Abstract
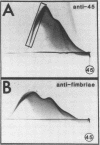
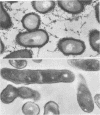
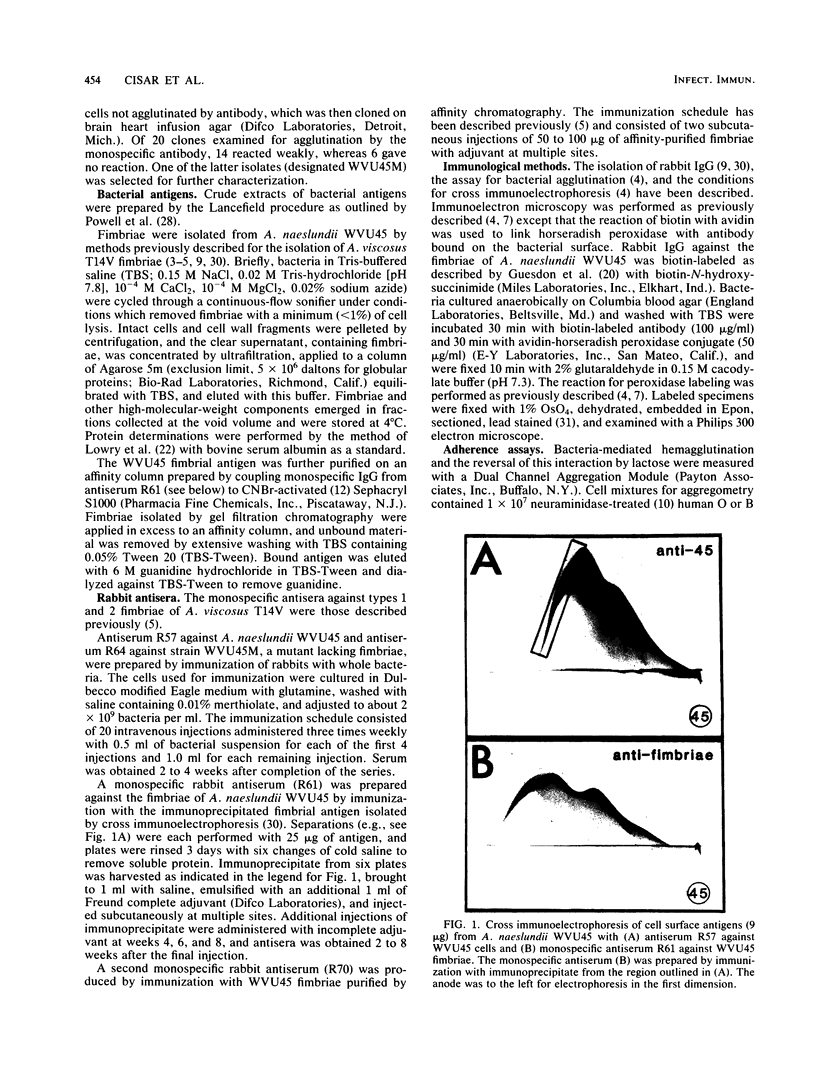
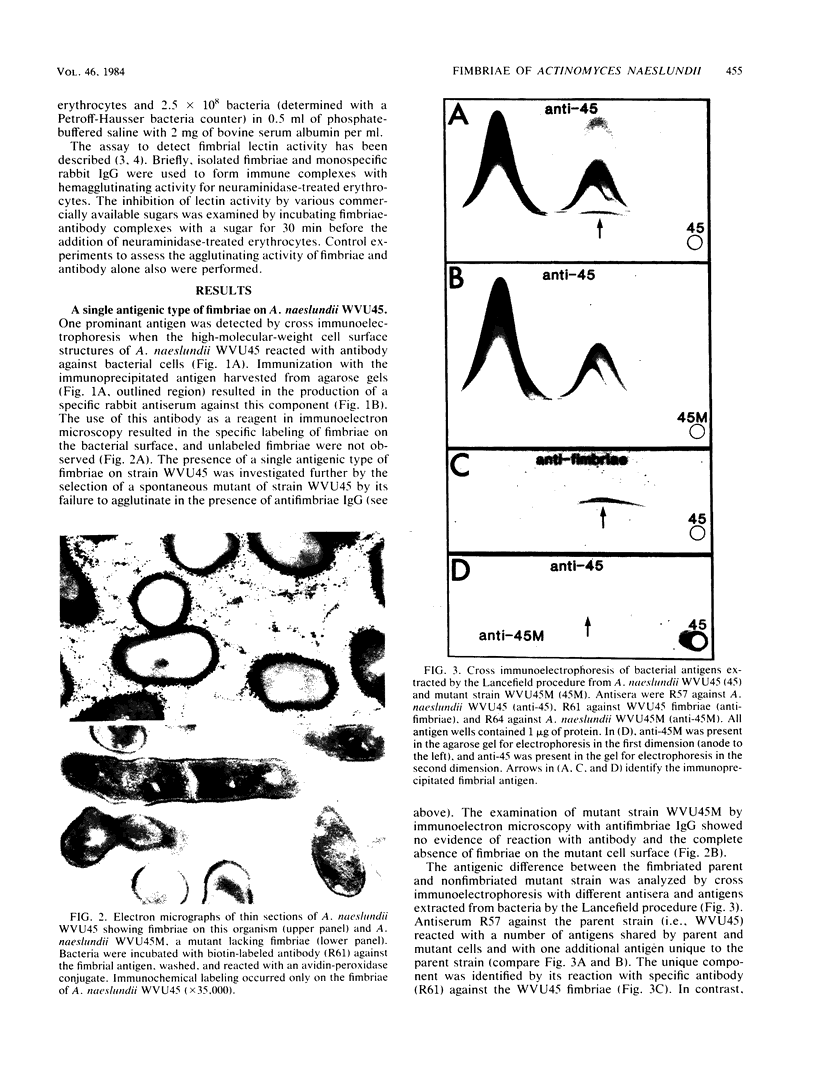
Lactose-sensitive fimbriae were identified as the only fimbriae present on Actinomyces naeslundii WVU45 (ATCC 12104). A single antigen reactive with antiserum against WVU45 cells was detected by cross immunoelectrophoresis of isolated fimbriae, and a monospecific antiserum against this antigen reacted with all fimbriae observed on the bacterial surface by immunoelectron microscopy. Moreover, the loss of one cell surface antigen by a spontaneous mutant of A. naeslundii WVU45 (WVU45M), isolated by its failure to react with a monospecific antibody against the fimbriae, was associated with the loss of all fimbriae. The functional involvement of the fimbriae in lactose-sensitive bacterial adherence was demonstrated by the ability of WVU45, but not WVU45M, cells to agglutinate neuraminidase-treated erythrocytes and by the lactose-sensitive hemagglutinating activity of immune complexes formed with isolated fimbriae and monospecific antibody. Bacterial agglutination assays with different monospecific antibodies revealed an antigenic similarity between the fimbriae of A. naeslundii WVU45 and the lactose-sensitive fimbriae (type 2) of Actinomyces viscosus T14V. In contrast, cross-reactivity was not observed between the WVU45 fimbriae and type 1 fimbriae, the structures involved in lactose-resistant adherence of strain T14V to saliva-treated hydroxyapatite. Functional differences between the fimbriae of A. naeslundii and A. viscosus strains may be correlated with well-established differences in the in vivo distribution of these organisms: namely, the preference of typical A. naeslundii for epithelial surfaces and of A. viscosus for tooth surfaces.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan M. J., Cisar J. O., Vatter A. E., Sandberg A. L. Lectin-dependent attachment of Actinomyces naeslundii to receptors on epithelial cells. Infect Immun. 1984 Nov;46(2):459–464. doi: 10.1128/iai.46.2.459-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Barsumian E. L., Curl S. H., Vatter A. E., Sandberg A. L., Siraganian R. P. Detection and localization of a lectin on Actinomyces viscosus T14V by monoclonal antibodies. J Immunol. 1981 Oct;127(4):1318–1322. [PubMed] [Google Scholar]
- Cisar J. O., Curl S. H., Kolenbrander P. E., Vatter A. E. Specific absence of type 2 fimbriae on a coaggregation-defective mutant of Actinomyces viscosus T14V. Infect Immun. 1983 May;40(2):759–765. doi: 10.1128/iai.40.2.759-765.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E., McIntire F. C. Identification of the virulence-associated antigen on the surface fibrils of Actinomyces viscosus T14. Infect Immun. 1978 Jan;19(1):312–319. doi: 10.1128/iai.19.1.312-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Webb E. L., Wheeler T. T., Fischlschweiger W., Birdsell D. C., Mansheim B. J. Role of surface fimbriae (fibrils) in the adsorption of Actinomyces species to saliva-treated hydroxyapatite surfaces. Infect Immun. 1981 Sep;33(3):908–917. doi: 10.1128/iai.33.3.908-917.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Wheeler T. T., Cisar J. O. Specific inhibition of adsorption of Actinomyces viscosus T14V to saliva-treated hydroxyapatite by antibody against type 1 fimbriae. Infect Immun. 1984 Feb;43(2):497–501. doi: 10.1128/iai.43.2.497-501.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Interbacterial aggregation of Actinomyces naeslundii and dental plaque streptococci. J Periodontal Res. 1977 Jan;12(1):11–20. doi: 10.1111/j.1600-0765.1977.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Ellen R. P. Establishment and distribution of Actinomyces viscosus and Actinomyces naeslundii in the human oral cavity. Infect Immun. 1976 Nov;14(5):1119–1124. doi: 10.1128/iai.14.5.1119-1124.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Fillery E. D., Chan K. H., Grove D. A. Sialidase-enhanced lectin-like mechanism for Actinomyces viscosus and Actinomyces naeslundii hemagglutination. Infect Immun. 1980 Feb;27(2):335–343. doi: 10.1128/iai.27.2.335-343.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Walker D. L., Chan K. H. Association of long surface appendages with adherence-related functions of the gram-positive species Actinomyces naeslundii. J Bacteriol. 1978 Jun;134(3):1171–1175. doi: 10.1128/jb.134.3.1171-1175.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillery E. D., Bowden G. H., Hardie J. M. A comparison of strains of bacteria designated Actinomyces viscosus and Actinomyces naeslundii. Caries Res. 1978;12(6):299–312. doi: 10.1159/000260349. [DOI] [PubMed] [Google Scholar]
- Girard A. E., Jacius B. H. Ultrastructure of Actinomyces viscosus and Actinomyces naeslundii. Arch Oral Biol. 1974 Jan;19(1):71–79. doi: 10.1016/0003-9969(74)90228-3. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Heeb M. J., Costello A. H., Gabriel O. Characterization of a galactose-specific lectin from Actinomyces viscosus by a model aggregation system. Infect Immun. 1982 Dec;38(3):993–1002. doi: 10.1128/iai.38.3.993-1002.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Masuda N., Ellen R. P., Fillery E. D., Grove D. A. Chemical and immunological comparison of surface fibrils of strains representing six taxonomic groups of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1983 Mar;39(3):1325–1333. doi: 10.1128/iai.39.3.1325-1333.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda N., Ellen R. P., Grove D. A. Purification and characterization of surface fibrils from taxonomically typical Actinomyces viscosus WVU627. J Bacteriol. 1981 Sep;147(3):1095–1104. doi: 10.1128/jb.147.3.1095-1104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Barlow J. J., Matta K. L. Structural preferences of beta-galactoside-reactive lectins on Actinomyces viscosus T14V and Actinomyces naeslundii WVU45. Infect Immun. 1983 Aug;41(2):848–850. doi: 10.1128/iai.41.2.848-850.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Vatter A. E. Inhibitors of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34: beta-galactosides, related sugars, and anionic amphipathic compounds. Infect Immun. 1982 Apr;36(1):371–378. doi: 10.1128/iai.36.1.371-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. T., Fischlschweiger W., Birdsell D. C. Modification of surface composition of Actinomyces viscosus T14V and T14AV. Infect Immun. 1978 Dec;22(3):934–944. doi: 10.1128/iai.22.3.934-944.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi J. V., Gibbons R. J. Differences in the adsorptive behavior of human strains of Actinomyces viscosus and Actinomyces naeslundii to saliva-treated hydroxyapatite surfaces. Infect Immun. 1981 Jan;31(1):261–266. doi: 10.1128/iai.31.1.261-266.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revis G. J., Vatter A. E., Crowle A. J., Cisar J. O. Antibodies against the Ag2 fimbriae of Actinomyces viscosus T14V inhibit lactose-sensitive bacterial adherence. Infect Immun. 1982 Jun;36(3):1217–1222. doi: 10.1128/iai.36.3.1217-1222.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. M., Miller C. H. Attachment of Actinomyces naeslundii to human buccal epithelial cells. Infect Immun. 1980 Sep;29(3):981–989. doi: 10.1128/iai.29.3.981-989.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. M., Miller C. H. Neuraminidase-activated attachment of Actinomyces naeslundii ATCC 12104 to human buccal epithelial cells. J Dent Res. 1983 Oct;62(10):1038–1040. doi: 10.1177/00220345830620100501. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Manganiello A. D., Propas D., Oram V., van Houte J. Bacteriological studies of developing supragingival dental plaque. J Periodontal Res. 1977 Mar;12(2):90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Wheeler T. T., Clark W. B. Fibril-mediated adherence of Actinomyces viscosus to saliva-treated hydroxyapatite. Infect Immun. 1980 May;28(2):577–584. doi: 10.1128/iai.28.2.577-584.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]