Abstract
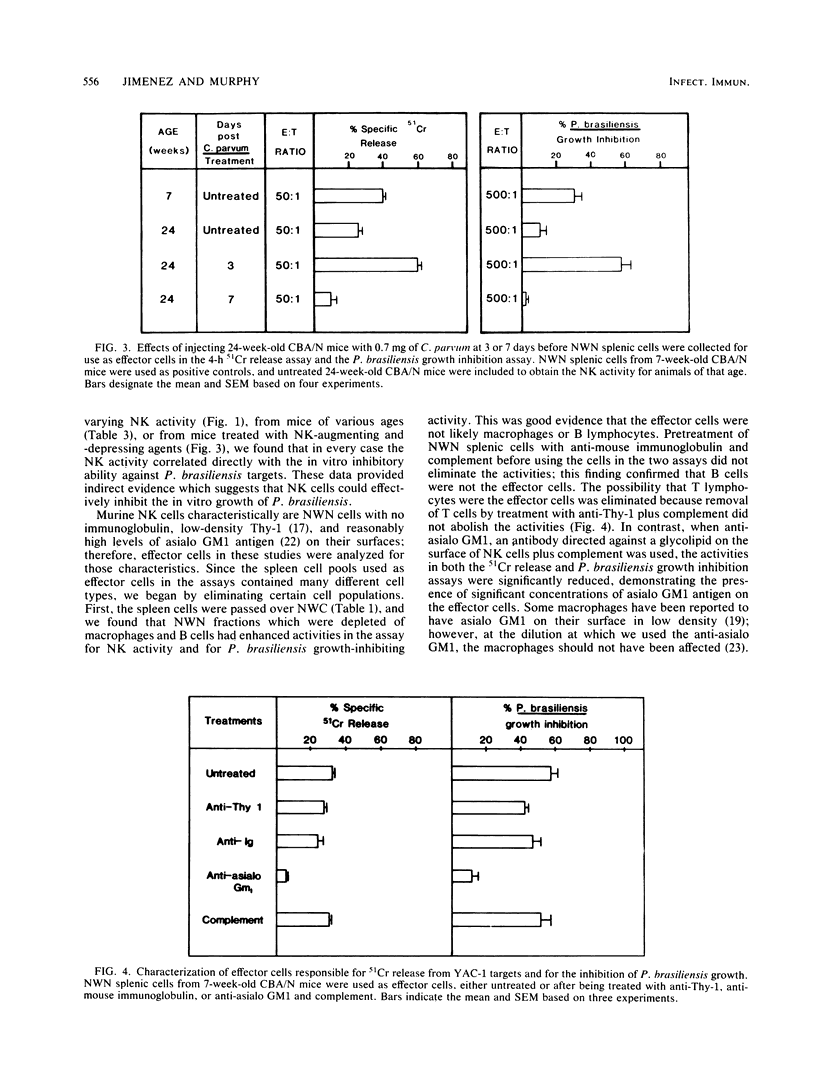
Recently, data have been reported suggesting natural killer (NK) cells may function in natural resistance against a fungus, Cryptococcus neoformans. The primary objective of this study was to examine the reactivity of murine splenic cells against another fungus, Paracoccidioides brasiliensis. Levels of NK activity in effector cell pools were varied by: (i) removing nylon wool-adherent cells, (ii) fractionating splenic cells on Percoll discontinuous gradients, (iii) using old and young effector cell donor mice, (iv) using donors from different strains, and (v) pretreating donors with NK-augmenting and -depressing agents. The various effector cell pools were simultaneously used in the 4-h 51Cr release assay with YAC-1 targets to determine the NK reactivity and in the in vitro growth inhibition assay against P. brasiliensis yeast phase targets. In each case, the level of NK reactivity correlated with the ability of the effector cells to inhibit the in vitro growth of P. brasiliensis. NK activity and P. brasiliensis growth-inhibiting ability could be augmented by fractionation of splenic cells through nylon wool or Percoll gradients. The effector cells responsible for the NK activity and P. brasiliensis growth inhibition were characterized as being nylon wool nonadherent, being found in the low-density fractions from Percoll discontinuous gradients, and having no detectable Thy-1 antigen or immunoglobulin but having asialo GM1 on their surface. These data support the contention that NK or NK-like cells are responsible for limiting the in vitro growth of P. brasiliensis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastardo de Albornoz M. C., Albornoz R. Estudio de la sensibilidad especifica en residentes de un area endemica a la paracoccidioidomicosis en Venezuela. Mycopathol Mycol Appl. 1971 Oct 13;45(2):65–75. doi: 10.1007/BF02059246. [DOI] [PubMed] [Google Scholar]
- Bistoni F., Baccarini M., Blasi E., Marconi P., Puccetti P., Garaci E. Correlation between in vivo and in vitro studies of modulation of resistance to experimental Candida albicans infection by cyclophosphamide in mice. Infect Immun. 1983 Apr;40(1):46–55. doi: 10.1128/iai.40.1.46-55.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Albornoz M. B. Isolation of Paracoccidioides brasiliensis from rural soil in Venezuela. Sabouraudia. 1971 Nov;9(3):248–253. [PubMed] [Google Scholar]
- Fava Netto C. Imunologia da paracoccidioidomicose. Rev Inst Med Trop Sao Paulo. 1976 Jan-Feb;18(1):42–53. [PubMed] [Google Scholar]
- Giraldo R., Restrepo A., Gutiérrez F., Robledo M., Londoño F., Hernández H., Sierra F., Calle G. Pathogenesis of paracoccidioidomycosis: a model based on the study of 46 patients. Mycopathologia. 1976 Jun 18;58(2):63–70. doi: 10.1007/BF00707174. [DOI] [PubMed] [Google Scholar]
- Glimcher L., Shen F. W., Cantor H. Identification of a cell-surface antigen selectively expressed on the natural killer cell. J Exp Med. 1977 Jan 1;145(1):1–9. doi: 10.1084/jem.145.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goihman-Yahr M., Essenfeld-Yahr E., Albornoz M. C., Yarzábal L., de Gómez M. H., San Martín B., Ocanto A., Convit J. New method for estimating digestion of Paracoccidioides brasiliensis by phagocytic cells in vitro. J Clin Microbiol. 1979 Sep;10(3):365–370. doi: 10.1128/jcm.10.3.365-370.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goihman-Yahr M., Essenfeld-Yahr E., de Albornoz M. C., Yarzábal L., de Gómez M. H., San Martín B., Ocanto A., Gil F., Convit J. Defect of in vitro digestive ability of polymorphonuclear leukocytes in paracoccidioidomycosis. Infect Immun. 1980 May;28(2):557–566. doi: 10.1128/iai.28.2.557-566.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna N. Inhibition of experimental tumor metastasis by selective activation of natural killer cells. Cancer Res. 1982 Apr;42(4):1337–1342. [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E. Destruction of Trypanosoma cruzi by Natural killer cells. Science. 1982 Oct 15;218(4569):295–296. doi: 10.1126/science.6812218. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975 Aug 15;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Iabuki K., Montenegro M. R. Experimental paracoccidioidomycosis in the Syrian hamster: morphology, ultrastructure and correlation of lesions with presence of specific antigens and serum levels of antibodies. Mycopathologia. 1979 Jul 16;67(3):131–141. doi: 10.1007/BF00470745. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kasai M., Iwamori M., Nagai Y., Okumura K., Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980 Mar;10(3):175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- Keller R., Bächi T., Okumura K. Discrimination between macrophage-and NK-type tumoricidal activities via anti-asialo GM1 antibody. Exp Cell Biol. 1983;51(3):158–164. [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Koo G. C., Jacobson J. B., Hammerling G. J., Hammerling U. Antigenic profile of murine natural killer cells. J Immunol. 1980 Sep;125(3):1003–1006. [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Suzuki R., Hinuma S., Saitoh F. Studies of murine large granular lymphocytes. I. Identification as effector cells in NK and K cytotoxicities. J Immunol. 1982 Jul;129(1):388–394. [PubMed] [Google Scholar]
- Linares L. I., Friedman L. Experimental paracoccidioidomycosis in mice. Infect Immun. 1972 May;5(5):681–687. doi: 10.1128/iai.5.5.681-687.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini W., Boraschi D., Alberti S., Aleotti A., Tagliabue A. Morphological characterization of a cell population responsible for natural killer activity. Immunology. 1981 Aug;43(4):663–668. [PMC free article] [PubMed] [Google Scholar]
- Mok P. W., Greer D. L. Cell-mediated immune responses in patients with paracoccidioidomycosis. Clin Exp Immunol. 1977 Apr;28(1):89–98. [PMC free article] [PubMed] [Google Scholar]
- Moorhead J. W. Tolerance and contact sensitivity to DNFA in mice. VIII. Identification of distinct T cell subpopulations that mediate in vivo and in vitro manifestations of delayed hypersensitivity. J Immunol. 1978 Jan;120(1):137–144. [PubMed] [Google Scholar]
- Moorhead J. W., Walters C. S., Claman H. N. Immunologic reactions to haptens on autologous carriers. I. Participation of both thymus-derived and bone marrow-derived cells in the secondary in vitro response. J Exp Med. 1973 Feb 1;137(2):411–423. doi: 10.1084/jem.137.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Murphy J. W., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982 Jan;128(1):276–283. [PubMed] [Google Scholar]
- Musatti C. C., Rezkallah M. T., Mendes E., Mendes N. F. In vivo and in vitro evaluation of cell-mediated immunity in patients with paracoccidiodomycosis. Cell Immunol. 1976 Jun 15;24(2):365–378. doi: 10.1016/0008-8749(76)90220-3. [DOI] [PubMed] [Google Scholar]
- Negroni P. El "paracoccidioides brasiliensis" vive saprofíticamente en el suelo argentino. Prensa Med Argent. 1966 Sep 30;53(39):2381–2382. [PubMed] [Google Scholar]
- Nencioni L., Villa L., Boraschi D., Berti B., Tagliabue A. Natural and antibody-dependent cell-mediated activity against Salmonella typhimurium by peripheral and intestinal lymphoid cells in mice. J Immunol. 1983 Feb;130(2):903–907. [PubMed] [Google Scholar]
- Phanuphak P., Moorhead J. W., Claman H. N. Tolerance and contact sensitivity to DNFB in mice. I. In vivo detection by ear swelling and correlation with in vitro cell stimulation. J Immunol. 1974 Jan;112(1):115–123. [PubMed] [Google Scholar]
- Puccetti P., Santoni A., Riccardi C., Herberman R. B. Cytotoxic effector cells with the characteristics of natural killer cells in the lungs of mice. Int J Cancer. 1980 Jan 15;25(1):153–158. doi: 10.1002/ijc.2910250121. [DOI] [PubMed] [Google Scholar]
- Restrepo A. A reappraisal of the microscopical appearance of the mycelial phase of Paracoccidioides brasiliensis. Sabouraudia. 1970 Aug;8(2):141–144. doi: 10.1080/00362177085190771. [DOI] [PubMed] [Google Scholar]
- Restrepo A., Jiménez B. E. Growth of Paracoccidioides brasiliensis yeast phase in a chemically defined culture medium. J Clin Microbiol. 1980 Aug;12(2):279–281. doi: 10.1128/jcm.12.2.279-281.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo A., Moncada L. H., Quintero M. Effect of hydrogen ion concentration and of temperature on the growth of Paracoccidioides brasiliensis in soil extracts. Sabouraudia. 1969 Oct;7(3):207–215. doi: 10.1080/00362177085190371. [DOI] [PubMed] [Google Scholar]
- Restrepo A., Restrepo M., de Restrepo F., Aristizábal L. H., Moncada L. H., Vélez H. Immune responses in paracoccidioidomycosis. A controlled study of 16 patients before and after treatment. Sabouraudia. 1978 Jun;16(2):151–163. [PubMed] [Google Scholar]
- Restrepo A., Robledo M., Ospina S., Restrepo M., Correa A. Distribution of paracoccidioidin sensitivity in Colombia. Am J Trop Med Hyg. 1968 Jan;17(1):25–37. [PubMed] [Google Scholar]
- Restrepo A., Vélez H. Efectos de la fagocitosis in vitro sobre el Paracoccidioides brasiliensis. Sabouraudia. 1975 Mar;13(Pt 1):10–21. [PubMed] [Google Scholar]
- Reynolds C. W., Timonen T., Herberman R. B. Natural killer (NK) cell activity in the rat. I. Isolation and characterization of the effector cells. J Immunol. 1981 Jul;127(1):282–287. [PubMed] [Google Scholar]
- Savary C. A., Lotzová E. Suppression of natural killer cell cytotoxicity by splenocytes from Corynebacterium parvum-injected, bone marrow-tolerant, and infant mice. J Immunol. 1978 Jan;120(1):239–243. [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R., Devens B. H., Raff H. V., Walford R. L. Influence of dietary restriction and aging on natural killer cell activity in mice. J Immunol. 1983 Feb;130(2):993–996. [PubMed] [Google Scholar]


