Abstract
Signal transduction in outer segments of vertebrate photoreceptors is mediated by a series of reactions among multiple polypeptides that form protein-protein complexes within or on the surface of the disk and plasma membranes. The individual components in the activation reactions include the photon receptor rhodopsin and the products of its absorption of light, the three subunits of the G protein, transducin, the four subunits of the cGMP phosphodiesterase, PDE6 and the four subunits of the cGMP-gated cation channel. Recovery involves membrane complexes with additional polypeptides including the Na+/Ca2+, K+ exchanger, NCKX2, rhodopsin kinases RK1 and RK7, arrestin, guanylate cyclases, guanylate cyclase activating proteins GCAP1 and GCAP2, and the GTPase accelerating complex of RGS9-1, Gβ5L, and membrane anchor R9AP. Modes of membrane binding by these polypeptides include transmembrane helices, fatty acyl or isoprenyl modifications, polar interactions with lipid head groups, non-polar interactions of hydrophobic sides chains with lipid hydrocarbon phase, and both polar and non-polar protein-protein interactions. In the course of signal transduction, complexes among these polypeptides form and dissociate, and undergo structural rearrangements that are coupled to their interactions with and catalysis of reactions by small molecules and ions, including guanine nucleotides, ATP, Ca2+, Mg2+, and lipids. The substantial progress that has been made in understanding the composition and function of these complexes is reviewed, along with the more preliminary state of our understanding of the structures of these complexes and the challenges and opportunities that present themselves for deepening our understanding of these complexes, and how they work together to convert a light signal into an electrical signal.
Introduction
The physiological function of rod and cone outer segments is the conversion of a light signal into an electrical signal. The biochemical cascade responsible for this conversion is known as the phototransduction cascade, and the major events in it are carried out by complexes of multiple polypeptides embedded in or attached to the surface of disk membranes and plasma membranes of the outer segments. Additional membrane complexes maintain the structures of these highly specialized membranes, and establish their proper spatial relationships.
Over the past two decades considerable progress has been made in identifying the major membrane proteins that make up these complexes and carry out their functions. Many of them have been purified from the retina, and all of their gene sequences are now known, allowing them to be studied in heterologous expression systems and in genetically engineered animals. Thus, despite the formidable challenges that continue to be faced in the biochemical characterization and structure determination of membrane proteins, much headway has been made in understanding the structure and function of membrane complexes important for phototransduction and outer segment structure. Understanding their structure-function relationships is important both for deepening our appreciation of the molecular mechanisms of vision, and for understanding the diseases that develop as consequences of disruption of the structures and functions of these complexes. The impact of progress in studying membrane complexes of the photoreceptors is felt well beyond the field of vision research, as these complexes have served as powerful models for understanding membrane complexes that mediate signaling pathways and membrane structures throughout the central nervous system and the rest of the body. One of the most striking examples is the enormous impact of the crystal structure of rhodopsin (Palczewski, et al., 2000) on the fields of G protein coupled receptors and membrane proteins.
An ongoing challenge and source of fascination is the role of the lipid milieu in which these complexes function. They have evolved to work optimally in an environment formed by a membrane bilayer with a highly specialized lipid composition. Most structural approaches and many biochemical studies begin by removing the complexes from the lipid bilayer, and one of the areas of research focus in the immediate future will be finding and exploiting creative approaches for determining structure and function in the membrane environment, and understanding the influence of the lipids on the behavior of the protein complexes.
The G protein, transducin, and its multiple membrane complexes
The photon receptor protein, rhodopsin, is a G protein-coupled receptor, and phototransduction is a prototypical G protein-mediated signaling cascade. At the center of this cascade lies the heterotrimeric G protein, transducin, Gαtβ1γ1, (rods) a peripheral membrane protein (the similar but distinct subunits of the rod and cone subunits will be generically referred to here as Gαβγ, with distinctions between rods and cones noted as needed). As the first G protein and the first component of the phototransduction cascade to have its structure determined, its structure and function have been extensively reviewed (Arshavsky, et al., 2002, Birnbaumer, 2007, Bohm, et al., 1997, Chen, 2005, Coleman & Sprang, 1996, Downs, et al., 2006, Hargrave, et al., 1993, Hofmann, 1999, Shichida & Morizumi, 2007, Sprang, 1997a, Sprang, 1997b, Sprang, 2000, Sprang, et al., 2007). Much of this work has focused on soluble forms of transducin and its component subunits, whereas the focus here is on its membrane dependent complexes.
Lipid modifications & structure of membrane-bound heterotrimer in GDP state
The α subunit of transducin, Gαt, is the more dynamic half of the heterotrimer, moving rapidly among at least three conformational states, and shuttling back and forth between binding partners on the membrane surface. It has one of four different 12- or 14-carbon fatty acids (DeMar, et al., 1999, DeMar, et al., 1996, Kokame, et al., 1992, Neubert, et al., 1992, Yang & Wensel, 1992) attached in an amide linkage to its N-terminal glycine residue, and these provide for modest membrane binding affinity, which varies somewhat depending on the hydrophobicity of the fatty acid (Johnson, et al., 1994, Lobanova, et al., 2007, Neubert & Hurley, 1998, Neubert et al., 1992). However, it is its interactions with other membrane proteins that keep it tethered to the disk membrane in rods under dim light conditions (where it functions in signaling) and likely membrane-bound in cones over most illumination conditions (Coleman & Semple-Rowland, 2005, Kennedy, et al., 2004); however, see (Chen, et al., 2007). In its inactive GDP-bound form, which predominates in the dark, the arrangement of its “switch” domains favors binding to its partner subunits, Gβγ. Gβ and Gγ bind to one another very tightly and have a mutual dependence for proper folding and stability. The intrinsic affinity of Gβγ for the disk membrane is higher than that of Gαt and is partly mediated by the presence of two hydrophobic modifications on Gγ: the cysteine residue which is the fourth residue from the carboxyl terminus in the initial translation product is methyl esterified after the last three residues are proteolytically cleaved, and a farnesyl group is attached in a thioether linkage to this same residue (Bigay, et al., 1994, Fukada, 1995, Fukada, et al., 1990, Lai, et al., 1990, Ohguro, et al., 1991). Both Gα and Gβγ bind membranes, with a higher affinity displayed by Gβγ than Gα-GDP (Bigay et al., 1994), which binds more tightly than Gα-GTP. However, for the heterotrimer, it is Gα-GDP that provides most of the membrane binding interactions (Seitz, et al., 1999, Zhang, et al., 2004b).
Studies with reconstituted vesicles or with spin-labeled lipids in disk membranes have revealed specificity in the interaction of transducin complexes with phospholipids (He, et al., 2004, Hessel, et al., 2003, Malinski & Wensel, 1992, Melia, et al., 2000, Melia, et al., 1999, Murray, et al., 2001).
A structure of the Gαβγ complex bound to GDP and a membrane bilayer was determined by cryo-electron microscopy of two-dimensional (helical) crystals of the complex bound to tubules of lipid bilayers (Melia et al., 1999, Zhang et al., 2004b). The structure reveals lipid interactions of both the amino-terminal and carboxyl terminal regions of Gα, and of the carboxyl terminus of Gγ , with no apparent lipid contact with Gβ. Two caveats of this structure are that it reveals a static picture, whereas in reality, in the absence of crystal contacts there is likely considerable dynamic motion of the hydrophilic surface of the heterotrimer with respect to the membrane surface, and that the contacts shown may be biased in favor of those favored by electrostatic attraction to positively charged lipids used for crystallization. Studies of transducin complexes in micelles and vesicles suggest that a lipid-like milieu enhances the effective affinity of Gβγ and Gα for one another, likely as a result of their both having lipid moieties. The combination of interactions of the two lipid tails with the membranes and of the Gα and Gβγ polypeptides with one another, produces a cooperativity of membrane binding of the two subunits (Bigay et al., 1994).
Complex with rhodopsin and photoexcited rhodopsin (R*)- progress and challenges
The affinity of transducin for disk membranes is not likely to be due entirely to its interactions with lipids. Although it can diffuse freely between photoreceptor outer and inner segments on a timescale of minutes, in the dark all three subunits of rod transducin are found almost exclusively in the outer segment. When a substantial portion of rhodopsin is bleached (i.e., to a level well beyond the point of saturation for rod vision), all three subunits translocate passively to the inner segment, most likely as separate Gα-GTP and Gβγ units(Lobanova et al., 2007, Rosenzweig, et al., 2007). One possible explanation for these results is a higher affinity of Gα for dark disk membranes than for partially bleached membranes, which would suggest that the relatively low affinity of the heterotrimeric form of transducin for the dark state of rhodopsin (Alves, et al., 2005) is sufficient to allow sequestration of the G protein to the outer segments in the dark. This rhodopsin-transducin-GDP complex has received relatively little attention, and is deserving of more thorough characterization. Alternatively, the lower membrane affinities of Gα and Gβγ separately for membranes, as compared to the higher membrane affinity of the heterotrimer, may be important in the net translocation.
The most attention has been focused on the complex between photoexcited rhodopsin, metarhodopsin II or R*, and the transducin heterotrimer (Fig. 1). Likely there are multiple states of this complex, and currently there are high resolution structures for none of them. The highest affinity form seems to involve the nucleotide-free form of Gαβγ , which is a key intermediate in the nucleotide exchange reaction (release of GDP and binding of GTP) catalyzed by R* as its central role in phototransduction. However, even this complex may exist in multiple conformations, and the complexes involving bound GDP or GTP must be considered as well. The structural and kinetic characterization of all of these complexes will be an important area of research in the coming years. Significant insights into their properties have been obtained by electron paramagnetic resonance (EPR) studies of the related protein Gi bound to rhodopsin in detergent, and by NMR and fluorescence studies of complex formation(Abdulaev, et al., 2006, Abdulaev, et al., 2005, Brabazon, et al., 2003, Knierim, et al., 2007, Medkova, et al., 2002, Oldham, et al., 2006, Ridge, et al., 2006). Kinetic studies using light-scattering and other methods (Bayburt, et al., 2007, Ernst, et al., 2007, Heck & Hofmann, 2001, Herrmann, et al., 2004, Herrmann, et al., 2006a, Herrmann, et al., 2006b, Oprian, 1992) have also shed light on the mechanisms of transducin activation by R*, but much remains to be done. There is reason for optimism that structures derived from three-dimensional or two-dimensional crystals of R*-transducin complexes will be forthcoming in the near future.
Figure 1.
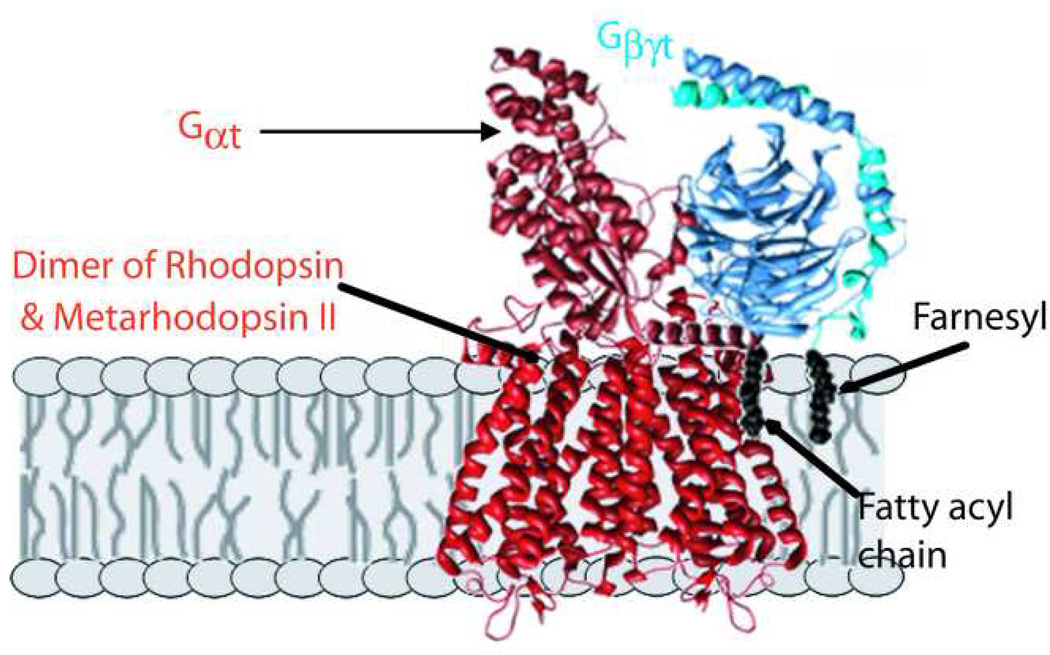
Proposed model for first complex formed in photoactivation. Following absorption of light and photoisomerization by one subunit of the rhodopsin dimer, a heterodimer of rhodopsin and Metarhodopsin II (R*) is formed, which quickly complexes with the G protein, transducin, in its heterotrimeric form. A conformational change within Gαt allows release of bound GDP, which then allows binding of GTP and dissociation of activated Gαt–GTP. The representation of the complex is purely schematic, as only indirect low resolution information is available on the structures of the actual complexes. The major contacts with R* are provided by Gαt, with both its carboxyl and fatty acylated amino termini known to be involved. Gβγ are also important for R* binding, with potential interactions with the membrane hydrocarbon phase provided by the farnesyl group on Gγ. For making the structure images shown, PDB files 1U19.pdb(Okada, et al., 2004) and 1GOT.pdb(Lambright, et al., 1996) were used with UCSF Chimera (Meng, et al., 2006, Pettersen, et al., 2004).
PDE6, and complexes with Gαt-GTP
The only known physiological function of activated GTP-bound Gα, is activation of its downstream effector enzyme, the cGMP-specific phosphodiesterase, PDE6. PDE6 is yet another lipidated peripheral membrane protein of the phototransduction cascade. It consists of four subunits of three kinds with a stoichiometry of PDE6αβγγ. PDE6α and PDE6β are catalytic subunits with similar structures; in cones there are two identical copies of a single type of catalytic subunit, PDE6α’, most closely related to PDE6β. These catalytic subunits have modifications identical to those described above for Gγt, except that PDE6α is farnesylated, whereas PDE6β is geranylgeranylated in mammals (Anant, et al., 1992, Catty & Deterre, 1991,Ong, et al., 1989, Qin, et al., 1992, Qin N, 1992), while the situation is reversed in frogs (Yamazaki, et al., 2002). These modifications at the carboxyl termini are critical for positioning PDE6 on the membrane surface where it interacts with activated GTP-for Gαt, and proteolytic removal of the carboxyl termini, or binding of a prenyl binding protein(Zhang, et al., 2004a), also known, somewhat misleadingly, as PDE6δ, releases PDE6 from disk membranes (Cook, etal., 2000, Cook, et al., 2001, Deterre, et al., 1988, Florio, et al., 1996, Li, et al., 1998, Norton, etal., 2005, Wensel & Stryer, 1986). The PDE6γ subunit is a 9.7 kDa inhibitory polypeptide that keeps PDE6 catalytic activity at a very low level in the dark. Much of the action of Gα–GTP on the activity of PDE6 is mediated through its interactions with the PDE6γ subunit, and complexes of PDE6γ with either GDP-form or GTPγS form PDE6γ have been studied in solution(Antonny, et al., 1993, Artemyev, et al., 1993, Skiba, et al., 1995, Slepak, et al., 1995). Indirect evidence suggests that two molecules of Gα–GTP bind to each PDE6 heterotetramer. In solution, the affinity of Gα–GTP for holo-PDE6 is relatively low, but phospholipid surfaces enhance their interactions dramatically, leading to nearly stoichiometric complex formation when both are membrane bound. This lipid-mediated interaction is enhanced by either positively charged lipids, which are not found in rods, or by phosphoinositides, especially phosphatidylinositol (4,5) bisphosphate (PIP2) which is present in disk membranes at low levels (He et al., 2004, Melia etal., 2000, Womack, et al., 2000). The physiological significance of the PIP2 interactions remains to be determined.
Low resolution structures of PDE6 have been determined by electron microscopy of the complex in heavy metal negative stain(Kajimura, et al., 2002, Kameni Tcheudji, et al., 2001). Electron microscope images of PDE6 in frozen solution or bound to vesicles have been recently obtained, as have images of quasi-crystalline arrays of PD6 bound to GTPγS-form Gαt. (Z. Zhang, F. He and T. Wensel, unpublished observations), so that low- to medium resolution structures of these membrane complexes should be forthcoming in the near future.
RGS9-1-G β5L-R9AP and its complex with G αt· –GTP
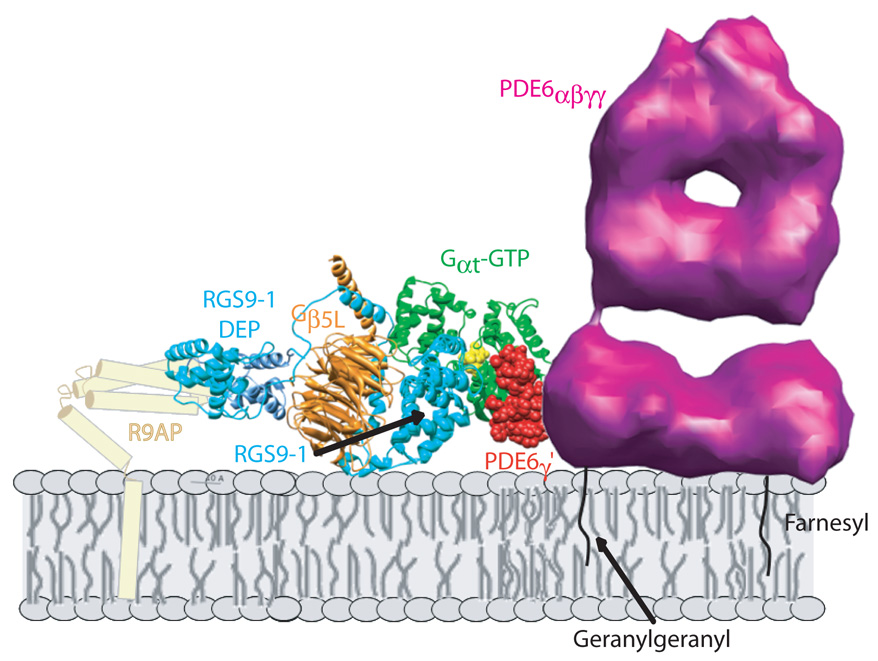
The other important membrane-associated complex formed by Gαt in the course of phototransduction is its complex with the GTPase accelerating complex consisting of the GTPase accelerating protein (GAP) RGS9-1, Gβ5L, and the membrane anchor, R9AP (Fig. 2). Indirect evidence suggests that under physiological conditions this complex may also be bound to PDE6, so that assuming a 2:1 stoichiometric ratio of Gα and its bound GAP complex to PDE6, one such complex could have a minimum of 12 membrane-associated polypeptides. Although high resolution structures have been determined by x-ray crystallography for a complex of Gα with a C-terminal fragment of PDE6γ and the catalytic core of RGS9-1 (Slep, et al., 2001, Sowa, et al., 2001), as well as for a nearly full-length complex of RGS9-1 with Gβ5L, (Cheever, et al., 2008), we are far from knowing how this large multi-subunit membrane complex is organized and how it is positioned with respect to the membrane surface. It is clear that association of RGS9-1 with its membrane anchor, R9AP, a member of the syntaxin super-family with a single transmembrane helix(Hu & Wensel, 2002, Hu & Wensel, 2004, Hu, et al., 2003), not only dramatically enhances its affinity for the membrane, but also is critical for its catalytic GAP activity and for its stability in rod cells(Baker, et al., 2006, Keresztes, et al., 2004, Keresztes, etal., 2003, Krispel, et al., 2006, Nishiguchi, et al., 2004).
Figure 2.
A multi-subunit complex essential for normal photoresponse recovery kinetics. Sub-second GTP hydrolysis by activated Gαt–GTP is catalyzed by the GTPase accelerating complex of RGS9-1, Gβ5L, and single-pass trans-membrane anchor protein, R9AP. Indirect evidence suggests that formation of this complex occurs while Gαt–GTP is bound to its effector PDE6, largely through interactions with PDE6γ, whose C-terminal fragment, PDE6γ’, (Slep et al., 2001) is shown in red space-filling representation. For making the structure images shown, the RGS domains from PDB files FQJ.pdb(Slep et al., 2001) and 2PBI(Cheever et al., 2008) were ligned in UCSF Chimera (Meng et al., 2006, Pettersen et al., 2004) and the resulting assembly of models positioned next to a model of holo PDE6, based on unpublished cryo-electron microscopy data kindly provided by Dr. Zhixian Zhang. The schematic representation of R9AP was loosely derived from a model presented previously(Cheever et al., 2008) The spatial relationships of PDE6γ to holo PDE6, and of all of the polypeptides with respect to the membrane are not intended to be accurate; however, the attachment of the complex to the membrane via insertion of the isoprenyl tails of PDE6 and of the transmembrane segment of R9AP into the membrane is based on substantial biochemical evidence.
Additional Complexes important for recovery
There are several membrane associated complexes that are critical for the recovery phase of phototransduction. As their structures remain to be determined, and they have been recently reviewed, they will only be discussed briefly here.
R*-Rhodopsin Kinase
An essential step for a return to the dark state of the phototransduction cascade is phosphorylation of R* by rhodopsin kinase, (GRK1; in some species a related kinase, GRK7 is found in cones), which belong to a family of serine/threonine kinases specific for activated states of G protein-coupled receptors (Arshavsky, 2002, Hurley, et al., 1998, Maeda, et al., 2003, Penn, et al., 2000, Pitcher, et al., 1998, Premont & Gainetdinov, 2007). The phosphorylation sites are found in the carboxyl-terminal tail of rhodopsin, which lies near the surface of the lipid membrane, and the transient complex between rhodopsin kinase and rhodopsin is a membrane-dependent kinase. Both GRK1 and GRK7 are isoprenylated, and also have the other covalent modifications at their C-termini described for Gγt above, with GRK1 being predominantly farnesylated (C15) and GRK7 having a C-terminal sequence directing geranylgeranylation (C20). An intriguing possibility is that the faster inactivation of cone pigments by phosphorylation as compared to rhodopsin (Tachibanaki, et al., 2005, Wada, et al., 2006) is related to differences in membrane binding between GRK1 and GRK7. An unresolved question in photoresponse termination is the role of the calcium binding protein recoverin, of the neuronal calcium binding protein family and calmodulin superfamily(Chen, 2002, Chen, et al., 1995,Kawamura & Tachibanaki, 2002, Klenchin & Bounds, 1995, Otto-Bruc, et al., 1998). It has been proposed that recoverin plays a key role in allowing lowered intracellular calcium concentration to serve as a feedback signal for activation of rhodopsin kinase in response to photoactivation. However, results of biochemical studies with permeabilized rods challenge this role(Otto-Bruc et al., 1998), and the effects of a knockout of the recoverin gene on photoresponse recovery kinetics, while in the right direction to support this hypothesis, are rather subtle(Makino, et al., 2004, Sampath, et al., 2005). One feature of recoverin’s interactions with Ca2+ is clear: binding of Ca2+ induces a conformational change termed a “myristoyl switch” which causes extrusion of an N-terminal fatty acyl group from a pocket within the protein structure to an exposed state which would be expected to maximize interactions with the hydrocarbon phase of the phospholipids membrane. (Ames, et al., 1996, Zozulya & Stryer, 1992).
The ability of rhodopsin kinase to effect R* inactivation depends to a large degree on the action of the capping protein, visual arrestin(Vishnivetskiy, et al., 2007). Although there has been relatively little attention paid to interactions of arrestin with membrane lipids, an intriguing observation is that incorporation of acidic phospholipids, which are relatively abundant in disk membranes, enhances the interactions between arrestin and phosphorylated R* in detergent micelles (Sommer, et al., 2006).
Guanylate cyclase-GCAPs
Another membrane complex between transmembrane and peripheral membrane proteins is the complex between the single pass transmembrane protein, photoreceptor guanylate cyclase (GC; GC1 and GC2), and its associated guanylate cyclase-activating proteins or GCAPs (Baehr, et al., 2007, Koch, 2002, Koch, et al., 2002, Palczewski, et al., 1994, Pugh, et al., 1997, Yu, etal., 1999). Unlike recoverin, deletion of the GCAP genes has a dramatic effect on photoresponse recovery kinetics, as well as on the peak amplitudes of dim flash photoresponses (Burns, et al.,2002, Howes, et al., 2002, Mendez, et al., 2001). GCAP complexes are necessarily formed at the membrane-cytoplasm interface with GC, which is an integral membrane protein. Like recoverin, they have an N-terminal sequence directing fatty acylation, but do not seem to undergo a “Ca2+-myristoyl switch” mechanism. Structural studies by x-ray crystallography and NMR have revealed that the myristoyl group is embedded in the protein structure of GCAP1, but that the fatty acyl group of GCAP2 inserts into the membrane bilayer.(Stephen, et al., 2007, Vogel, et al.,2007) Myristoylation influences the affinity of GCAP1 for GC and its Ca2+ sensitivity, but not that of GCAP2 (Hwang & Koch, 2002a, Hwang & Koch, 2002b, Olshevskaya, et al., 1997). In photoreceptor membranes, guanylate cyclase dimerizes with itself as well as binding GCAPs, and these interactions are critical to its activation when intracellular calcium concentrations fall in response to light (Hwang, et al., 2003, Olshevskaya, et al., 1999, Ramamurthy, et al., 2001, Tucker, et al., 1999, Yu et al., 1999).
Plasma membrane complexes
Multi-subunit protein complexes mediate the light regulated ionic fluxes through the plasma membrane of photoreceptor outer segments which are integral to light responses (Kaupp& Altenhofen, 1992, Molday, et al., 1999). The cyclic GMP-gated cation channel is a heterotetrameric complex of three α (CNGA1) and one β (CNGCB1) subunits(Trudeau & Zagotta, 2002, Weitz, et al., 2002, Zheng, et al., 2002, Zhong, et al., 2002); cones have a similar but apparently symmetric assembly of two CNGA3 and two CNGB3 subunits (Peng, et al., 2004). These complexes are in turn associated with the major calcium extrusion protein of outer segments, the Na+/Ca2+,K+ exchanger (Bauer, 2002, Schnetkamp, 1989). Although progress has been made in establishing the stoichiometry of the participants in this complex, and in the functional roles of specific domains and residues within them (Bradley, et al., 2005, Kaupp & Seifert, 2002, Matulef & Zagotta, 2003, Shibukawa, et al., 2007), little is known about their structural arrangement. Electron microscopy and single-particle analysis have been used to determine a low resolution structure of the CNG channel(Higgins, et al., 2002), and there is a high resolution structure of a cyclic nucleotide-binding domain similar to that of the photoreceptor CNG channel(Zagotta, et al., 2003).
In addition to its association with the Na/Ca exchanger, the CNG channel also binds calmodulin (Molday, 1996, Trudeau & Zagotta, 2002) at a site between the N-terminal GARP (glutamic acid-rich protein) domain and the transmembrane domain of the β subunit (CNGB1) , and may be involved in interactions with an alternative protein product of the channel β subunit gene in which the glutamic acid rich protein or GARP (Korschen, et al., 1999, Molday & Molday, 1998, Pentia, et al., 2006) is expressed as a soluble protein without the transmembrane and cyclic nucleotide-binding domians. GARP (there are two splice variants of GARP, a minor form, GARP1, and a major form, GARP2) has been proposed to bind to multiple membrane proteins of both the plasma and disk membranes and may be involved in forming some of the connections between these non-continuous membranes (see below).
Membrane Complexes of the disk rims
An additional membrane compartment, in addition to the planar surface of the disk membranes or the gently curved surface of the plasma membrane is the rim region of the disk and the corresponding region of the cone plasma membranes where there is an extraordinarily low radius of curvature and a unique set of membrane protein complexes. The tetraspanin proteins peripherin/rds and ROM1 are important for maintaining the unusual membrane structure in this region (Goldberg, 2006, Molday, et al., 1987, Molday et al., 1999). These proteins do not act as monomers, but rather as multimers, with a non-covalent heterotetramer of peripherin/rds and ROM1forming higher order multimers through covalent disulfide linkages. These disulfide linkages may form in the disk lumen and serve as molecular “staples” to hold together the closely apposed bilayers of either side of each disk membrane. While several studies have described the interactions among these proteins and their ability to induce sharp membrane curvature into heterologous membranes (Wrigley, et al., 2000) little is known about the three dimensional structure of these important membrane protein complexes. Also confined to the disk rims is a photoreceptor-specific member of the ATP binding cassette family known as ABCR or ABCA4(Allikmets, et al., 1997, Azarian & Travis, 1997, Illing, et al., 1997, Shroyer, et al., 2001, Sun & Nathans, 1997, Wiszniewski, et al., 2005). Rather than playing a structural role, this transport protein has been proposed to serve as a lipid-flippase, possibly for the covalent Schiff’s base adduct between all-trans-retinaldehyde and the amino group of phosphatidylethanolamine (APE). It seems likely that localization to the disk rims is mediated by complex formation between ABCR and other rim-specific proteins such as peripherin/RDS and ROM-1. Although some structural information has been obtained for other members of the ATP binding cassette family which ABCR likely resembles in many of its structural features, its three-dimensional structure and the structure or even existence of its complexes with other rim proteins remain to be determined. This determination may be facilitated by the existence of experimental protocols for expression and purification of ABCR in functional form (Ahn & Molday, 2000, Sun, et al., 1999).
Mystery Proteins Controlling Membrane Structure
There may be additional membrane protein complexes important for photoreceptor structure and function whose components have yet to be identified. Freeze-etch electron microscopy has revealed connections, likely formed by membrane-associated proteins, between the disks and the plasma membranes, and between the rims of adjacent disks(Roof & Heuser, 1982, Roof, et al., 1982). A recent study of mouse disk membranes by cryo-electron tomography revealed very large protein complexes connecting adjacent disks, randomly distributed over the disk plane(Nickell, et al., 2007). Identifying the constituents of all these intermembrane complexes remains a fascinating challenge to be overcome in the next few years.
Influence of membranes on kinetics and thermodynamics of protein-protein interactions
A recurring theme that has emerged in the study of membrane protein complexes in phototransduction is that the membranes do much more than simply organize the protein components. By greatly increasing the local concentrations of protein binding partners and by reducing their conformational and orientational freedom they dramatically increase their effective affinities for one another. These effects are evident not only for the heteromeric complexes, but likely also for the rhodopsin homo-dimer, since the form it takes in disk membranes is likely incompatible with those it assumes in detergent micelles and reconstituted membranes, where it can be found either as dimers or monomers, (Bayburt et al., 2007, Fotiadis, et al., 2003, Jastrzebska, et al., 2004, Li, et al., 2004, Liang, et al., 2003, Schertler, et al., 1993). It is worth noting that the existence and/or functional importance of rhodopsin dimers remains a subject of some controversy (Bayburt et al., 2007, Chabre, et al., 2003, Chabre & le Maire, 2005, Fotiadis et al., 2003, Hanson, et al., 2007).
In principle membranes can have a similar effect on kinetics both by the concentration effect and by the reduction in dimensionality from three to two dimensions. At this point the diffusion kinetics in photoreceptors have been reported only for rhodopsin (Cone, 1972, Liebman, et al., 1982, Montal, 1976, Poo & Cone, 1973, Poo & Cone, 1974, Wey, et al., 1981), so it will be important to document the dynamic behavior of the other membrane protein components of the phototransduction cascade within intact photoreceptors. It seems likely that the varying lipid environments presented by lipid microdomains within disk membranes will exert varying effects on behavior of the protein complexes (Boesze-Battaglia, et al., 2002, Martin, et al., 2005, Nair, et al., 2002, Nickell et al., 2007, Senin, et al., 2004, Seno, et al., 2001). Extensive evidence exists for the modulation of rhodopsin’s behavior, as well as that of the downstream phototransduction complexes by lipid composition(Alves et al., 2005, Botelho, et al., 2006, Brown, 1994, He et al., 2004, Koenig, et al., 2002, Litman, et al., 2001, Malinski & Wensel, 1992, Melia et al., 2000, Mitchell, et al., 2001, Mitchell, et al., 2003a, Mitchell, et al., 2003b, Niu, et al., 2001, Niu, et al., 2002, Womack et al., 2000)
Remaining challenges and future prospects
In reviewing the current state of our understanding of membrane protein complexes of photoreceptor outer segments it is clear that the protein composition of most of the major complexes has been determined, much is known about the biochemistry of the polypeptides involved, and through spontaneous or engineered mutations in animal models or humans, much is known about their physiological functions. The weakest link in our knowledge is in the structures of these complexes, especially as they exist in vivo, embedded in or attached to the surface of the photoreceptor membrane. Part of the reason is clearly that multi-subunit membrane complexes remain the most challenging subjects for x-ray crystallography, which has been by far the most commonly used approach for determination of protein structure. New approaches, or new applications of existing alternative approaches, are needed if progress on this front in the next decade is to exceed the progress made over the past decade. Some of these approaches, involving cryo-electron microscopy and electron cryo-tomography are especially well suited to determining membrane structure in the environment of a membrane bilayer. Spectroscopic techniques, including solid-state NMR, electron paramagnetic spectroscopy of spin-labeled proteins, and fluorescence energy transfer techniques are emerging as powerful methods for extracting structural information about membrane protein complexes over a range of spatial resolutions. Also promising in this regard is the development of miniature bilayer membranes stabilized by lipoproteins, known as nano-disks or bicelles (Bayburt, et al., 2006, Boldog, et al., 2007, De Angelis & Opella, 2007, Leitz, et al., 2006, McKibbin, et al., 2007, Prosser, et al., 2006, Struppe, et al., 1998) These have begun to be exploited for the study of photoreceptor proteins (Bayburt et al., 2007, McKibbin et al., 2007) and may offer a route to application of high resolution structural techniques to bilayer-embedded proteins. However, these alternative approaches are still in the process of development, and are being pursued by a relatively small number of laboratories. We can only hope that an appreciation of the importance of establishing and extending new approaches to membrane protein complexes will motivate sufficient support from funding agencies, scientific journals, research institutions and others to keep these efforts going through the awkward stages faced by all truly innovative scientific endeavors.
Acknowledgements
Work in the Wensel laboratory on signal transducing membrane complexes has been supported by the National Eye Institute, by NASA, and by the Welch Foundation (Q0035). Drs. Zhixian Zhang, Feng He and Qiong Wang provided access to unpublished data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulaev NG, Ngo T, Ramon E, Brabazon DM, Marino JP, Ridge KD. The receptor-bound "empty pocket" state of the heterotrimeric G-protein alpha-subunit is conformationally dynamic. Biochemistry. 2006;45(43):12986–12997. doi: 10.1021/bi061088h. [DOI] [PubMed] [Google Scholar]
- Abdulaev NG, Ngo T, Zhang C, Dinh A, Brabazon DM, Ridge KD, Marino JP. Heterotrimeric G-protein alpha-subunit adopts a "preactivated" conformation when associated with betagamma-subunits. J Biol Chem. 2005;280(45):38071–38080. doi: 10.1074/jbc.M505259200. [DOI] [PubMed] [Google Scholar]
- Ahn J, Molday RS. Purification and characterization of ABCR from bovine rod outer segments. Methods Enzymol. 2000;315:864–879. doi: 10.1016/s0076-6879(00)15887-2. [DOI] [PubMed] [Google Scholar]
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15(3):236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- Alves ID, Salgado GF, Salamon Z, Brown MF, Tollin G, Hruby VJ. Phosphatidylethanolamine enhances rhodopsin photoactivation and transducin binding in a solid supported lipid bilayer as determined using plasmon-waveguide resonance spectroscopy. Biophys J. 2005;88(1):198–210. doi: 10.1529/biophysj.104.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames JB, Tanaka T, Stryer L, Ikura M. Portrait of a myristoyl switch protein. Curr Opin Struct Biol. 1996;6(4):432–438. doi: 10.1016/s0959-440x(96)80106-0. [DOI] [PubMed] [Google Scholar]
- Anant JS, Ong OC, Xie HY, Clarke S, O'Brien PJ, Fung BK. In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem. 1992;267(2):687–690. [PubMed] [Google Scholar]
- Antonny B, Otto-Bruc A, Chabre M, Vuong TM. GTP hydrolysis by purified alpha-subunit of transducin and its complex with the cyclic GMP phosphodiesterase inhibitor. Biochemistry. 1993;32:8646–8653. doi: 10.1021/bi00084a036. [DOI] [PubMed] [Google Scholar]
- Arshavsky VY. Rhodopsin phosphorylation: from terminating single photon responses to photoreceptor dark adaptation. Trends Neurosci. 2002;25(3):124–126. doi: 10.1016/s0166-2236(00)02094-4. [DOI] [PubMed] [Google Scholar]
- Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- Artemyev NO, Mills JS, Thornburg KR, Knapp DR, Schey KL, Hamm HE. A site on transducin alpha-subunit of interaction with the polycationic region of cGMP phosphodiesterase inhibitory subunit. J Biol Chem. 1993;268(31):23611–23615. [PubMed] [Google Scholar]
- Azarian SM, Travis GH. The photoreceptor rim protein is an ABC transporter encoded by the gene for recessive Stargardt's disease (ABCR) FEBS Lett. 1997;409(2):247–252. doi: 10.1016/s0014-5793(97)00517-6. [DOI] [PubMed] [Google Scholar]
- Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM, Palczewski K. The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem. 2007;282(12):8837–8847. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Martemyanov KA, Shavkunov AS, Arshavsky VY. Kinetic mechanism of RGS9-1 potentiation by R9AP. Biochemistry. 2006;45(35):10690–10697. doi: 10.1021/bi060376a. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. The complex of cGMP-gated channel and Na+/Ca2+, K+ exchanger in rod photoreceptors. Adv Exp Med Biol. 2002;514:253–274. [PubMed] [Google Scholar]
- Bayburt TH, Grinkova YV, Sligar SG. Assembly of single bacteriorhodopsin trimers in bilayer nanodiscs. Arch Biochem Biophys. 2006;450(2):215–222. doi: 10.1016/j.abb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J Biol Chem. 2007;282(20):14875–14881. doi: 10.1074/jbc.M701433200. [DOI] [PubMed] [Google Scholar]
- Bigay J, Faurobert E, Franco M, Chabre M. Roles of lipid modifications of transducin subunits in their GDP-dependent association and membrane binding. Biochemistry. 1994;33(47):14081–14090. doi: 10.1021/bi00251a017. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. Expansion of signal transduction by G proteins. The second 15 years or so: from 3 to 16 alpha subunits plus betagamma dimers. Biochim Biophys Acta. 2007;1768(4):772–793. doi: 10.1016/j.bbamem.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Dispoto J, Kahoe MA. Association of a photoreceptor-specific tetraspanin protein, ROM-1, with triton X-100-resistant membrane rafts from rod outer segment disk membranes. J Biol Chem. 2002;277(44):41843–41849. doi: 10.1074/jbc.M207111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm A, Gaudet R, Sigler PB. Structural aspects of heterotrimeric G-protein signaling. Curr Opin Biotechnol. 1997;8(4):480–487. doi: 10.1016/s0958-1669(97)80072-9. [DOI] [PubMed] [Google Scholar]
- Boldog T, Li M, Hazelbauer GL. Using Nanodiscs to create water-soluble transmembrane chemoreceptors inserted in lipid bilayers. Methods Enzymol. 2007;423:317–335. doi: 10.1016/S0076-6879(07)23014-9. [DOI] [PubMed] [Google Scholar]
- Botelho AV, Huber T, Sakmar TP, Brown MF. Curvature and hydrophobic forces drive oligomerization and modulate activity of rhodopsin in membranes. Biophys J. 2006;91(12):4464–4477. doi: 10.1529/biophysj.106.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabazon DM, Abdulaev NG, Marino JP, Ridge KD. Evidence for structural changes in carboxyl-terminal peptides of transducin alpha-subunit upon binding a soluble mimic of light-activated rhodopsin. Biochemistry. 2003;42(2):302–311. doi: 10.1021/bi0268899. [DOI] [PubMed] [Google Scholar]
- Bradley J, Reisert J, Frings S. Regulation of cyclic nucleotide-gated channels. Curr Opin Neurobiol. 2005;15(3):343–349. doi: 10.1016/j.conb.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Brown MF. Modulation of rhodopsin function by properties of the membrane bilayer. Chem Phys Lipids. 1994;73(1–2):159–180. doi: 10.1016/0009-3084(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Burns ME, Mendez A, Chen J, Baylor DA. Dynamics of cyclic GMP synthesis in retinal rods. Neuron. 2002;36(1):81–91. doi: 10.1016/s0896-6273(02)00911-x. [DOI] [PubMed] [Google Scholar]
- Catty P, Deterre P. Activation and solubilization of the retinal cGMP-specific phosphodiesterase by limited proteolysis. Role of the C-terminal domain of the beta-subunit. Eur J Biochem. 1991;199(2):263–269. doi: 10.1111/j.1432-1033.1991.tb16119.x. [DOI] [PubMed] [Google Scholar]
- Chabre M, Cone R, Saibil H. Biophysics: is rhodopsin dimeric in native retinal rods? Nature. 2003;426(6962):30–31. doi: 10.1038/426030b. discussion 31. [DOI] [PubMed] [Google Scholar]
- Chabre M, le Maire M. Monomeric G-protein-coupled receptor as a functional unit. Biochemistry. 2005;44(27):9395–9403. doi: 10.1021/bi050720o. [DOI] [PubMed] [Google Scholar]
- Cheever ML, Snyder JT, Gershburg S, Siderovski DP, Harden TK, Sondek J. Crystal structure of the multifunctional Gbeta5-RGS9 complex. Nat Struct Mol Biol. 2008;15(2):155–162. doi: 10.1038/nsmb.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK. Recoverin and rhodopsin kinase. Adv Exp Med Biol. 2002;514:101–107. doi: 10.1007/978-1-4615-0121-3_6. [DOI] [PubMed] [Google Scholar]
- Chen CK. The vertebrate phototransduction cascade: amplification and termination mechanisms. Rev Physiol Biochem Pharmacol. 2005;154:101–121. doi: 10.1007/s10254-005-0004-0. [DOI] [PubMed] [Google Scholar]
- Chen CK, Inglese J, Lefkowitz RJ, Hurley JB. Ca(2+)-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem. 1995;270(30):18060–18066. doi: 10.1074/jbc.270.30.18060. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu M, Sezate SA, McGinnis JF. Light threshold-controlled cone alpha-transducin translocation. Invest Ophthalmol Vis Sci. 2007;48(7):3350–3355. doi: 10.1167/iovs.07-0126. [DOI] [PubMed] [Google Scholar]
- Coleman DE, Sprang SR. How G proteins work: a continuing story. Trends Biochem Sci. 1996;21(2):41–44. [PubMed] [Google Scholar]
- Coleman JE, Semple-Rowland SL. GC1 deletion prevents light-dependent arrestin translocation in mouse cone photoreceptor cells. Invest Ophthalmol Vis Sci. 2005;46(1):12–16. doi: 10.1167/iovs.04-0691. [DOI] [PubMed] [Google Scholar]
- Cone RA. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat New Biol. 1972;236(63):39–43. doi: 10.1038/newbio236039a0. [DOI] [PubMed] [Google Scholar]
- Cook TA, Ghomashchi F, Gelb MH, Florio SK, Beavo JA. Binding of the delta subunit to rod phosphodiesterase catalytic subunits requires methylated, prenylated C-termini of the catalytic subunits. Biochemistry. 2000;39(44):13516–13523. doi: 10.1021/bi001070l. [DOI] [PubMed] [Google Scholar]
- Cook TA, Ghomashchi F, Gelb MH, Florio SK, Beavo JA. The delta subunit of type 6 phosphodiesterase reduces light-induced cGMP hydrolysis in rod outer segments. J Biol Chem. 2001;276(7):5248–5255. doi: 10.1074/jbc.M004690200. [DOI] [PubMed] [Google Scholar]
- De Angelis AA, Opella SJ. Bicelle samples for solid-state NMR of membrane proteins. Nat Protoc. 2007;2(10):2332–2338. doi: 10.1038/nprot.2007.329. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Jr, Rundle DR, Wensel TG, Anderson RE. Heterogeneous N-terminal acylation of retinal proteins. Prog Lipid Res. 1999;38(1):49–90. doi: 10.1016/s0163-7827(98)00020-4. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Jr, Wensel TG, Anderson RE. Biosynthesis of the unsaturated 14-carbon fatty acids found on the N termini of photoreceptor-specific proteins. J Biol Chem. 1996;271(9):5007–5016. doi: 10.1074/jbc.271.9.5007. [DOI] [PubMed] [Google Scholar]
- Deterre P, Bigay J, Forquet F, Robert M, Chabre M. cGMP phosphodiesterase of retinal rods is regulated by two inhibitory subunits. Proc. Natl. Acad. Sci. U S A. 1988;85(8):2424–2428. doi: 10.1073/pnas.85.8.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs MA, Arimoto R, Marshall GR, Kisselev OG. G-protein alpha and beta-gamma subunits interact with conformationally distinct signaling states of rhodopsin. Vision Res. 2006;46(27):4442–4448. doi: 10.1016/j.visres.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Ernst OP, Gramse V, Kolbe M, Hofmann KP, Heck M. Monomeric G protein-coupled receptor rhodopsin in solution activates its G protein transducin at the diffusion limit. Proc Natl Acad Sci U S A. 2007;104(26):10859–10864. doi: 10.1073/pnas.0701967104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio SK, Prusti RK, Beavo JA. Solubilization of membrane-bound rod phosphodiesterase by the rod phosphodiesterase recombinant delta subunit. J Biol Chem. 1996;271(39):24036–24047. doi: 10.1074/jbc.271.39.24036. [DOI] [PubMed] [Google Scholar]
- Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K. Atomic-force microscopy: Rhodopsin dimers in native disc membranes. Nature. 2003;421(6919):127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- Fukada Y. Prenylation and carboxylmethylation of G-protein gamma subunit. Methods Enzymol. 1995;250:91–105. doi: 10.1016/0076-6879(95)50065-0. [DOI] [PubMed] [Google Scholar]
- Fukada Y, Takao T, Ohguro H, Yoshizawa T, Akino T, Shimonishi Y. Farnesylated gamma-subunit of photoreceptor G protein indispensable for GTP-binding. Nature. 1990;346(6285):658–660. doi: 10.1038/346658a0. [DOI] [PubMed] [Google Scholar]
- Goldberg AF. Role of peripherin/rds in vertebrate photoreceptor architecture and inherited retinal degenerations. Int Rev Cytol. 2006;253:131–175. doi: 10.1016/S0074-7696(06)53004-9. [DOI] [PubMed] [Google Scholar]
- Hanson SM, Gurevich EV, Vishnivetskiy SA, Ahmed MR, Song X, Gurevich VV. Each rhodopsin molecule binds its own arrestin. Proc Natl Acad Sci U S A. 2007;104(9):3125–3128. doi: 10.1073/pnas.0610886104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrave PA, Hamm HE, Hofmann KP. Interaction of rhodopsin with the G-protein, transducin. Bioessays. 1993;15(1):43–50. doi: 10.1002/bies.950150107. [DOI] [PubMed] [Google Scholar]
- He F, Mao M, Wensel TG. Enhancement of phototransduction g protein-effector interactions by phosphoinositides. J Biol Chem. 2004;279(10):8986–8990. doi: 10.1074/jbc.M311488200. [DOI] [PubMed] [Google Scholar]
- Heck M, Hofmann KP. Maximal rate and nucleotide dependence of rhodopsin-catalyzed transducin activation: initial rate analysis based on a double displacement mechanism. J Biol Chem. 2001;276(13):10000–10009. doi: 10.1074/jbc.M009475200. [DOI] [PubMed] [Google Scholar]
- Herrmann R, Heck M, Henklein P, Henklein P, Kleuss C, Hofmann KP, Ernst OP. Sequence of interactions in receptor-G protein coupling. J Biol Chem. 2004;279(23):24283–24290. doi: 10.1074/jbc.M311166200. [DOI] [PubMed] [Google Scholar]
- Herrmann R, Heck M, Henklein P, Hofmann KP, Ernst OP. Signal transfer from GPCRs to G proteins: role of the G alpha N-terminal region in rhodopsin-transducin coupling. J Biol Chem. 2006a;281(40):30234–30241. doi: 10.1074/jbc.M600797200. [DOI] [PubMed] [Google Scholar]
- Herrmann R, Heck M, Henklein P, Kleuss C, Wray V, Hofmann KP, Ernst OP. Rhodopsin-transducin coupling: role of the Galpha C-terminus in nucleotide exchange catalysis. Vision Res. 2006b;46(27):4582–4593. doi: 10.1016/j.visres.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Hessel E, Heck M, Muller P, Herrmann A, Hofmann KP. Signal transduction in the visual cascade involves specific lipid-protein interactions. J Biol Chem. 2003;278(25):22853–22860. doi: 10.1074/jbc.M302747200. [DOI] [PubMed] [Google Scholar]
- Higgins MK, Weitz D, Warne T, Schertler GF, Kaupp UB. Molecular architecture of a retinal cGMP-gated channel: the arrangement of the cytoplasmic domains. Embo J. 2002;21(9):2087–2094. doi: 10.1093/emboj/21.9.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann KP. Signalling states of photoactivated rhodopsin. Novartis Found Symp. 1999;224:158–175. doi: 10.1002/9780470515693.ch10. discussion 175–180. [DOI] [PubMed] [Google Scholar]
- Howes KA, Pennesi ME, Sokal I, Church-Kopish J, Schmidt B, Margolis D, Frederick JM, Rieke F, Palczewski K, Wu SM, Detwiler PB, Baehr W. GCAP1 rescues rod photoreceptor response in GCAP1/GCAP2 knockout mice. Embo J. 2002;21(7):1545–1554. doi: 10.1093/emboj/21.7.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Wensel TG. R9AP, a membrane anchor for the photoreceptor GTPase accelerating protein, RGS9-1. Proc Natl Acad Sci U S A. 2002;99(15):9755–9760. doi: 10.1073/pnas.152094799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Wensel TG. Characterization of R9AP, a membrane anchor for the photoreceptor GTPase-accelerating protein, RGS9-1. Methods Enzymol. 2004;390:178–196. doi: 10.1016/S0076-6879(04)90012-2. [DOI] [PubMed] [Google Scholar]
- Hu G, Zhang Z, Wensel TG. Activation of RGS9-1GTPase acceleration by its membrane anchor, R9AP. J Biol Chem. 2003;278(16):14550–14554. doi: 10.1074/jbc.M212046200. [DOI] [PubMed] [Google Scholar]
- Hurley JB, Spencer M, Niemi GA. Rhodopsin phosphorylation and its role in photoreceptor function. Vision Res. 1998;38(10):1341–1352. doi: 10.1016/s0042-6989(97)00459-8. [DOI] [PubMed] [Google Scholar]
- Hwang JY, Koch KW. Calcium- and myristoyl-dependent properties of guanylate cyclase-activating protein-1 and protein-2. Biochemistry. 2002a;41(43):13021–13028. doi: 10.1021/bi026618y. [DOI] [PubMed] [Google Scholar]
- Hwang JY, Koch KW. The myristoylation of the neuronal Ca2+ -sensors guanylate cyclase-activating protein 1 and 2. Biochim Biophys Acta. 2002b;1600(1–2):111–117. doi: 10.1016/s1570-9639(02)00451-x. [DOI] [PubMed] [Google Scholar]
- Hwang JY, Lange C, Helten A, Hoppner-Heitmann D, Duda T, Sharma RK, Koch KW. Regulatory modes of rod outer segment membrane guanylate cyclase differ in catalytic efficiency and Ca(2+)-sensitivity. Eur J Biochem. 2003;270(18):3814–3821. doi: 10.1046/j.1432-1033.2003.03770.x. [DOI] [PubMed] [Google Scholar]
- Illing M, Molday LL, Molday RS. The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J Biol Chem. 1997;272(15):10303–10310. doi: 10.1074/jbc.272.15.10303. [DOI] [PubMed] [Google Scholar]
- Jastrzebska B, Maeda T, Zhu L, Fotiadis D, Filipek S, Engel A, Stenkamp RE, Palczewski K. Functional characterization of rhodopsin monomers and dimers in detergents. J Biol Chem. 2004;279(52):54663–54675. doi: 10.1074/jbc.M408691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RS, Ohguro H, Palczewski K, Hurley JB, Walsh KA, Neubertx TA. Heterogeneous N-acylation is a tissue- and species-specific posttranslational modification. J Biol Chem. 1994;269(33):21067–21071. [PubMed] [Google Scholar]
- Kajimura N, Yamazaki M, Morikawa K, Yamazaki A, Mayanagi K. Three-dimensional structure of non-activated cGMP phosphodiesterase 6 and comparison of its image with those of activated forms. J Struct Biol. 2002;139(1):27–38. doi: 10.1016/s1047-8477(02)00502-6. [DOI] [PubMed] [Google Scholar]
- Kameni Tcheudji JF, Lebeau L, Virmaux N, Maftei CG, Cote RH, Lugnier C, Schultz P. Molecular organization of bovine rod cGMP-phosphodiesterase 6. J Mol Biol. 2001;310(4):781–791. doi: 10.1006/jmbi.2001.4813. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Altenhofen W. Cyclic nucleotide-gated channels of vertebrate photoreceptor cells and olfactory epithelium. Soc Gen Physiol Ser. 1992;47:133–150. [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82(3):769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Tachibanaki S. S-modulin. Adv Exp Med Biol. 2002;514:61–68. doi: 10.1007/978-1-4615-0121-3_4. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Dunn FA, Hurley JB. Visual pigment phosphorylation but not transducin translocation can contribute to light adaptation in zebrafish cones. Neuron. 2004;41(6):915–928. doi: 10.1016/s0896-6273(04)00086-8. [DOI] [PubMed] [Google Scholar]
- Keresztes G, Martemyanov KA, Krispel CM, Mutai H, Yoo PJ, Maison SF, Burns ME, Arshavsky VY, Heller S. Absence of the RGS9.Gbeta5 GTPase-activating complex in photoreceptors of the R9AP knockout mouse. J Biol Chem. 2004;279(3):1581–1584. doi: 10.1074/jbc.C300456200. [DOI] [PubMed] [Google Scholar]
- Keresztes G, Mutai H, Hibino H, Hudspeth AJ, Heller S. Expression patterns of the RGS9-1 anchoring protein R9AP in the chicken and mouse suggest multiple roles in the nervous system. Mol Cell Neurosci. 2003;24(3):687–695. doi: 10.1016/s1044-7431(03)00231-8. [DOI] [PubMed] [Google Scholar]
- Klenchin VACPD, Bounds MD. Inhibition of rhodopsin kinase by recoverin. J. Biol. Chem. 1995;270:16147–16152. doi: 10.1074/jbc.270.27.16147. [DOI] [PubMed] [Google Scholar]
- Knierim B, Hofmann KP, Ernst OP, Hubbell WL. Sequence of late molecular events in the activation of rhodopsin. Proc Natl Acad Sci U S A. 2007;104(51):20290–20295. doi: 10.1073/pnas.0710393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KW. Target recognition of guanylate cyclase by guanylate cyclase-activating proteins. Adv Exp Med Biol. 2002;514:349–360. doi: 10.1007/978-1-4615-0121-3_21. [DOI] [PubMed] [Google Scholar]
- Koch KW, Duda T, Sharma RK. Photoreceptor specific guanylate cyclases in vertebrate phototransduction. Mol Cell Biochem. 2002;230(1–2):97–106. [PubMed] [Google Scholar]
- Koenig BW, Kontaxis G, Mitchell DC, Louis JM, Litman BJ, Bax A. Structure and orientation of a G protein fragment in the receptor bound state from residual dipolar couplings. J Mol Biol. 2002;322(2):441–461. doi: 10.1016/s0022-2836(02)00745-3. [DOI] [PubMed] [Google Scholar]
- Kokame K, Fukada Y, Yoshizawa T, Takao T, Shimonishi Y. Lipid modification at the N terminus of photoreceptor G-protein alpha-subunit. Nature. 1992;359(6397):749–752. doi: 10.1038/359749a0. [DOI] [PubMed] [Google Scholar]
- Korschen HG, Beyermann M, Muller F, Heck M, Vantler M, Koch KW, Kellner R, Wolfrum U, Bode C, Hofmann KP, Kaupp UB. Interaction of glutamic-acid-rich proteins with the cGMP signalling pathway in rod photoreceptors. Nature. 1999;400(6746):761–766. doi: 10.1038/23468. [DOI] [PubMed] [Google Scholar]
- Krispel CM, Chen D, Melling N, Chen YJ, Martemyanov KA, Quillinan N, Arshavsky VY, Wensel TG, Chen CK, Burns ME. RGS expression rate-limits recovery of rod photoresponses. Neuron. 2006;51(4):409–416. doi: 10.1016/j.neuron.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Lai RK, Perez-Sala D, Canada FJ, Rando RR. The gamma subunit of transducin is farnesylated. Proc Natl Acad Sci U S A. 1990;87(19):7673–7677. doi: 10.1073/pnas.87.19.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. The 2.0 A crystal structure of a heterotrimeric G protein. Nature. 1996;379(6563):311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- Leitz AJ, Bayburt TH, Barnakov AN, Springer BA, Sligar SG. Functional reconstitution of Beta2-adrenergic receptors utilizing self-assembling Nanodisc technology. Biotechniques. 2006;40(5):601–602. doi: 10.2144/000112169. 604, 606, passim. [DOI] [PubMed] [Google Scholar]
- Li J, Edwards PC, Burghammer M, Villa C, Schertler GF. Structure of bovine rhodopsin in a trigonal crystal form. J Mol Biol. 2004;343(5):1409–1438. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- Li N, Florio SK, Pettenati MJ, Rao PN, Beavo JA, Baehr W. Characterization of human and mouse rod cGMP phosphodiesterase delta subunit (PDE6D) and chromosomal localization of the human gene. Genomics. 1998;49(1):76–82. doi: 10.1006/geno.1998.5210. [DOI] [PubMed] [Google Scholar]
- Liang Y, Fotiadis D, Filipek S, Saperstein DA, Palczewski K, Engel A. Organization of the G Protein-coupled Receptors Rhodopsin and Opsin in Native Membranes. J Biol Chem. 2003;278(24):21655–21662. doi: 10.1074/jbc.M302536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman PA, Weiner HL, Drzymala RE. Lateral diffusion of visual pigment in rod disk membranes. Methods Enzymol. 1982;81:660–668. doi: 10.1016/s0076-6879(82)81091-4. [DOI] [PubMed] [Google Scholar]
- Litman BJ, Niu SL, Polozova A, Mitchell DC. The role of docosahexaenoic acid containing phospholipids in modulating G protein-coupled signaling pathways: visual transduction. J Mol Neurosci. 2001;16(2–3):237–242. doi: 10.1385/JMN:16:2-3:237. discussion 279–284. [DOI] [PubMed] [Google Scholar]
- Lobanova ES, Finkelstein S, Song H, Tsang SH, Chen CK, Sokolov M, Skiba NP, Arshavsky VY. Transducin translocation in rods is triggered by saturation of the GTPase-activating complex. J Neurosci. 2007;27(5):1151–1160. doi: 10.1523/JNEUROSCI.5010-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Imanishi Y, Palczewski K. Rhodopsin phosphorylation: 30 years later. Prog Retin Eye Res. 2003;22(4):417–434. doi: 10.1016/s1350-9462(03)00017-x. [DOI] [PubMed] [Google Scholar]
- Makino CL, Dodd RL, Chen J, Burns ME, Roca A, Simon MI, Baylor DA. Recoverin regulates light-dependent phosphodiesterase activity in retinal rods. J Gen Physiol. 2004;123(6):729–741. doi: 10.1085/jgp.200308994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinski JA, Wensel TG. Membrane stimulation of cGMP phosphodiesterase activation by transducin: comparison of phospholipid bilayers to rod outer segment membranes. Biochemistry. 1992;31(39):9502–9512. doi: 10.1021/bi00154a024. [DOI] [PubMed] [Google Scholar]
- Martin RE, Elliott MH, Brush RS, Anderson RE. Detailed characterization of the lipid composition of detergent-resistant membranes from photoreceptor rod outer segment membranes. Invest Ophthalmol Vis Sci. 2005;46(4):1147–1154. doi: 10.1167/iovs.04-1207. [DOI] [PubMed] [Google Scholar]
- Matulef K, Zagotta WN. Cyclic nucleotide-gated ion channels. Annu Rev Cell Dev Biol. 2003;19:23–44. doi: 10.1146/annurev.cellbio.19.110701.154854. [DOI] [PubMed] [Google Scholar]
- McKibbin C, Farmer NA, Jeans C, Reeves PJ, Khorana HG, Wallace BA, Edwards PC, Villa C, Booth PJ. Opsin stability and folding: modulation by phospholipids bicelles. J Mol Biol. 2007;374(5):1319–1332. doi: 10.1016/j.jmb.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Medkova M, Preininger AM, Yu NJ, Hubbell WL, Hamm HE. Conformational changes in the amino-terminal helix of the G protein alpha(i1) following dissociation from Gbetagamma subunit and activation. Biochemistry. 2002;41(31):9962–9972. doi: 10.1021/bi0255726. [DOI] [PubMed] [Google Scholar]
- Melia TJ, Malinski JA, He F, Wensel TG. Enhancement of phototransduction protein interactions by lipid surfaces. J. Biol. Chem. 2000;275(05):3535–3542. doi: 10.1074/jbc.275.5.3535. [DOI] [PubMed] [Google Scholar]
- Melia TJ, Sowa ME, Schutze L, Wensel TG. Formation of helical protein assemblies of IgG and transducin on varied lipid tubules. J Struct Biol. 1999;128(1):119–130. doi: 10.1006/jsbi.1999.4151. [DOI] [PubMed] [Google Scholar]
- Mendez A, Burns ME, Sokal I, Dizhoor AM, Baehr W, Palczewski K, Baylor DA, Chen J. Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc Natl Acad Sci U S A. 2001;98(17):9948–9953. doi: 10.1073/pnas.171308998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng EC, Pettersen EF, Couch GS, Huang CC, Ferrin TE. Tools for integrated sequence-structure analysis with UCSF Chimera. BMC Bioinformatics. 2006;7(1):339. doi: 10.1186/1471-2105-7-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DC, Niu SL, Litman BJ. Optimization of receptor-G protein coupling by bilayer lipid composition I: kinetics of rhodopsin-transducin binding. J Biol Chem. 2001;276(46):42801–42806. doi: 10.1074/jbc.M105772200. [DOI] [PubMed] [Google Scholar]
- Mitchell DC, Niu SL, Litman BJ. DHA-Rich phospholipids optimize G-Protein-coupled signaling. J Pediatr. 2003a;143(4 Suppl):S80–S86. doi: 10.1067/s0022-3476(03)00405-0. [DOI] [PubMed] [Google Scholar]
- Mitchell DC, Niu SL, Litman BJ. Enhancement of G protein-coupled signaling by DHA phospholipids. Lipids. 2003b;38(4):437–443. doi: 10.1007/s11745-003-1081-1. [DOI] [PubMed] [Google Scholar]
- Molday RS. Calmodulin regulation of cyclic-nucleotide-gated channels. Curr Opin Neurobiol. 1996;6(4):445–452. doi: 10.1016/s0959-4388(96)80048-1. [DOI] [PubMed] [Google Scholar]
- Molday RS, Hicks D, Molday L. Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest Ophthalmol Vis Sci. 1987;28(1):50–61. [PubMed] [Google Scholar]
- Molday RS, Molday LL. Molecular properties of the cGMP-gated channel of rod photoreceptors. Vision Res. 1998;38(10):1315–1323. doi: 10.1016/s0042-6989(97)00409-4. [DOI] [PubMed] [Google Scholar]
- Molday RS, Warren R, Loewen C, Molday L. Cyclic GMP-gated channel and peripherin/rds-rom-1 complex of rod cells. Novartis Found Symp. 1999;224:249–261. doi: 10.1002/9780470515693.ch14. discussion 261-244. [DOI] [PubMed] [Google Scholar]
- Montal M. Rhodopsin in bilayer membranes. Biochem Soc Trans. 1976;4(4):560–561. doi: 10.1042/bst0040560. [DOI] [PubMed] [Google Scholar]
- Murray D, McLaughlin S, Honig B. The role of electrostatic interactions in the regulation of the membrane association of G protein beta gamma heterodimers. J Biol Chem. 2001;276(48):45153–45159. doi: 10.1074/jbc.M101784200. [DOI] [PubMed] [Google Scholar]
- Nair KS, Balasubramanian N, Slepak VZ. Signal-dependent translocation of transducin, RGS9-1-Gbeta5L complex, and arrestin to detergent-resistant membrane rafts in photoreceptors. Curr Biol. 2002;12(5):421–425. doi: 10.1016/s0960-9822(02)00691-7. [DOI] [PubMed] [Google Scholar]
- Neubert TA, Hurley JB. Functional heterogeneity of transducin alpha subunits. FEBS Lett. 1998;422(3):343–345. doi: 10.1016/s0014-5793(98)00037-4. [DOI] [PubMed] [Google Scholar]
- Neubert TA, Johnson RS, Hurley JB, Walsh KA. The rod transducin alpha subunit amino terminus is heterogeneously fatty acylated. J Biol Chem. 1992;267(26):18274–18277. [PubMed] [Google Scholar]
- Nickell S, Park PS, Baumeister W, Palczewski K. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol. 2007;177(5):917–925. doi: 10.1083/jcb.200612010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi KM, Sandberg MA, Kooijman AC, Martemyanov KA, Pott JW, Hagstrom SA, Arshavsky VY, Berson EL, Dryja TP. Defects in RGS9 or its anchor protein R9AP in patients with slow photoreceptor deactivation. Nature. 2004;427(6969):75–78. doi: 10.1038/nature02170. [DOI] [PubMed] [Google Scholar]
- Niu SL, Mitchell DC, Litman BJ. Optimization of receptor-G protein coupling by bilayer lipid composition II: formation of metarhodopsin II-transducin complex. J Biol Chem. 2001;276(46):42807–42811. doi: 10.1074/jbc.M105778200. [DOI] [PubMed] [Google Scholar]
- Niu SL, Mitchell DC, Litman BJ. Manipulation of cholesterol levels in rod disk membranes by methyl-beta-cyclodextrin: effects on receptor activation. J Biol Chem. 2002;277(23):20139–20145. doi: 10.1074/jbc.M200594200. [DOI] [PubMed] [Google Scholar]
- Norton AW, Hosier S, Terew JM, Li N, Dhingra A, Vardi N, Baehr W, Cote RH. Evaluation of the 17-kDa prenyl-binding protein as a regulatory protein for phototransduction in retinal photoreceptors. J Biol Chem. 2005;280(2):1248–1256. doi: 10.1074/jbc.M410475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohguro H, Fukada Y, Takao T, Shimonishi Y, Yoshizawa T, Akino T. Carboxyl methylation and farnesylation of transducin gamma-subunit synergistically enhance its coupling with metarhodopsin II. Embo J. 1991;10(12):3669–3674. doi: 10.1002/j.1460-2075.1991.tb04934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J Mol Biol. 2004;342(2):571–583. doi: 10.1016/j.jmb.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Oldham WM, Van Eps N, Preininger AM, Hubbell WL, Hamm HE. Mechanism of the receptor-catalyzed activation of heterotrimeric G proteins. Nat Struct Mol Biol. 2006;13(9):772–777. doi: 10.1038/nsmb1129. [DOI] [PubMed] [Google Scholar]
- Olshevskaya EV, Ermilov AN, Dizhoor AM. Dimerization of guanylyl cyclase-activating protein and a mechanism of photoreceptor guanylyl cyclase activation. J Biol Chem. 1999;274(36):25583–25587. doi: 10.1074/jbc.274.36.25583. [DOI] [PubMed] [Google Scholar]
- Olshevskaya EV, Hughes RE, Hurley JB, Dizhoor AM. Calcium binding, but not a calcium-myristoyl switch, controls the ability of guanylyl cyclase-activating protein GCAP-2 to regulate photoreceptor guanylyl cyclase. J Biol Chem. 1997;272(22):14327–14333. doi: 10.1074/jbc.272.22.14327. [DOI] [PubMed] [Google Scholar]
- Ong OC, Ota IM, Clarke S, Fung BK. The membrane binding domain of rod cGMP phosphodiesterase is posttranslationally modified by methyl esterification at a C-terminal cysteine. Proc Natl Acad Sci U S A. 1989;86(23):9238–9242. doi: 10.1073/pnas.86.23.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprian DD. Molecular determinants of spectral properties and signal transduction in the visual pigments. Curr Opin Neurobiol. 1992;2(4):428–432. doi: 10.1016/0959-4388(92)90175-k. [DOI] [PubMed] [Google Scholar]
- Otto-Bruc AE, Fariss RN, Van Hooser JP, Palczewski K. Phosphorylation of photolyzed rhodopsin is calcium-insensitive in retina permeabilized by alpha-toxin. Proc Natl Acad Sci U S A. 1998;95(25):15014–15019. doi: 10.1073/pnas.95.25.15014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289(5480):739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Subbaraya I, Gorczyca WA, Helekar BS, Ruiz CC, Ohguro H, Huang J, Zhao X, Crabb JW, Johnson RS, et al. Molecular cloning and characterization of retinal photoreceptor guanylyl cyclase-activating protein. Neuron. 1994;13(2):395–404. doi: 10.1016/0896-6273(94)90355-7. [DOI] [PubMed] [Google Scholar]
- Peng C, Rich ED, Varnum MD. Subunit configuration of heteromeric cone cyclic nucleotide-gated channels. Neuron. 2004;42(3):401–410. doi: 10.1016/s0896-6273(04)00225-9. [DOI] [PubMed] [Google Scholar]
- Penn RB, Pronin AN, Benovic JL. Regulation of G protein-coupled receptor kinases. Trends Cardiovasc Med. 2000;10(2):81–89. doi: 10.1016/s1050-1738(00)00053-0. [DOI] [PubMed] [Google Scholar]
- Pentia DC, Hosier S, Cote RH. The glutamic acid-rich protein-2 (GARP2) is a high affinity rod photoreceptor phosphodiesterase (PDE6)-binding protein that modulates its catalytic properties. J Biol Chem. 2006;281(9):5500–5505. doi: 10.1074/jbc.M507488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Poo M, Cone RA. Lateral diffusion of rhodopsin in the visual receptor membrane. J Supramol Struct. 1973;1(4):354. doi: 10.1002/jss.400010411. [DOI] [PubMed] [Google Scholar]
- Poo M, Cone RA. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974;247(441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- Prosser RS, Evanics F, Kitevski JL, Al-Abdul-Wahid MS. Current applications of bicelles in NMR studies of membrane-associated amphiphiles and proteins. Biochemistry. 2006;45(28):8453–8465. doi: 10.1021/bi060615u. [DOI] [PubMed] [Google Scholar]
- Pugh EN, Jr, Duda T, Sitaramayya A, Sharma RK. Photoreceptor guanylate cyclases: a review. Biosci Rep. 1997;17(5):429–473. doi: 10.1023/a:1027365520442. [DOI] [PubMed] [Google Scholar]
- Qin N, Pittler SJ, Baehr W. In vitro isoprenylation and membrane association of mouse rod photoreceptor cGMP phosphodiesterase alpha and beta subunits expressed in bacteria. J Biol. Chem. 1992;267:8458–8463. [PubMed] [Google Scholar]
- Qin N PSJBW. In vitro isoprenylation and membrane association of mouse rod photoreceptor cGMP phosphodiesterase alpha and beta subunits expressed in bacteria. J Biol. Chem. 1992;267:8458–8463. [PubMed] [Google Scholar]
- Ramamurthy V, Tucker C, Wilkie SE, Daggett V, Hunt DM, Hurley JB. Interactions within the coiled-coil domain of RetGC-1 guanylyl cyclase are optimized for regulation rather than for high affinity. J Biol Chem. 2001;276(28):26218–26229. doi: 10.1074/jbc.M010495200. [DOI] [PubMed] [Google Scholar]
- Ridge KD, Marino JP, Ngo T, Ramon E, Brabazon DM, Abdulaev NG. NMR analysis of rhodopsin-transducin interactions. Vision Res. 2006;46(27):4482–4492. doi: 10.1016/j.visres.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Roof DJ, Heuser JE. Surfaces of rod photoreceptor disk membranes: integral membrane components. J Cell Biol. 1982;95(2 Pt 1):487–500. doi: 10.1083/jcb.95.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof DJ, Korenbrot JI, Heuser JE. Surfaces of rod photoreceptor disk membranes: light-activated enzymes. J Cell Biol. 1982;95(2 Pt 1):501–509. doi: 10.1083/jcb.95.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig DH, Nair KS, Wei J, Wang Q, Garwin G, Saari JC, Chen CK, Smrcka AV, Swaroop A, Lem J, Hurley JB, Slepak VZ. Subunit dissociation and diffusion determine the subcellular localization of rod and cone transducins. J Neurosci. 2007;27(20):5484–5494. doi: 10.1523/JNEUROSCI.1421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath AP, Strissel KJ, Elias R, Arshavsky VY, McGinnis JF, Chen J, Kawamura S, Rieke F, Hurley JB. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron. 2005;46(3):413–420. doi: 10.1016/j.neuron.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Schertler GF, Villa C, Henderson R. Projection structure of rhodopsin. Nature. 1993;362(6422):770–772. doi: 10.1038/362770a0. [DOI] [PubMed] [Google Scholar]
- Schnetkamp PP. Na-Ca or Na-Ca-K exchange in rod photoreceptors. Prog Biophys Mol Biol. 1989;54(1):1–29. doi: 10.1016/0079-6107(89)90007-2. [DOI] [PubMed] [Google Scholar]
- Seitz HR, Heck M, Hofmann KP, Alt T, Pellaud J, Seelig A. Molecular determinants of the reversible membrane anchorage of the G- protein transducin. Biochemistry. 1999;38(25):7950–7960. doi: 10.1021/bi990298+. [DOI] [PubMed] [Google Scholar]
- Senin II, Hoppner-Heitmann D, Polkovnikova OO, Churumova VA, Tikhomirova NK, Philippov PP, Koch KW. Recoverin and rhodopsin kinase activity in detergent-resistant membrane rafts from rod outer segments. J Biol Chem. 2004;279(47):48647–48653. doi: 10.1074/jbc.M402516200. [DOI] [PubMed] [Google Scholar]
- Seno K, Kishimoto M, Abe M, Higuchi Y, Mieda M, Owada Y, Yoshiyama W, Liu H, Hayashi F. Light- and guanosine 5'-3-O-(thio)triphosphate-sensitive localization of a G protein and its effector on detergent-resistant membrane rafts in rod photoreceptor outer segments. J Biol Chem. 2001;276(24):20813–20816. doi: 10.1074/jbc.C100032200. [DOI] [PubMed] [Google Scholar]
- Shibukawa Y, Kang KJ, Kinjo TG, Szerencsei RT, Altimimi HF, Pratikhya P, Winkfein RJ, Schnetkamp PP. Structure-function relationships of the NCKX2 Na+/Ca2+-K+ exchanger. Ann N Y Acad Sci. 2007;1099:16–28. doi: 10.1196/annals.1387.054. [DOI] [PubMed] [Google Scholar]
- Shichida Y, Morizumi T. Mechanism of G-protein activation by rhodopsin. Photochem Photobiol. 2007;83(1):70–75. doi: 10.1562/2006-03-22-IR-854. [DOI] [PubMed] [Google Scholar]
- Shroyer NF, Lewis RA, Yatsenko AN, Wensel TG, Lupski JR. Cosegregation and functional analysis of mutant ABCR (ABCA4) alleles in families that manifest both Stargardt disease and age-related macular degeneration. Hum Mol Genet. 2001;10(23):2671–2678. doi: 10.1093/hmg/10.23.2671. [DOI] [PubMed] [Google Scholar]
- Skiba NP, Artemyev NO, Hamm HE. The carboxyl terminus of the gamma-subunit of rod cGMP phosphodiesterase contains distinct sites of interaction with the enzyme catalytic subunits and the alpha-subunit of transducin. J Biol Chem. 1995;270(22):13210–13215. doi: 10.1074/jbc.270.22.13210. [DOI] [PubMed] [Google Scholar]
- Slep KC, Kercher MA, He W, Cowan CW, Wensel TG, Sigler PB. Structural determinants for regulation of phosphodiesterase by a G protein at 2.0 A. Nature. 2001;409(6823):1071–1077. doi: 10.1038/35059138. [DOI] [PubMed] [Google Scholar]
- Slepak VZ, Artemyev NO, Zhu Y, Dumke CL, Sabacan L, Sondek J, Hamm HE, Bownds MD, Arshavsky VY. An effector site that stimulates G-protein GTPase in photoreceptors. J Biol Chem. 1995;270(24):14319–14324. doi: 10.1074/jbc.270.24.14319. [DOI] [PubMed] [Google Scholar]
- Sommer ME, Smith WC, Farrens DL. Dynamics of arrestin-rhodopsin interactions: acidic phospholipids enable binding of arrestin to purified rhodopsin in detergent. J Biol Chem. 2006;281(14):9407–9417. doi: 10.1074/jbc.M510037200. [DOI] [PubMed] [Google Scholar]
- Sowa ME, He W, Slep KC, Kercher MA, Lichtarge O, Wensel TG. Prediction and confirmation of a site critical for effector regulation of RGS domain activity. Nat Struct Biol. 2001;8(3):234–237. doi: 10.1038/84974. [DOI] [PubMed] [Google Scholar]
- Sprang SR. G protein mechanisms: insights from structural analysis. Annu Rev Biochem. 1997a;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- Sprang SR. G proteins, effectors and GAPs: structure and mechanism. Curr Opin Struct Biol. 1997b;7(6):849–856. doi: 10.1016/s0959-440x(97)80157-1. [DOI] [PubMed] [Google Scholar]
- Sprang SR. Conformational display: a role for switch polymorphism in the superfamily of regulatory GTPases. Sci STKE. 2000;2000(50) doi: 10.1126/stke.2000.50.pe1. PE1. [DOI] [PubMed] [Google Scholar]
- Sprang SR, Chen Z, Du X. Structural basis of effector regulation and signal termination in heterotrimeric Galpha proteins. Adv Protein Chem. 2007;74:1–65. doi: 10.1016/S0065-3233(07)74001-9. [DOI] [PubMed] [Google Scholar]
- Stephen R, Bereta G, Golczak M, Palczewski K, Sousa MC. Stabilizing function for myristoyl group revealed by the crystal structure of a neuronal calcium sensor, guanylate cyclase-activating protein 1. Structure. 2007;15(11):1392–1402. doi: 10.1016/j.str.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struppe J, Komives EA, Taylor SS, Vold RR. 2H NMR studies of a myristoylated peptide in neutral and acidic phospholipid bicelles. Biochemistry. 1998;37(44):15523–15527. doi: 10.1021/bi981326b. [DOI] [PubMed] [Google Scholar]
- Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem. 1999;274(12):8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- Sun H, Nathans J. Stargardt's ABCR is localized to the disc membrane of retinal rod outer segments. Nat Genet. 1997;17(1):15–16. doi: 10.1038/ng0997-15. [DOI] [PubMed] [Google Scholar]
- Tachibanaki S, Arinobu D, Shimauchi-Matsukawa Y, Tsushima S, Kawamura S. Highly effective phosphorylation by G protein-coupled receptor kinase 7 of light-activated visual pigment in cones. Proc Natl Acad Sci U S A. 2005;102(26):9329–9334. doi: 10.1073/pnas.0501875102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau MC, Zagotta WN. Mechanism of calcium/calmodulin inhibition of rod cyclic nucleotide-gated channels. Proc Natl Acad Sci U S A. 2002;99(12):8424–8429. doi: 10.1073/pnas.122015999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker CL, Woodcock SC, Kelsell RE, Ramamurthy V, Hunt DM, Hurley JB. Biochemical analysis of a dimerization domain mutation in RetGC-1 associated with dominant cone-rod dystrophy. Proc Natl Acad Sci U S A. 1999;96(16):9039–9044. doi: 10.1073/pnas.96.16.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Raman D, Wei J, Kennedy MJ, Hurley JB, Gurevich VV. Regulation of arrestin binding by rhodopsin phosphorylation level. J Biol Chem. 2007;282(44):32075–32083. doi: 10.1074/jbc.M706057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A, Schroder T, Lange C, Huster D. Characterization of the myristoyl lipid modification of membrane-bound GCAP-2 by 2H solid-state NMR spectroscopy. Biochim Biophys Acta. 2007;1768(12):3171–3181. doi: 10.1016/j.bbamem.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Wada Y, Sugiyama J, Okano T, Fukada Y. GRK1 and GRK7: unique cellular distribution and widely different activities of opsin phosphorylation in the zebrafish rods and cones. J Neurochem. 2006;98(3):824–837. doi: 10.1111/j.1471-4159.2006.03920.x. [DOI] [PubMed] [Google Scholar]
- Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB. Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron. 2002;36(5):881–889. doi: 10.1016/s0896-6273(02)01098-x. [DOI] [PubMed] [Google Scholar]
- Wensel TG, Stryer L. Reciprocal control of retinal rod cyclic GMP phosphodiesterase by its gamma subunit and transducin. Proteins. 1986;1(1):90–99. doi: 10.1002/prot.340010114. [DOI] [PubMed] [Google Scholar]
- Wey CL, Cone RA, Edidin MA. Lateral diffusion of rhodopsin in photoreceptor cells measured by fluorescence photobleaching and recovery. Biophys J. 1981;33(2):225–232. doi: 10.1016/S0006-3495(81)84883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiszniewski W, Zaremba CM, Yatsenko AN, Jamrich M, Wensel TG, Lewis RA, Lupski JR. ABCA4 mutations causing mislocalization are found frequently in patients with severe retinal dystrophies. Hum Mol Genet. 2005;14(19):2769–2778. doi: 10.1093/hmg/ddi310. [DOI] [PubMed] [Google Scholar]
- Womack KB, Gordon SE, He F, Wensel TG, Lu CC, Hilgemann DW. Do phosphatidylinositides modulate vertebrate phototransduction? J Neurosci. 2000;20(8):2792–2799. doi: 10.1523/JNEUROSCI.20-08-02792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley JD, Ahmed T, Nevett CL, Findlay JB. Peripherin/rds influences membrane vesicle morphology. Implications for retinopathies. J Biol Chem. 2000;275(18):13191–13194. doi: 10.1074/jbc.c900853199. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Li N, Bondarenko VA, Yamazaki RK, Baehr W, Yamazaki A. Binding of cGMP to GAF domains in amphibian rod photoreceptor cGMP phosphodiesterase (PDE). Identification of GAF domains in PDE alphabeta subunits and distinct domains in the PDE gamma subunit involved in stimulation of cGMP binding to GAF domains. J Biol Chem. 2002;277(43):40675–40686. doi: 10.1074/jbc.M203469200. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wensel TG. N-myristoylation of the rod outer segment G protein, transducin, in cultured retinas. J Biol Chem. 1992;267(32):23197–23201. [PubMed] [Google Scholar]
- Yu H, Olshevskaya E, Duda T, Seno K, Hayashi F, Sharma RK, Dizhoor AM, Yamazaki A. Activation of retinal guanylyl cyclase-1 by Ca2+-binding proteins involves its dimerization. J Biol Chem. 1999;274(22):15547–15555. doi: 10.1074/jbc.274.22.15547. [DOI] [PubMed] [Google Scholar]
- Zagotta WN, Olivier NB, Black KD, Young EC, Olson R, Gouaux E. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature. 2003;425(6954):200–205. doi: 10.1038/nature01922. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu XH, Zhang K, Chen CK, Frederick JM, Prestwich GD, Baehr W. Photoreceptor cGMP phosphodiesterase delta subunit (PDEdelta) functions as a prenyl-binding protein. J Biol Chem. 2004a;279(1):407–413. doi: 10.1074/jbc.M306559200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Melia TJ, He F, Yuan C, McGough A, Schmid MF, Wensel TG. How a G protein binds a membrane. J Biol Chem. 2004b;279(32):33937–33945. doi: 10.1074/jbc.M403404200. [DOI] [PubMed] [Google Scholar]
- Zheng J, Trudeau MC, Zagotta WN. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron. 2002;36(5):891–896. doi: 10.1016/s0896-6273(02)01099-1. [DOI] [PubMed] [Google Scholar]
- Zhong H, Molday LL, Molday RS, Yau KW. The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature. 2002;420(6912):193–198. doi: 10.1038/nature01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zozulya S, Stryer L. Calcium-myristoyl protein switch. Proc Natl Acad Sci U S A. 1992;89(23):11569–11573. doi: 10.1073/pnas.89.23.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]