Abstract
Autophagy has been shown to contribute to defense against intracellular bacteria and parasites. In comparison, the ability of such pathogens to manipulate host cell autophagy to their advantage has not been examined. Here we present evidence that infection by Toxoplasma gondii, an intracellular protozoan parasite, induces host cell autophagy in both HeLa cells and primary fibroblasts, via a mechanism dependent on host Atg5 but independent of host mammalian target of rapamycin suppression. Infection led to the conversion of LC3 to the autophagosome-associated form LC3-II, to the accumulation of LC3-containing vesicles near the parasitophorous vacuole, and to the relocalization toward the vacuole of structures labeled by the phosphatidylinositol 3-phosphate indicator YFP-2×FYVE. The autophagy regulator beclin 1 was concentrated in the vicinity of the parasitophorous vacuole in infected cells. Inhibitor studies indicated that parasite-induced autophagy is dependent on calcium signaling and on abscisic acid. At physiologically relevant amino acid levels, parasite growth became defective in Atg5-deficient cells, indicating a role for host cell autophagy in parasite recovery of host cell nutrients. A flow cytometric analysis of cell size as a function of parasite content revealed that autophagy-dependent parasite growth correlates with autophagy-dependent consumption of host cell mass that is dependent on parasite progression. These findings indicate a new role for autophagy as a pathway by which parasites may effectively compete with the host cell for limiting anabolic resources.
Macroautophagy (hereafter referred to as autophagy) is a major catabolic process in which cytosolic constituents are sequestered within double-membraned vesicles (autophagosomes) and subsequently delivered to lysosomes for degradation. Current evidence indicates at least two distinct functions for this process. On the one hand, autophagy can be up-regulated under nutrient-limiting conditions to increase nutrient supply via recycling of the products of autophagic degradation, which may be exported from the lysosome (1). The up-regulation of autophagy upon starvation is thought to be mediated by the suppression of signaling through the mTOR pathway (2). On the other hand, autophagy can serve to maintain cellular homeostasis by facilitating the removal of damaged or deleterious elements, such as misfolded protein aggregates (3). An important example of the latter function is the role of autophagy in restricting the growth of intracellular pathogens, including both free bacteria that have escaped into host cytosol, such as group A Streptococcus, and pathogens, such as Mycobacterium tuberculosis, that reside in parasitophorous vacuoles in macrophages (4, 5). In macrophages infected with Toxoplasma gondii, fusion of the parasitophorous vacuole with lysosomes can be induced in an autophagy-dependent manner when host cell anti-parasitic function is activated via CD40 (6). Autophagy as a component of host defense may be up-regulated by inflammatory agents such as lipopolysaccharide (7) and interferon-γ (8).
Although the clearance function of autophagy may enhance pathogen killing in host cells that have been activated to generate antimicrobial or antiparasitic function, in permissive host cells, in which the pathogen is less susceptible to sequestration by the autophagosome, autophagy may conceivably play a quite different role. Modulation of the balance between anabolic and catabolic processes may affect the outcome of competition between pathogen and host cell for limiting nutrients. In particular, the nutritive function of autophagy could favor pathogen expansion by providing greater access to host cell biomass. The intracellular apicomplexan parasite, T. gondii, is a suitable agent for the investigation of this hypothesis, because it has been shown to be highly dependent on its host cell for the supply of several nutrients, including amino acids (9), lipids (10), and purines (11). T. gondii replicates within a parasitophorous vacuole that, in permissive host cells, is protected from lysosomal fusion. Recent evidence indicates that in such permissive cells, in which the parasite can differentiate into bradyzoites associated with chronic infection, the pathogen is able to actively sequester host cell lysosome-derived vesicles, thereby potentially gaining access to their contents (12).
The ability of intracellular parasites to regulate host cell autophagy has been little examined, and there is also little information with respect to the impact of these pathogens on host cell signals that potentially affect the autophagic pathway. In addition to mTOR, these include calcium ions, which have been implicated in autophagy induced by endoplasmic reticulum stress (13). In this study, we provide evidence that T. gondii induces host cell autophagy by a mechanism dependent on calcium but independent of mTOR and that it exploits the nutritive function of host autophagy to enhance its proliferation.
EXPERIMENTAL PROCEDURES
Parasite and Cell Culture—T. gondii RH strain was maintained in primary human foreskin fibroblasts (HFFs).2 T. gondii expressing yellow fluorescent protein (YFP) was a kind gift of Dr. Boris Striepen (University of Georgia). Atg5-deficient and control mouse embryonic fibroblasts (MEFs) were kind gifts of Dr. Noboru Mizushima (Tokyo Medical and Dental University). TSC2-deficient and control MEFs were kind gifts of Dr. David Kwiatkowski (Harvard University). All of the cell lines were maintained in Dulbecco's minimal essential medium (DMEM) containing 10% fetal bovine serum in the absence of antibiotics at 5% CO2. For infection, cells were seeded at a density of 10,000 cells/cm2 and after overnight incubation infected with freshly harvested T. gondii.
Immunoblotting Analysis—After washing twice with cold PBS, cell monolayers were lysed in radioimmune precipitation assay buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS in PBS) supplemented with protease inhibitor and phosphatase inhibitor cocktails (Sigma). Ten to twenty micrograms of protein extracts were resolved by SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and probed with the relevant antibodies. The blots were developed using enhanced chemiluminescence (Pierce) and exposed to x-ray film. Anti-LC3 was a generous gift from Dr. Ron Kopito (Stanford University). Anti-YFP was obtained from Becton Dickson. Primary antibodies obtained from Cell Signaling Technology include anti-pAkt (S473) (catalog number 9271), anti-total Akt (9272), anti-pGsk3β(S9) (9336), anti-pS6K1 (T389) (9234), anti-total S6K1 (9202), anti-pS6 (S235/236) (2211), anti-total S6 (2217), and anti-4E-BP1 (9644). Densitometry of blots was performed using ImageJ.
Transfection—The GFP-tagged human LC3 construct was a kind gift from Dr. Karla Kierkegaard (Stanford University). The YFP-tagged tandem FYVE construct was a kind gift from Drs. Marc Fivaz (Duke University) and Harald Stenmark (The Norwegian Radium Hospital). The FLAG-tagged Beclin 1 construct was a kind gift from Dr. Beth Levine (University of Texas Southwestern Medical Center). The cells were transfected with endotoxin-free DNA (Qiagen) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. One day post-transfection, the cells were trypsinized and seeded. After overnight incubation, the cells were infected with T. gondii for another 24 h, followed by fixation with paraformaldehyde and microscopic observation.
siRNA Knockdown—The oligoribonucleotide targeting the cDNA sequence of beclin 1 (CAGTTTGGCACAATCAATA), as well as nonspecific control siRNA, were generous gifts from Dr. Michael Lenardo (National Institutes of Health). HeLa cells in 24-well dishes were transfected with nonspecific or beclin 1 siRNA (50 pmol/well) using Lipofectamine 2000 (Invitrogen). 48 h after transfection, the cells were infected with T. gondii for 24 h before the preparation of protein extracts.
Immunoprecipitation—HeLa cells were seeded in 100-mm dishes (800,000 cells/dish) and on the second day infected or not with T. gondii at a multiplicity of infection of 8. Twenty-four h post-infection, the cells were washed with cold PBS and incubated in 1 ml of lysis buffer (150 mm NaCl, 50 mm HEPES, pH 7.5, 0.1% Triton X-100) supplemented with protease inhibitor and phosphatase inhibitor cocktails. After clearing, aliquots of the lysates (equalized for total protein, 0.5–1 mg) were incubated with goat anti-Beclin 1 (1–2 μg; Santa Cruz) for 90 min on ice and subsequently with protein G-Sepharose for 1 h. The immunoprecipitates were washed three times with lysis buffer prior to SDS-PAGE and immunoblot analysis with anti-Vps34. One-tenth of each sample was run separately on the gel for probing with anti-beclin 1.
Flow Cytometry—Trypsinized single cell suspensions were placed on ice, fixed with 4% buffered paraformaldehyde, resuspended in PBS containing 0.5% bovine serum albumin, and analyzed by flow cytometry using a FACSCalibur (Becton Dickinson). The number of parasites per infected cell was estimated by dividing the FL-1 value (YFP intensity) of infected cells by the mean value obtained for free parasites (readily discriminated by size) in the same sample. The relationship between YFP intensity and parasite content is near linear (14). The data were analyzed using either CellQuest (Becton Dickinson) or FCSExpress (De Novo). Loess smoothing was performed using SigmaPlot (Jandel).
RESULTS
Infection by T. gondii Induces Host Cell Autophagy—Autophagosomes are formed from a cup-shaped single-membrane structure known as the isolation membrane or preautophagosome. A key event in the maturation of this structure is the Atg7 + Atg3-mediated conversion of the ubiquitin-like protein, LC3, from a diffuse cytosolic form (LC3-I) to a lipidated form (LC3-II) that specifically associates with the isolation membrane and remains associated with the inner autophagosomal membrane (15, 16). These observations have led to the development of two widely used indicators for autophagy: the accumulation of LC3-II, detected by its increased mobility in SDS-PAGE relative to LC3-I, and the detection of punctate vesicular LC3 by fluorescence microscopy. We used these assays to examine the impact of T. gondii infection on the level of host cell autophagy.
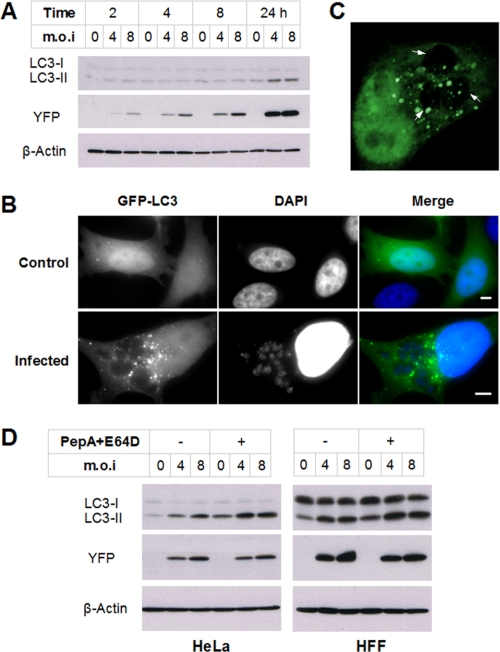
As shown in Fig. 1A, infection of HeLa cells with YFP-expressing parasites led to a progressive increase in intracellular parasite content (as measured by YFP level) that was consistent with the expected parasite replication time of 6–8 h. LC3-II levels remained constant from 2 to 8 h post-infection but were clearly elevated at 24 h. Consistent with this finding, infection of HeLa cells expressing GFP-LC3 led to a striking increase in punctate fluorescence (Fig. 1B). This increase was not observed in neighboring uninfected cells (data not shown), demonstrating that the induction of autophagy was cell-autonomous and did not result from induced secretion of mediators. Moreover, GFP-LC3-labeled vesicles displayed a specific localization to the vicinity of the parasitophorous vacuoles (Fig. 1B), as confirmed by z-stack reconstruction (Fig. 1C).
FIGURE 1.
Toxoplasma infection induces host cell autophagy. A, HeLa cells were infected with YFP-expressing T. gondii at the indicated multiplicity of infection (m.o.i) for 2, 4, 8, or 24 h. The cell lysates were subjected to immunoblotting analysis. B, HeLa cells were transfected with GFP-LC3 and then infected with wild-type T. gondii for 24 h. The cells were fixed and stained with DAPI prior to fluorescent imaging. Scale bars, 5 μm. C, cells were treated as in B. To detect signal both adjacent to and overlying the parasitophorous vacuole, a reconstruction was performed with confocal z-stack images. The arrows indicate parasitophorous vacuoles. D, HeLa or HFF cells were infected with YFP-expressing T. gondii for 22 h and then incubated with or without 10 μg/ml pepstatin A and 10 μg/ml E64D (PepA+E64D) for 2 h. The protein extracts were analyzed by immunoblotting using the indicated antibodies.
Because LC3-II is partially degraded by lysosomal hydrolases following lysosome fusion, increased LC3-II might represent a reduced rate of fusion and LC3-II clearance rather than increased autophagy (17). To address this question, we examined LC3-II levels in infected and control HeLa cells in which LC3-II clearance was prevented using cathepsin inhibitors (pepstatin A and E64D) for 2 h prior to harvest. As shown in Fig. 1D, an infection-dependent increase in LC3-II was observed both in the presence and in the absence of inhibitors, indicating that the data represent a genuine increase in the rate of autophagosome generation in infected cells. Similar data were obtained using HFFs, indicating that T. gondii can also up-regulate autophagy in normal untransformed cells.
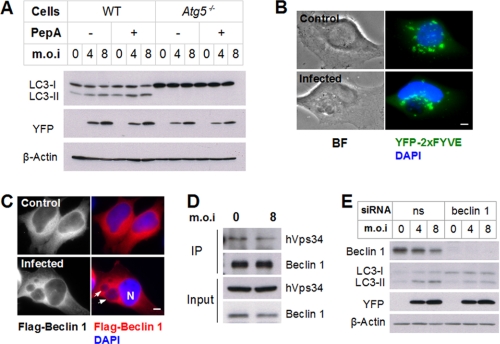
Role of Autophagy Genes in Toxoplasma-induced Autophagy— The lipidation of LC3-I to form LC3-II is dependent on the prior formation of a complex of three autophagy gene products, Atg5, Atg12, and Atg16 (18). Consequently, Atg5-deficient cells display a severe defect in starvation-induced autophagosome formation (19). Atg5 dependence of the generation of LC3 puncta has been observed in cells infected with viral and bacterial pathogens (20, 21). We examined the involvement of Atg5 in autophagy induced by T. gondii infection. In wild-type MEFs, we again observed infection-induced LC3-II accumulation in both the presence and the absence of a cathepsin inhibitor (Fig. 2A). In contrast, no LC3-II was detectable in either infected or uninfected atg5-/- MEFs, indicating that Atg5 is essential for infection-induced autophagy.
FIGURE 2.
Roles of autophagic pathway components in T. gondii-induced autophagy. A, control (WT) or atg5-/- MEFs were infected with YFP-expressing T. gondii for 22 h. The indicated samples were treated with pepstatin A (PepA) for 2 h prior to immunoblotting. B, HeLa cells were transfected with YFP-2×FYVE and infected with wild-type T. gondii for 24 h at a multiplicity of infection (m.o.i) of 4. The cells were fixed and stained with DAPI prior to fluorescent imaging. The asterisk indicates a parasitophorous vacuole. Scale bar, 5 μm. C, HeLa cells were transfected with FLAG-Beclin 1 and infected with wild-type T. gondii for 24 h at a multiplicity of infection of 4. The cells were fixed and stained with DAPI prior to fluorescent imaging. Arrows indicate parasitophorous vacuoles. N, host nucleus. Scale bar, 5 μm. D, lysates of infected and control HeLa cells were subjected to immunoprecipitation (IP) using anti-Beclin 1. Input (20 μg) and IP samples were resolved by SDS-PAGE and probed with the indicated antibodies. E, HeLa cells were transfected with either non-specific (ns) or beclin 1 siRNA. After 2 days, the cells were infected with T. gondii for 24 h and analyzed by immunoblotting.
A second complex involved in the maturation of the autophagosome, at least in response to starvation, is comprised of Beclin 1 (Atg6) and Vps34, a class III phosphatidylinositol 3-kinase (22, 23). The product of Vps34, phosphatidylinositol 3-phosphate (PI3P), is involved in multiple vesicular trafficking events and can be detected by fluorescent markers linked to a PI3P-binding domain (FYVE) (24). Beclin 1 association appears to specifically direct Vps34 activity in connection with autophagy; for example, a study in glioblastoma cells showed that beclin 1 knockdown prevented starvation-induced autophagy but left other Vps34 functions unimpaired (22). To probe the role of Vps34/beclin in T. gondii-induced autophagy, we first examined the effect of the parasite on the accumulation and distribution of PI3P in HeLa cells expressing the PI3P indicator, YFP-2×FYVE. As shown in Fig. 2B, infection did not noticeably affect the overall intensity of the YFP-2×FYVE signal. However, there was a marked alteration in the subcellular localization of the signal. In uninfected cells, perinuclear labeling was distributed isotropically around the nucleus, whereas in infected cells, label was concentrated around the parasitophorous vacuole. A similar shift in localization was observed for beclin 1 (Fig. 2C). The level of beclin 1 association with Vps34 was not altered by infection (Fig. 2D). These findings suggest that parasite-induced autophagy may depend less on global up-regulation of Vps34/beclin 1 activity than on allocation of these complexes to sites of autophagosome generation.
We then asked whether beclin 1 was necessary for Toxoplasma-induced autophagy. As shown in Fig. 2E, siRNA interference efficiently decreased beclin 1 protein expression. Knockdown of beclin 1 significantly reduced the LC3-II/LC3-I ratio in both infected and uninfected cells, but this ratio was still at least partly susceptible to elevation by the parasite in the absence of beclin 1. These data imply that parasite-induced autophagy proceeds via a beclin-dependent pathway, but the effect of the parasite is likely to lie either downstream or parallel to the beclin-regulated step.
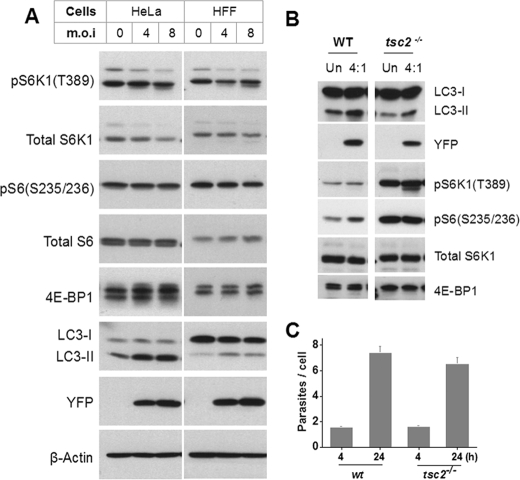
T. gondii-induced Autophagy Is Independent of mTOR Signaling—The inactivation of mTOR, a central regulator of cell growth and metabolism, mediates starvation-induced autophagy (2). A recent report suggests that calcium-induced autophagy is also dependent on suppression of mTOR signaling (25). We asked whether Toxoplasma-mediated induction of autophagy correlates with down-regulation of mTOR activity. The downstream sequelae of mTOR activation include the phosphorylation of S6 kinase-1 (S6K1) at T389, the phosphorylation of the eIF4E inhibitor, 4E-BP, at multiple sites (detected by mobility shift in SDS-PAGE), and the phosphorylation of ribosomal protein S6 by activated S6K1. As shown in Fig. 3A, infection of either HeLa or HFF cells did not lead to any alteration in the phosphorylation state of host 4E-BP1 or S6, whereas there was at most a modest reduction in S6K1 activation. These data indicate that suppression of host mTOR is unlikely to be the mechanism by which T. gondii induces host cell autophagy. Consistent with this finding, we have, in a separate study, observed that infection of serum-starved cells, in which basal mTOR activity is low, leads to a robust, sustained activation of host mTOR as detected by rapamycin-sensitive S6 phosphorylation.3
FIGURE 3.
T. gondii-induced autophagy is independent of mTOR. A, protein extracts from control or infected HeLa or HFF cells were analyzed with the indicated antibodies. B, wild-type or tsc2-/- MEFs were infected with YFP-expressing T. gondii for 24 h. The protein extracts were resolved by SDS-PAGE and probed with the indicated antibodies. For 4E-BP1, increased phosphorylation in tsc2-/- MEFs is reflected by the shift of intensity to the uppermost band. C, cells were infected as in B for 4 or 24 h, followed by trypsinization and fixation. Parasite proliferation was determined by flow cytometry as the number of parasites per infected cell. m.o.i, multiplicity of infection; Un, uninfected.
To confirm that infection-induced autophagy is independent of mTOR inactivation, we asked whether T. gondii was able to induce autophagy in cells in which mTOR signaling was constitutively hyperstimulated by loss of the negative regulator Tsc2. Tsc2 acts as a GTPase-activating protein toward the small GTPase Rheb, which activates mTOR (26). As expected, we found that the phosphorylation of S6K1, S6, and 4E-BP1 was markedly elevated in tsc2-/- MEFs (Fig. 3B). Nevertheless, infection led to comparable increases in LC3-II level in both wild-type and mutant MEFs. The induction of autophagy in tsc2-/- MEFs was not due to enhanced parasite proliferation (Fig. 3C). Therefore, we conclude that parasite-induced autophagy is mTOR-independent.
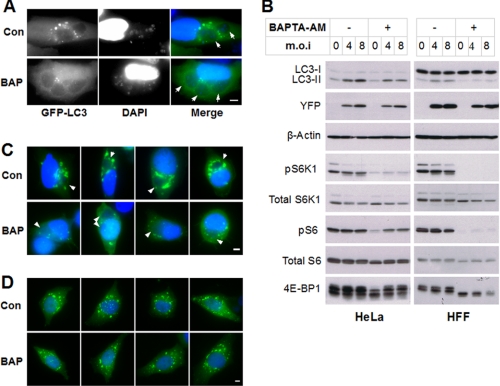
The Role of Calcium in T. gondii-induced Autophagy—Calcium signaling has been implicated in both basal and endoplasmic reticulum stress-induced autophagy (27, 28). In addition, agents that induce prolonged elevation of cytosolic calcium can up-regulate autophagy (25). We therefore asked whether cytosolic calcium levels contribute to T. gondii-induced autophagy. The calcium chelator 1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester (BAPTA-AM) is cell-permeant, but after cytosolic entry is rapidly converted by cellular esterases to a membrane-impermeant product. It therefore acts to chelate host cell cytosolic calcium but is not expected to modulate calcium levels either in organellar host stores or in intracellular parasites. HeLa or HFF cells, infected for 22 h, were treated for 2 h with BAPTA-AM or vehicle and assessed for autophagic signaling. Calcium chelation strongly suppressed the generation of GFP-LC3 puncta in infected cells (Fig. 4A) and also reduced the level of LC3-II relative to LC3-I (Fig. 4B). The LC3-II level in uninfected cells also appeared to be reduced by BAPTA-AM treatment, so that a degree of parasite-induced LC3-II elevation was still observable in the presence of the chelator. These results imply that T. gondii-induced autophagy is dependent on a calcium-sensitive step, but it is not clear from these data whether that step is regulated by the parasite. To gain further insight into this issue, we examined the effect of calcium chelation on host cell PI3P. As shown in Fig. 4C, treatment with BAPTA-AM markedly reduced YFP-2x-FYVE intensity in infected cells but did not alter the parasite-induced relocalization of the label to the parasitophorous vacuole. Importantly, the chelator had no effect on YFP-2x-FYVE signal in uninfected cells (Fig. 4D), implying that the T. gondii controls the YFP-2x-FYVE signal via a calcium-dependent mechanism. Finally, we observed that calcium chelation strongly suppressed signaling through the mTOR pathway in both infected and uninfected cells (Fig. 4B). This result is consistent with a recent study of the calcium dependence of TORC1 signaling (29) and further confirms that T. gondii-induced autophagy does not arise from mTOR suppression.
FIGURE 4.
T. gondii-induced autophagy is calcium-dependent. A, HeLa cells were transfected with GFP-LC3, infected with YFP-expressing T. gondii for 22 h, and then treated with either Me2SO vehicle (0.2%, v/v) or 20 μm BAPTA-AM (BAP) for 2 h. The cells were fixed and stained with DAPI prior to fluorescent imaging, Scale bar, 5 μm. B, HeLa or HFF cells were infected and treated with vehicle or BAPTA-AM as in A. The immunoblots were probed with the indicated antibodies. C and D, HeLa cells (transfected with YFP-2×FYVE) were either infected with T. gondii for 22 h (C) or incubated in medium (D). The cells were then treated with vehicle (Con) or BAPTA-AM (BAP) for 2 h, followed by fixation, DAPI staining, and fluorescent imaging. The arrows indicate parasitophorous vacuoles. Scale bar, 5 μm. m.o.i, multiplicity of infection.
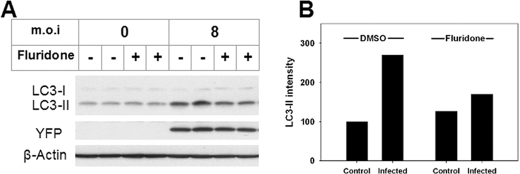
A recent study indicates that Toxoplasma has the capacity to regulate calcium signals through the synthesis of abscisic acid (ABA), a membrane-permeant (30) phytohormone that induces the release of intracellular calcium stores via the generation of cyclic AMP ribose (31). ABA has recently been detected as a product of human granulocytes (32), pancreatic islet cells (33), and other cell types (34), and it can initiate calcium-dependent signaling in these cells (32, 33). Parasite- or host cell-derived ABA represents a potential initiator of calcium-mediated host cell autophagy. Synthesis of ABA can be blocked with fluridone, an inhibitor of phytoene desaturase, the initial enzyme in the ABA biosynthetic pathway (35). We examined the effect of ABA blockade on parasite-induced autophagy. As shown in Fig. 5, treatment with fluridone inhibited LC3-II generation in infected cells, whereas it had no effect on the level of either LC3-I or LC3-II in uninfected cells. This result suggests that ABA is a potential initiating agent of T. gondii-induced autophagy.
FIGURE 5.
Fluridone inhibits T. gondii-induced autophagy. HeLa cells were infected or not with YFP-expressing T. gondii for 4 h. After washing away free parasites, the cells were treated with vehicle (0.1% dimethyl sulfoxide (DMSO)) or 50 μm fluridone in medium for 20 h. A, protein extracts were immunoblotted and probed with the indicated antibodies. The blot includes replicate samples from a single experiment. B, the intensity of the LC3-II band in immunoblots was measured by densitometry and normalized (untreated control = 100). Each bar represents the mean value from two independent experiments. m.o.i, multiplicity of infection.
The Role of Host Cell Autophagy in Parasite Growth—To assess the impact of host cell autophagy on parasite growth, we infected wild-type or atg5-/- MEFs with YFP-expressing T. gondii and measured intracellular parasite content by flow cytometry. Initial experiments using MEFs cultured in complete medium showed no Atg5-dependent effect on parasite proliferation (data not shown). We reasoned that if the role of autophagy were to provide nutritive support to the parasite, this would not be evident when nutrients were provided in excess. The amino acid (AA) concentration in DMEM is approximately eight times that of normal human plasma. Such concentrations are unlikely to ever occur in nature, because the maximum obtainable increase or decrease in plasma AA level (driven, respectively, by high protein diet or starvation) is only ∼2-fold (36–39). We therefore repeated the experiment using DMEM diluted with Hanks saline to progressively lower AA concentration through the physiological range. The MEFs were first rendered quiescent by serum deprivation to minimize host cell AA utilization and maintain an approximately constant AA concentration throughout the culture interval. Quiescent cells also provide a more appropriate model of the host cells encountered by the parasite in vivo.
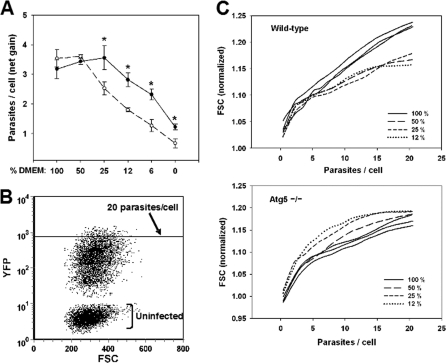
We observed that parasite growth in wild-type MEFs was not adversely affected by reduction of AA levels to a range approximating the plasma level in healthy mice or humans (12–25% of complete DMEM) (Fig. 6A). There was in fact a reproducible optimum in growth seen at 25% DMEM, which was also observed for HFFs (data not shown). In contrast, reduction of AA concentration to the physiological range resulted in a 30–50% decrease in parasite growth in atg5-/- MEFs relative to wild type. These data imply that host cell autophagy is required for T. gondii to maintain an optimal rate of proliferation when AA are physiologically limiting, consistent with the notion that the host autophagic pathway provides nutritive support to the parasite.
FIGURE 6.
Role of host cell autophagy in parasite growth. A, autophagy enhances growth at physiological amino acid levels. MEFs, either wild-type (filled circles) or Atg5-/- (open circles), were deprived of serum for 24 h, infected with YFP-expressing T. gondii for 4 h, rinsed to remove free parasites, and then incubated with the indicated dilutions of DMEM in Hanks buffer for 16 h. The cells were trypsinized, fixed, and analyzed by flow cytometry to measure parasite number/cell. This number was used to calculate parasite net gain by subtracting the values obtained at 4 h. The asterisks indicate p values < 0.02. B and C, infection under limiting amino acids progressively reduces host cell size via autophagy. The cells analyzed in A were assessed for forward scatter (FSC) as a function of parasite content (YFP). B, dot plot displaying a representative sample of wt host cells in 100% DMEM after overnight infection. C, loess smoothing was applied to dot plot data for cells containing 1–20 parasites. Forward scatter values were normalized to the mean values of uninfected cells in each sample. Each curve represents data from the dot plot of one sample. For simplicity, only one representative sample (of three total) is displayed for cells cultured in 12, 25, or 50% DMEM. The data are representative of two similar experiments.
These findings suggest that Toxoplasma may exploit host autophagy to compete with the host cell for limiting anabolic resources. We therefore asked whether autophagy results in a net transfer of biomass from the host cell to the parasite. The data from the experiment in Fig. 6A were analyzed with respect to host cell size (measured by forward scatter) as a function of parasite content. As expected, the cells containing more parasites (and therefore enlarged parasitophorous vacuoles) have increased size (Fig. 6C). However, for wild-type cells bearing more than approximately five parasites, the extent of the gain in size becomes noticeably reduced when AA concentration is lowered to 12–50% DMEM. This reduction in size is entirely dependent on host cell autophagy, because it is eliminated in atg5-/- MEFs, in which the reduction of AA levels actually leads to a slight enhancement of host cell size gain as a function of increasing parasite content. These data imply that, at physiological AA levels, the parasite competes for amino acids and in effect consumes the host cell in an autophagy-dependent manner.
DISCUSSION
Autophagy provides the cell with a mechanism both to destroy unwanted elements and to replenish scarce nutrients. In the context of intracellular infection with either bacteria or Toxoplasma, several studies have documented the contribution of the destructive function of host cell autophagy to host defense (4, 5). In contrast, a pro-pathogen role for autophagy has been described in the case of infection by a variety of RNA viruses (40–43), for which the autophagosome is thought to provide a scaffold membrane that facilitates viral replication. However the current study is the first to provide evidence for a pro-pathogen role based on the nutritive function of autophagy. The basis for this inference is the observation that the disabling of host cell autophagy by deletion of Atg5 abrogates the ability of the parasite to maintain optimal proliferation when AA levels become limiting and also completely eliminates the ability of the parasite to respond to AA limitation by capturing additional biomass from the host cell. If parasitism is defined as the ability of an organism to gain nourishment at the expense of its host, then these data provide direct evidence for parasitism at the cellular level.
That the dependence of Toxoplasma on host cell autophagy is manifest only at physiological AA concentration is consistent with many other studies that show that autophagy is largely dispensable for anabolic processes in the absence of challenge but becomes essential under conditions of high nutrient demand or stress. For example, autophagy promotes tolerance of starvation in yeast, slime molds, nematodes, plants, and mice (3). It is possible, however, that the role of autophagy under nutrient-replete conditions has been underestimated, because many of these studies rely on the use of autophagy-deficient mutant strains in which compensatory mechanisms may have developed. A recent study of the role of autophagy in the prevention of cardiomyopathy provides evidence, suggesting that such compensation may be considerable (44). The authors assessed hearts in which Atg5 had been depleted either acutely in the adult mouse or else beginning in early development. Under long term depletion, cardiac function was normal until stress was introduced by pressure overload; in contrast, acute depletion led to heart failure in the absence of challenge. Because atg5-/- MEF cells represent a comparable circumstance of long term depletion, it is possible that the dependence of T. gondii on autophagy is understated by our results, because of the presence of compensatory pathways of protein turnover operating in the mutant cells.
A second novel aspect of this study is the demonstration that infection by an intracellular parasite can induce autophagy in the host cell. The delayed kinetics of this response, which occurs between 8 and 24 h post-infection, are suggestive of an event triggered by increased nutrient demand rather than by the initial contact with and invasion of the host cell. This notion can be tested in future using an auxotrophic parasite strain whose intracellular growth can be arrested (45). On the other hand, the finding that mTOR suppression, which has been implicated in many studies as the mechanism of autophagic stimulation by nutrient limitation, plays no role in T. gondii-induced autophagy argues against this interpretation and suggests that the up-regulated autophagy is more likely due to an active trigger resulting from infection. The capacity of Toxoplasma to export signaling molecules to the host cell has been clearly demonstrated, although it is not yet well understood (46). The parasite contains specialized secretory vesicles, termed rhoptries, that release multiple parasite-encoded proteins into the host cell during or shortly after invasion (47). These proteins include kinases and phosphatases, at least one of which (Rop16, a putative kinase) has been shown to have significant effects on host cell signaling (48). It is possible that one or more of these exported proteins regulates host cell autophagy.
Alternatively, the finding that fluridone inhibits parasite-induced autophagy suggests that the process may be initiated by ABA, whose concentration in the parasite is reported to increase significantly during the first 10 h post-invasion (31). However, recent data indicate that human fibroblasts contain approximately micromolar concentrations of ABA (34), so it is currently unclear whether this substance is to be regarded as a potential parasite- or host-derived signal. In either case, our data suggest that the role of ABA is specific to the parasite-triggered autophagic mechanism, because fluridone had no effect on LC3 processing in uninfected cells.
Parasite-triggered, ABA-dependent autophagy might be mediated by calcium signaling, because ABA generates elevated cytosolic calcium in granulocytes (32) and stimulates calcium-dependent protein secretion in both T. gondii (31) and mammalian cells (32). Our data imply that T. gondii-induced autophagy most likely involves at least two steps that are dependent on intracellular calcium. One of these steps, indicated by the BAPTA-AM effect on LC3-II levels in both uninfected and infected cells, is probably antecedent or parallel to the parasite-initiated mechanism. In contrast, the other calcium-dependent event, the maintenance of high levels of PI3P, is entirely specific to infected cells and therefore suggests a calcium-dependent step that is regulated by T. gondii. This calcium-dependent step is apparently not essential for the restriction of PI3P-containing vesicles to the vicinity of the parasitophorous vacuole, because the residual YFP-2×FYVE signal in BAPTA-treated infected cells remains concentrated near the vacuole. Nevertheless, the absolute amount of PI3P localized to the vacuole is greatly reduced by BAPTA treatment, consistent with a role for PI3P relocalization in T. gondii-induced autophagy.
The finding that calcium dependence of PI3P is observed only in infected cells, whereas relocalization of PI3P is independent of calcium, suggests two potential explanations. One is that infection results in modulation of the signals governing Vps34 activity, rendering this activity calcium-dependent. The nature of this hypothesized parasite effect is unclear. It does not involve alteration of beclin-Vps34 association, which was not affected by the parasite. The second possibility is that relocalization of vesicles to the vacuole is in fact calcium-dependent and that these vesicles only acquire PI3P after relocalization has been completed. This notion is consistent with our observation of beclin 1 localization to the vacuole, suggesting that Vps34 may be similarly localized. Under this second hypothesis, the reduction of YFP-2x-FYVE intensity in BAPTA-treated infected cells is explained by the highly dynamic nature of the PI3P-containing vesicular compartment.
The relocalization of PI3P and beclin 1 correlated with the appearance of LC3 puncta in the vicinity of the vacuole. These findings suggest that parasite control of the localization of the host cell autophagic apparatus, rather than global up-regulation of host cell autophagy, might be the primary means by which T. gondii enhances its capture of host nutrients. Conceivably, relocalization facilitates the up-regulation by increasing the local concentration of autophagic components. However, a potentially more important consequence of this localization is to enhance the flow of autophagic products to the vacuole and thence to the parasite. Coppens et al. (12) have observed that host cell lysosomes cluster around the parasitophorous vacuole and also enter the vacuole by way of host microtubule-mediated tubular invaginations of the vacuolar membrane. The study also provided evidence that these invaginations can be pinched off to generate vesicles laden with host-derived material for potential delivery to the parasite. We propose that this host-derived material may include either autophagosomes or their derivatives after lysosome fusion. Under this mechanism, parasite capture of autophagy products, rather than reflecting nutrient release from lysosomes in host cytosol (followed by diffusion to the vacuole, which is permeant to nutrients (49)), would instead result from a more efficient process of directed vesicular import to the parasite.
Our study does not address the mechanism of relocalization, although it appears to be at least partly calcium-independent. The study of Coppens et al. (12) demonstrated a reorganization of the host cell microtubule network around the parasitophorous vacuole, and it is likely that microtubule-vesicle interaction is an important component of relocalization. The nature of the signals that influence this interaction in connection with the autophagic pathway are the subject of ongoing investigation in our laboratory.
The findings in this study represent an initial step in the elucidation of a link between the nutritive, recycling function of autophagy and the anabolic competition between parasites and their host cells. The data indicate that the mechanistic basis of this link is likely to have multiple aspects, including the parasite-triggered up-regulation of autophagy (via a mediator such as abscisic acid), the spatial reorganization of the subcellular components of autophagy, and the mechanism of nutrient capture by the parasite. The host-parasite system therefore presents a novel opportunity for the coordinated investigation of protein metabolism, autophagic signals, cytoskeletal function, and Toxoplasma pathogenesis. T. gondii is highly amenable to genetic manipulation and screening, and future experiments will seek to exploit this capability to obtain mechanistic insight into the ability of the parasite to manipulate the autophagic machinery of the host cell.
Acknowledgments
We acknowledge Drs. Jonathan Backer, Ana Maria Cuervo, Marc Fivaz, Jae Jung, Karla Kierkegaard, Ron Kopito, David Kwiatkowski, Beth Levine, Michael Lenardo, Noboru Mizushima, Harald Stenmark, Boris Striepen, and Junying Yuan for valuable reagents and suggestions. We also thank Yanfen Ma for assistance with parasite preparation.
This work was supported, in whole or in part, by National Institutes of Health Grants AI-55358 (to A. O.) and AI-39454 (to L. M. W.). This work was also supported by the Flow Cytometry Core of the Center for AIDS Research Grant AI-51519. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HFF, human foreskin fibroblast; MEF, mouse embryonic fibroblast; DMEM, Dulbecco's minimal essential medium; YFP, yellow fluorescent protein; PI3P, phosphatidylinositol 3-phosphate; S6K1, S6-kinase 1; ABA, abscisic acid; AA, amino acid; PBS, phosphate-buffered saline; GFP, green fluorescent protein; siRNA, small interfering RNA; DAPI, 4′,6′-diamino-2-phenylindole; mTOR, mammalian target of rapamycin; BAPTA-AM, 1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester.
Y. Wang, L. M. Weiss, and A. Orlofsky, manuscript in preparation.
References
- 1.Mizushima, N., and Klionsky, D. J. (2007) Annu. Rev. Nutr. 27 19-40 [DOI] [PubMed] [Google Scholar]
- 2.Wullschleger, S., Loewith, R., and Hall, M. N. (2006) Cell 124 471-484 [DOI] [PubMed] [Google Scholar]
- 3.Levine, B., and Kroemer, G. (2008) Cell 132 27-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmid, D., and Munz, C. (2007) Immunity 27 11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Py, B. F., Lipinski, M. M., and Yuan, J. Y. (2007) Autophagy 3 117-125 [DOI] [PubMed] [Google Scholar]
- 6.Andrade, R. M., Wessendarp, M., Gubbels, M. J., Striepen, B., and Subauste, C. S. (2006) J. Clin. Investig. 116 2366-2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu, Y., Jagannath, C., Liu, X. D., Sharafkhaneh, A., Kolodziejska, K. E., and Eissa, N. T. (2007) Immunity 27 135-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez, M. G., Master, S. S., Singh, S. B., Taylor, G. A., Colombo, M. I., and Deretic, V. (2004) Cell 119 753-766 [DOI] [PubMed] [Google Scholar]
- 9.Fox, B. A., Gigley, J. P., and Bzik, D. J. (2004) Int. J. Parasitol. 34 323-331 [DOI] [PubMed] [Google Scholar]
- 10.Mazumdar, J., and Striepen, B. (2007) Eukaryot. Cell. 6 1727-1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gherardi, A., and Sarciron, M. E. (2007) Trends Parasitol. 23 384-389 [DOI] [PubMed] [Google Scholar]
- 12.Coppens, I., Dunn, J. D., Romano, J. D., Pypaert, M., Zhang, H., Boothroyd, J. C., and Joiner, K. A. (2006) Cell 125 261-274 [DOI] [PubMed] [Google Scholar]
- 13.Sakaki, K., Wu, J., and Kaufman, R. J. (2008) J. Biol. Chem. 283 15370-15380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrestha, S. P., Tomita, T., Weiss, L. M., and Orlofsky, A. (2006) Int. J. Parasitol. 36 433-441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabeya, Y., Mizushima, N., Uero, T., Yamamoto, A., Kirisako, T., Noda, T., Kominami, E., Ohsumi, Y., and Yoshimori, T. (2000) EMBO J. 19 5720-5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichimura, Y., Kirisako, T., Takao, T., Satomi, Y., Shimonishi, Y., Ishihara, N., Mizushima, N., Tanida, I., Kominami, E., Ohsumi, M., Noda, T., and Ohsumi, Y. (2000) Nature 408 488-492 [DOI] [PubMed] [Google Scholar]
- 17.Tanida, I., Minematsu-Ikeguchi, N., Ueno, T., and Kominami, E. (2005) Autophagy 1 84-91 [DOI] [PubMed] [Google Scholar]
- 18.Hanada, T., Noda, N. N., Satomi, Y., Ichimura, Y., Fujioka, Y., Takao, T., Inagaki, F., and Ohsumi, Y. (2007) J. Biol. Chem. 282 37298-37302 [DOI] [PubMed] [Google Scholar]
- 19.Mizushima, N., Yamamoto, A., Hatano, M., Kobayashi, Y., Kabeya, Y., Suzuki, K., Tokuhisa, T., Ohsumi, Y., and Yoshimori, T. (2001) J. Cell Biol. 152 657-667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jounai, N., Takeshita, F., Kobiyama, K., Sawano, A., Miyawaki, A., Xin, K. Q., Ishii, K. J., Kawaii, T., Akira, S., Suzuki, K., and Okuda, K. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 14050-14055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogawa, M., Yoshimori, T., Suzuki, T., Sagara, H., Mizushima, N., and Sasakawa, C. (2005) Science 307 727-731 [DOI] [PubMed] [Google Scholar]
- 22.Zeng, X. H., Overmeyer, J. H., and Maltese, W. A. (2006) J. Cell Sci. 119 259-270 [DOI] [PubMed] [Google Scholar]
- 23.Cao, Y., and Klionsky, D. J. (2007) Cell Res. 17 839-849 [DOI] [PubMed] [Google Scholar]
- 24.Gillooly, D. J., Morrow, I. C., Lindsay, M., Gould, R., Bryant, N. J., Gaullier, J. M., Parton, R. G., and Stenmark, H. (2000) EMBO J. 19 4577-4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyer-Hansen, M., Bastholm, L., Szyniarowski, P., Campanella, M., Szabadkai, G., Farkas, T., Bianchi, K., Fehrenbacher, N., Elling, F., Rizzuto, R., Mathiasen, I. S., and Jaattela, M. (2007) Mol. Cell 25 193-205 [DOI] [PubMed] [Google Scholar]
- 26.Huang, J., and Manning, B. D. (2008) Biochem. J. 412 179-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao, W. T., Ding, W. X., Stolz, D. B., and Yin, X. M. (2008) Autophagy 4 754-761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon, P. B., Holen, I., Fosse, M., Rotnes, J. S., and Seglen, P. O. (1993) J. Biol. Chem. 268 26107-26112 [PubMed] [Google Scholar]
- 29.Gulati, P., Gaspers, L. D., Dann, S. G., Joaquin, M., Nobukuni, T., Natt, F., Kozma, S. C., Thomas, A. P., and Thomas, G. (2008) Cell Metabol. 7 456-465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz, A., Wu, W. H., Tucker, E. B., and Assmann, S. M. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 4019-4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagamune, K., Hicks, L. M., Fux, B., Brossier, F., Chini, E. N., and Sibley, L. D. (2008) Nature 451 207-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruzzone, S., Moreschi, I., Usai, C., Guida, L., Damonte, G., Salis, A., Scarfi, S., Millo, E., De Flora, A., and Zocchi, E. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 5759-5764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruzzone, S., Bodrato, N., Usai, C., Guida, L., Moreschi, I., Nano, R., Antonioli, B., Fruscione, F., Magnone, M., Scarfi, S., De Flora, A., and Zocchi, E. (2008) J. Biol. Chem. [DOI] [PubMed]
- 34.Zocchi, E., Guida, L., Buzzone, S., Scarfi, S., Magnone, M., Basile, G., Benatti, U., DeFlora, A., Moreschi, I., Franco, L., and Salis, A. PCT/IB2006/053669 (06/10/2006) Fluridone as an Anti-inflammatory Agent (October 6, 2006)
- 35.Wasilewska, A., Vlad, F., Sirichandra, C., Redko, Y., Jammes, F., Valon, C., Frey, N. F. D., and Leung, J. (2008) Mol. Plant 1 198-217 [DOI] [PubMed] [Google Scholar]
- 36.Palou, A., Remesar, X., Arola, L., Herrera, E., and Alemany, M. (1981) Horm. Metab. Res. 13 326-330 [DOI] [PubMed] [Google Scholar]
- 37.Aoki, T. T., Muller, W. A., Brennan, M. F., and Cahill, G. F., Jr. (1975) Am. J. Clin. Nutr. 28 507-511 [DOI] [PubMed] [Google Scholar]
- 38.Yokogoshi, H., and Wurtman, R. J. (1986) Metabolism 35 837-842 [DOI] [PubMed] [Google Scholar]
- 39.Calbet, J. A. L., and MacLean, D. A. (2002) J. Nutr. 132 2174-2182 [DOI] [PubMed] [Google Scholar]
- 40.Brass, A. L., Dykxhoorn, D. M., Benita, Y., Yan, N., Engelman, A., Xavier, R. J., Lieberman, J., and Elledge, S. J. (2008) Science 319 921-926 [DOI] [PubMed] [Google Scholar]
- 41.Jackson, W. T., Giddings, T. H., Taylor, M. P., Mulinyawe, S., Rabinovitch, M., Kopito, R. R., and Kirkegaard, K. (2005) PLoS Biol. 3 861-871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prentice, E., Jerome, W. G., Yoshimori, T., Mizushima, N., and Denison, M. R. (2004) J. Biol. Chem. 279 10136-10141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong, J., Zhang, J. C., Si, X. N., Gao, G., Mao, I., McManus, B. M., and Luo, H. L. (2008) J. Virol. 82 9143-9153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakai, A., Yamaguchi, O., Takeda, T., Higuchi, Y., Hikoso, S., Taniike, M., Omiya, S., Mizote, I., Matsumura, Y., Asahi, M., Nishida, K., Hori, M., Mizushima, N., and Otsu, K. (2007) Nat. Med. 13 619-624 [DOI] [PubMed] [Google Scholar]
- 45.Fox, B. A., and Bzik, D. J. (2002) Nature 415 926-929 [DOI] [PubMed] [Google Scholar]
- 46.Ravindran, S., and Boothroyd, J. C. (2008) Traffic 9 647-656 [DOI] [PubMed] [Google Scholar]
- 47.Boothroyd, J. C., and Dubremetz, J. F. (2008) Nat. Rev. Microbiol. 6 79-88 [DOI] [PubMed] [Google Scholar]
- 48.Saeij, J. P. J., Coller, S., Boyle, J. P., Jerome, M. E., White, M. W., and Boothroyd, J. C. (2007) Nature 445 324-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwab, J. C., Beckers, C. J. M., and Joiner, K. A. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 509-513 [DOI] [PMC free article] [PubMed] [Google Scholar]