucidate the molecular requirements for designing effective peptide mercenaries. Mimetics of the DNA repair factor, Ku70, yielded crucial residues for binding to BAX and inhibiting apoptosis.61 However, as a single target inhibitor of apoptosis, the Ku70 peptide had a limited application in preventing cell death by inhibiting BAX. Conversely, the development of BH3 mimetics, concisely reviewed by Zhang et al,62 is an attempt to exploit the pro-apoptotic function of native BH3-only proteins. As example, Goldsmith et al, using the active helical sequence of BID and BAD proteins, generated a peptide with an arginine homo-polymer to make them permeable to cells.63 These peptidomimetics demonstrated in vitro and in vivo effectiveness against neuroblastoma by disrupting the protein-protein interactions between BCL-2 and endogenous BH3 proteins. Using a novel approach, Li et al fused the antennapedia peptide transduction domain (ANT), to BAX, BAK and BAD BH3 sequences and tested the peptides against head and neck squamous cell carcinoma.64 These peptides demonstrated an improvement over the polyarginine transduction motif. Though promising, these peptidomimetics target only individual anti-apoptotic proteins. This is a problem because not all cancers are alike and thus require customized therapies. To address this, the single target limitations of BH3 peptidomimetics was exploited in concept of BH3 profiling,65 a method used to identify cancers that are amenable to specific BH3 peptidomimetics. This was successfully used to identify cancers most responsive to the small molecule ABT-737.65 BH3 peptidomimetics are thereby most useful, not for direct induction of apoptosis, but for predicting sensitivities to already developed therapeutic agents.65
The current trend to target the BCL2 family of proteins in order to control apoptosis follows logically from the notion of targeting the first cause. However, thinking linearly about pathways in terms of upstream and downstream events not only limits possible outcomes but confounds data interpretation as well. The strengths and limitations of BH3 peptidomimetics are a reflection of the complexity of the intertwining pathways that characterize cellular destruction. Wading through the flood of experimental data relating to apoptosis, it is becomes evident that apoptosis is not a linear event, but a network of spatial-temporal events centered about the mitochondria. The mitochondria cannot be viewed just as scaffold for crucial cellular processes, or a reservoir for apoptotic proteins, but rather an integral part that is at the crossroads of life and death itself.
THE MITOCHONDRION
In order to understand the apoptotic process and design novel therapeutic approaches targeting the BCL-2 family, one must understand the architecture of the mitochondrion and how the different BCL-2 proteins interact with this most unique of organelles. In most cells, mitochondria are analogous to major metropolitan centers. Like a macroscopic metropolis, the essential services of a thriving community converge within the mitochondria. Here, crucial cellular processes such as oxidative phosphorylation, lipid metabolism, and porphyrin and steroid hormone synthesis are housed. Calcium is transiently stored within the mitochondrial matrix, enabling the mitochondrion to function as a signal transduction rheostat. In addition to its primary function, ATP synthesis via the citric acid cycle, the mitochondrion participates in cell cycling, growth and differentiation. It is not surprising then, that determinants that lead to the commitment to apoptosis intersect at the mitochondria.
Like the high density of skyscrapers in an urban metropolis, the high protein to lipid ratio of the outer mitochondria membrane (OMM), approximately 1 protein to 45 lipids, leaves very little membrane surface area exposed. This feature is critical, and must be taken into consideration, when designing drugs, small molecules or peptides that target mitochondria. The skyline of the mitochondrial surface is dotted with large protein complexes such as VDAC (voltage dependent anion selective channel) and signaling complexes anchored by AKAP121/8466 and TOM (translocase of the outer membrane) as well as smaller integral proteins like porins, which form small diameter pores that are permeable to molecules no larger than 5kDa. The protein density of the inner mitochondrial membrane (IMM) is even greater with approximately 1 protein for 15 lipids, slightly more dense than the OMM. The phospholipid composition is the same as the OMM with the exception of the presence of cardiolipin, an unusual phospholipid that has four acyl chains and carries two negative charges in its head-group. The presence of cardiolipin in the inner membrane makes the IMM nearly impermeable to ions. It requires another energy-driven protein complex, TIM (translocase of the inner membrane), to facilitate transport across the IMM.
The unique mitochondrial membranes not only sequester individual components of mitochondrial processes, but they also function as integral elements of those processes. For example, an electrochemical gradient is set up by the active translocation of protons out of the matrix, through the IMM and into the inter-membrane space. Thus the inner membrane acts as an electronic capacitor as a result of the asymmetric charge distribution on either side of that membrane. The energy potential of the capacitor drives the production of ATP by protein complexes imbedded in the inner leaflet of the membrane's phospholipid bilayer. The ATP production potential of the mitochondria is greatly enhanced by the convoluted folding of the inner membrane into an elaborate labyrinth of ATP producing niches. The resultant folded structures are called Cristae and the space in between the cristae is the mitochondrial matrix, the storage compartment for ATP. The two membranes meet at junctions where VDAC and TOM complexes converge. It is here that the unique phospholipid, cardiolipin, is found on the outer leaflet of the inner membrane. Mitochondria are thus the energy factories of a cell, and it is therefore not surprising that the same machinery that gives life is subverted during the apoptotic process to cause cell death.
THE MITOCHONDRIAL-DEPENDENT APOPTOTIC PATHWAY
The two main pathways for apoptosis are known as the extrinsic or death receptor pathway and the intrinsic or mitochondria-dependent pathway. Though both pathways are important, the intrinsic pathway is most responsive to external or environmental cues and DNA damage. The intrinsic apoptotic pathway follows a prescribed sequence of events that center on the mitochondria. One of the earliest observable events is the structural remodeling of mitochondrial cristae,67 which is followed by the translocation of cardiolipin from the inner leaflet of the IMM to the outer leaflet of the OMM.68,69 This translocation is reminiscent of the exposure of phosphatidyl-serine (PS) and annexin-I from the intercellular leaflet to the extracellular face of the plasma membrane. The translocation event coincides with the cristae junction remodeling event reported by Zhang et al.70 Cytochrome c, a highly positively charged heme-containing protein that is involved in electron transport and oxidative phosphorylation, is anchored to cardiolipin in the mitochondrial matrix and presumably translocates with cardiolipin to the intermembrane space. Cristae reorganization leads to the disruption of the mitochondrial inner membrane potential.71 These events are quickly followed by the OMM permeabilization (MOMP) and release of mitochondrial proteins from the intermembrane space, specifically cytochrome c.
Once released, cytochrome c associates with Apaf-1 and procaspase 9 to form the apoptosome. Activated caspase 9 is released from the apoptosome, initiating the caspase cascade. Other apoptotic factors released from mitochondria include Smac/DIABLO (second mitochondria-derived activator of caspases/direct IAP-binding protein with low pI), AIF (apoptosis inducing factor), EndoG (Endonuclease G), and HtrA2/Omi (high temperature requirement protein A2). Each protein has a specific apoptotic function, which is temporally coordinated by MOMP. For example, EndoG orchestrates the nuclear condensation and chromatin (DNA) fragmentation. Apoptosis culminates with the packaging of cellular contents into apoptotic bodies that are engulfed by the cell's "haz-mat" team, the macrophages. Thus concludes a series of highly orchestrated events whose conductors are members of the BCL-2 family, apoptotic proteins whose principal binding sites are postulated to be in the very crowded OMM.
THE NEXT STEP: THE MULTI-DISCIPLINE APPROACH FOR DESIGNING NOVEL THERAPEUTICS
Returning to the metropolis analogy, targeting a single type of structure (such as one BCL-2 family member) may or may not initiate the cascade that demolishes the entire city (or cell). The consequence of targeting that structure depends on the number, function and redundancy of that structure. Rather than targeting the redundant structures with the intent of setting off a cascade of events leading to the implosion of the foundation, perhaps the foundation itself should be the target. In the case of the BCL-2 family - the foundation is the mitochondrion. There is evidence that the mitochondria itself can act independently of BAX to promote apoptosis through the disruption of micro-domains72 or changes in its ultrastructure.73 Additionally, specific mitochondrial membrane components, for example cholesterol and sphingomyelin, have been implicated as mediators of apoptosis74,75 as well as the physical properties of those components, such as fluidity and topography.75
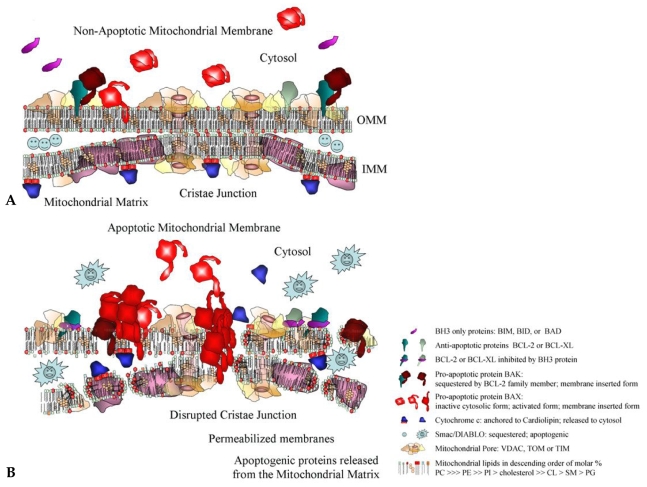
In terms of the BCL-2 family, apoptosis-driven mitochondrial changes can impact upon their activation. We found that in experiments combining cytosol, containing transfected-BAX, and mitochondria, isolated from apoptotic or non-apoptotic BAX-deficient HCT116 cells, the predominant fraction of transfected BAX spontaneously translocated to apoptotic mitochondria (Tschammer et al. unpublished results). This is shown diagrammatically in Fig. 1, in which the mitochondrial membrane from apoptotic cells contains a very different array of proteins and arrangement of lipids in comparison to non-apoptotic cells. The mitochondrial landscape is therefore dramatically altered under apoptotic compared to non-apoptotic conditions. This is an important consideration when designing therapeutics to modify the activity of the BCL-2 proteins, in particular when designing novel compounds that produce the similar lethal effects upon mitochondria without the need for first identifying specificity (as with the BH3 mimetics).
Fig. 1.
Mitochondrial membranes are sites of dynamic events during apoptosis. (A). In non-apoptotic cells the cytosol contains monomeric BAX (light red), while in the outer mitochondrial membrane (OMM), membrane-associated BAX monomers (dark red) are found in close association with anti-apoptotic proteins like BCL-2 (blue-green) or BCL-XL (green). Membrane-associated BAK (dark red) is found in complex with VDAC2. Soluble BH3-only (lavender) proteins like BAD or BIM are sequestered by distinct regulatory mechanisms in the cytosol. The mitochondrial cristae junction is denoted by the two pores that span both mitochondrial membranes. ATP synthesis proteins (pink) are embedded in the inner mitochondrial membrane (IMM). Cholesterol (yellow) spans both bilayers. Cardiolipin (double red squares) has bound cytochrome c (dark blue) and together these are located in inner face of the IMM. SMAC/Diablo (light blue smiley faces), as examples of mitochondrial apoptotic factors, is found in the intermembrane spaces. (B) In apoptotic cells, cardiolipin (double red squares), flips from the IMM inner leaflet to the OMM outer leaflet, carrying cytochrome c. Initiation of apoptosis activates cytosolic BAX (light red) which is recruited to the OMM to form oligomers (dark red). This is enabled by membrane-associated BAX (dark red) and BAK monomers that are no longer sequestered by BCL-2, BCL-XL or VDAC2 and can act as nuclei for the initiation of BAX/BAK oligomer formation. This process is enabled by soluble BH3 proteins (lavender) that bind to and inactivate BCL-2 (blue-green) and BCL-XL (green). The cristae junction is spanned by BAX/BAK oligomeric complexes and mitochondrial membrane is disrupted, leading to the release of cytochrome c (dark blue) and SMAC/Diablo (light blue) into the cytosol.
In the attempt to harness cellular death to cure disease, it is imperative to keep in mind the final outcome of the avalanche that is being triggered. The most important difference between damage-induced necrosis and apoptosis is that death by necrosis triggers an inflammatory immune response, an unwanted result in ischemic insult but perhaps a necessary one when total annihilation of a cancer is desired. Consequently, the limited, linear perspective of protein-protein interactions, as has dominated the development of BH3 peptidomimetics, must be broadened to include all the permutations of possible contributors from the mitochondrial microenvironment. This includes not only the large number of protein complexes crowding the mitochondrial surface (Fig. 1) but the complexity of the lipids that make up the membrane milieu as well.
In conclusion, the repertoire of potential structures in our arsenal must include more than the hydrophobic alpha helices that have been the focus of so much study and development. The contribution of electrostatics, and more importantly lipophilicity, must also be considered in the development of drugs, small molecules and peptidomimetics that target components of mitochondrial-dependent apoptosis in human diseases. In the field of apoptosis, a large gap is slowly being bridge between the biological and physical approaches that rely on knowledge bases unique to each discipline. Great strides have been made already and by encouraging the addition of a biophysical perspective to create an interdisciplinary research base perhaps more of the missing pieces to the puzzle that is the BCL-2 family will be found.
Footnotes
This work was supported in part by an NIH/NIGMS grant GM083324 (Khaled) and an NIH/NCI grant CA109524 (Khaled).
References
- 1.Metzstein MM, Stanfield GM, Horvitz HR. Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet. 1998;14:410–416. doi: 10.1016/s0168-9525(98)01573-x. [DOI] [PubMed] [Google Scholar]
- 2.Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 3.Yuan J, Horvitz HR. The Caenorhabditis elegans cell death gene ced-4 encodes a novel protein and is expressed during the period of extensive programmed cell death. Development. 1992;116:309–320. doi: 10.1242/dev.116.2.309. [DOI] [PubMed] [Google Scholar]
- 4.Hengartner MO, Horvitz HR. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994;76:665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- 5.Conradt B, Horvitz HR. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell. 1998;93:519–529. doi: 10.1016/s0092-8674(00)81182-4. [DOI] [PubMed] [Google Scholar]
- 6.Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 7.Bakhshi A, Jensen JP, Goldman P, Wright JJ, McBride OW, Epstein AL, et al. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985;41:899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- 8.Graninger WB, Seto M, Boutain B, Goldman P, Korsmeyer SJ. Expression of Bcl-2 and Bcl-2-Ig fusion transcripts in normal and neoplastic cells. J Clin Invest. 1987;80:1512–1515. doi: 10.1172/JCI113235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seto M, Jaeger U, Hockett RD, Graninger W, Bennett S, Goldman P, et al. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988;7:123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boise LH, González-García M, Postema CE, Ding L, Lindsten T, Turka LA, et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 12.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 14.Farrow SN, White JH, Martinou I, Raven T, Pun KT, Grinham CJ, et al. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature. 1995;374:731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- 15.Kiefer MC, Brauer MJ, Powers VC, Wu JJ, Umansky SR, Tomei LD, et al. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature. 1995;374:736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- 16.Hsu SY, Kaipia A, McGee E, Lomeli M, Hsueh AJ. Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc Natl Acad Sci U S A. 1997;94:12401–12406. doi: 10.1073/pnas.94.23.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 18.Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 21.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3:932–939. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 23.Pellegrini M, Bouillet P, Robati M, Belz GT, Davey GM, Strasser A. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J Exp Med. 2004;200:1189–1195. doi: 10.1084/jem.20041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zha H, Aimé-Sempé C, Sato T, Reed JC. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem. 1996;271:7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- 25.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 27.Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, et al. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 2000;6:41–51. [PubMed] [Google Scholar]
- 28.U M, Miyashita T, Shikama Y, Tadokoro K, Yamada M. Molecular cloning and characterization of six novel isoforms of human Bim, a member of the proapoptotic Bcl-2 family. FEBS Lett. 2001;509:135–141. doi: 10.1016/s0014-5793(01)03145-3. [DOI] [PubMed] [Google Scholar]
- 29.Puthalakath H, Huang DC, O'Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 30.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 31.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 32.Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci U S A. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucken-Ardjomande S, Martinou JC. Newcomers in the process of mitochondrial permeabilization. J Cell Sci. 2005;118:473–483. doi: 10.1242/jcs.01654. [DOI] [PubMed] [Google Scholar]
- 35.Khaled AR, Kim K, Hofmeister R, Muegge K, Durum SK. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci U S A. 1999;96:14476–14481. doi: 10.1073/pnas.96.25.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khaled AR, Moor AN, Li A, Kim K, Ferris DK, Muegge K, et al. Trophic factor withdrawal: p38 mitogen-activated protein kinase activates NHE1, which induces intracellular alkalinization. Mol Cell Biol. 2001;21:7545–7557. doi: 10.1128/MCB.21.22.7545-7557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grenier AL, Abu-Ihweij K, Zhang G, Moore S, Boohaker R, Slepkov E, et al. Apoptosis-induced alkalinization by the NA+/H+ exchanger Isoform 1 is mediated through phosphorylation of amino acids SER726 and SER729. Am J Physiol Cell Physiol. 2008;295:C883–C896. doi: 10.1152/ajpcell.00574.2007. In press 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khaled AR, Li WQ, Huang J, Fry TJ, Khaled AS, Mackall CL, et al. Bax deficiency partially corrects interleukin-7 receptor alpha deficiency. Immunity. 2002;17:561–573. doi: 10.1016/s1074-7613(02)00450-8. [DOI] [PubMed] [Google Scholar]
- 39.Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002;1:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- 40.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 41.Gibson ME, Han BH, Choi J, Knudson CM, Korsmeyer SJ, Parsadanian M, et al. BAX contributes to apoptotic-like death following neonatal hypoxia-ischemia: evidence for distinct apoptosis pathways. Mol Med. 2001;7:644–655. [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 43.Vila M, Jackson-Lewis V, Vukosavic S, Djaldetti R, Liberatore G, Offen D, et al. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson\'s disease. Proc Natl Acad Sci USA. 2001;98:2837–2842. doi: 10.1073/pnas.051633998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharief MK, Matthews H, Noori MA. Expression ratios of the Bcl-2 family proteins and disease activity in multiple sclerosis. J Neuroimmunol. 2003;134:158–165. doi: 10.1016/s0165-5728(02)00400-9. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi O, Fisher J, Suh H, Harada H, Malynn BA, Korsmeyer SJ. Essential role of BAX,BAK in B cell homeostasis and prevention of autoimmune disease. Proc Natl Acad Sci U S A. 2005;102:11272–11277. doi: 10.1073/pnas.0504783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 47.Sax JK, Fei P, Murphy ME, Bernhard E, Korsmeyer SJ, El-Deiry WS. BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol. 2002;4:842–849. doi: 10.1038/ncb866. [DOI] [PubMed] [Google Scholar]
- 48.Weiss LM, Warnke RA, Sklar J, Cleary ML. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987;317:1185–1189. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- 49.Hermine O, Haioun C, Lepage E, d'Agay MF, Briere J, Lavignac C, et al. Prognostic significance of bcl-2 protein expression in aggressive non-Hodgkin's lymphoma. Groupe d'Etude des Lymphomes de l'Adulte (GELA) Blood. 1996;87:265–272. [PubMed] [Google Scholar]
- 50.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 51.Heiser D, Labi V, Erlacher M, Villunger A. The Bcl-2 protein family and its role in the development of neoplastic disease. Exp Gerontol. 2004;39:1125–1135. doi: 10.1016/j.exger.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP, et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci U S A. 2004;101:6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eischen CM, Roussel MF, Korsmeyer SJ, Cleveland JL. Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutations during Myc-mediated lymphomagenesis. Mol Cell Biol. 2001;21:7653–7662. doi: 10.1128/MCB.21.22.7653-7662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 56.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 57.Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol Cell. 1999;3:159–167. doi: 10.1016/s1097-2765(00)80307-x. [DOI] [PubMed] [Google Scholar]
- 58.Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marani M, Tenev T, Hancock D, Downward J, Lemoine NR. Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis. Mol Cell Biol. 2002;22:3577–3589. doi: 10.1128/MCB.22.11.3577-3589.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gomez JA, Gama V, Yoshida T, Sun W, Hayes P, Leskov K, et al. Bax-inhibiting peptides derived from Ku70 and cell-penetrating pentapeptides. Biochem Soc Trans. 2007;35:797–801. doi: 10.1042/BST0350797. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Ming L, Yu J. BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updat. 2007;10:207–217. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldsmith KC, Liu X, Dam V, Morgan BT, Shabbout M, Cnaan A, et al. BH3 peptidomimetics potently activate apoptosis and demonstrate single agent efficacy in neuroblastoma. Oncogene. 2006;25:4525–4533. doi: 10.1038/sj.onc.1209489. [DOI] [PubMed] [Google Scholar]
- 64.Li R, Boehm AL, Miranda MB, Shangary S, Grandis JR, Johnson DE. Targeting antiapoptotic Bcl-2 family members with cell-permeable BH3 peptides induces apoptosis signaling and death in head and neck squamous cell carcinoma cells. Neoplasia. 2007;9:801–811. doi: 10.1593/neo.07394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Feliciello A, Gottesman ME, Avvedimento EV. cAMP-PKA signaling to the mitochondria: protein scaffolds, mRNA and phosphatases. Cell Signal. 2005;17:279–287. doi: 10.1016/j.cellsig.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 68.Garcia Fernandez M, Troiano L, Moretti L, Nasi M, Pinti M, Salvioli S, et al. Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell Growth Differ. 2002;13:449–455. [PubMed] [Google Scholar]
- 69.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang D, Lu C, Whiteman M, Chance B, Armstrong JS. The mitochondrial permeability transition regulates cytochrome c release for apoptosis during endoplasmic reticulum stress by remodeling the cristae junction. J Biol Chem. 2008;283:3476–3486. doi: 10.1074/jbc.M707528200. [DOI] [PubMed] [Google Scholar]
- 71.De Giorgi F, Lartigue L, Bauer MK, Schubert A, Grimm S, Hanson GT, et al. The permeability transition pore signals apoptosis by directing Bax translocation and multimerization. FASEB J. 2002;16:607–609. doi: 10.1096/fj.01-0269fje. [DOI] [PubMed] [Google Scholar]
- 72.Garofalo T, Giammarioli AM, Misasi R, Tinari A, Manganelli V, Gambardella L, et al. Lipid microdomains contribute to apoptosis-associated modifications of mitochondria in T cells. Cell Death Differ. 2005;12:1378–1389. doi: 10.1038/sj.cdd.4401672. [DOI] [PubMed] [Google Scholar]
- 73.Cross JR, Postigo A, Blight K, Downward J. Viral pro-survival proteins block separate stages in Bax activation but changes in mitochondrial ultrastructure still occur. Cell Death Differ. 2008;15:997–1008. doi: 10.1038/cdd.2008.14. [DOI] [PubMed] [Google Scholar]
- 74.Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem J. 2005;386:445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lucken-Ardjomande S, Montessuit S, Martinou JC. Contributions to Bax insertion and oligomerization of lipids of the mitochondrial outer membrane. Cell Death Differ. 2008;15:929–937. doi: 10.1038/cdd.2008.9. [DOI] [PubMed] [Google Scholar]