Abstract
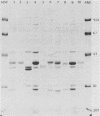
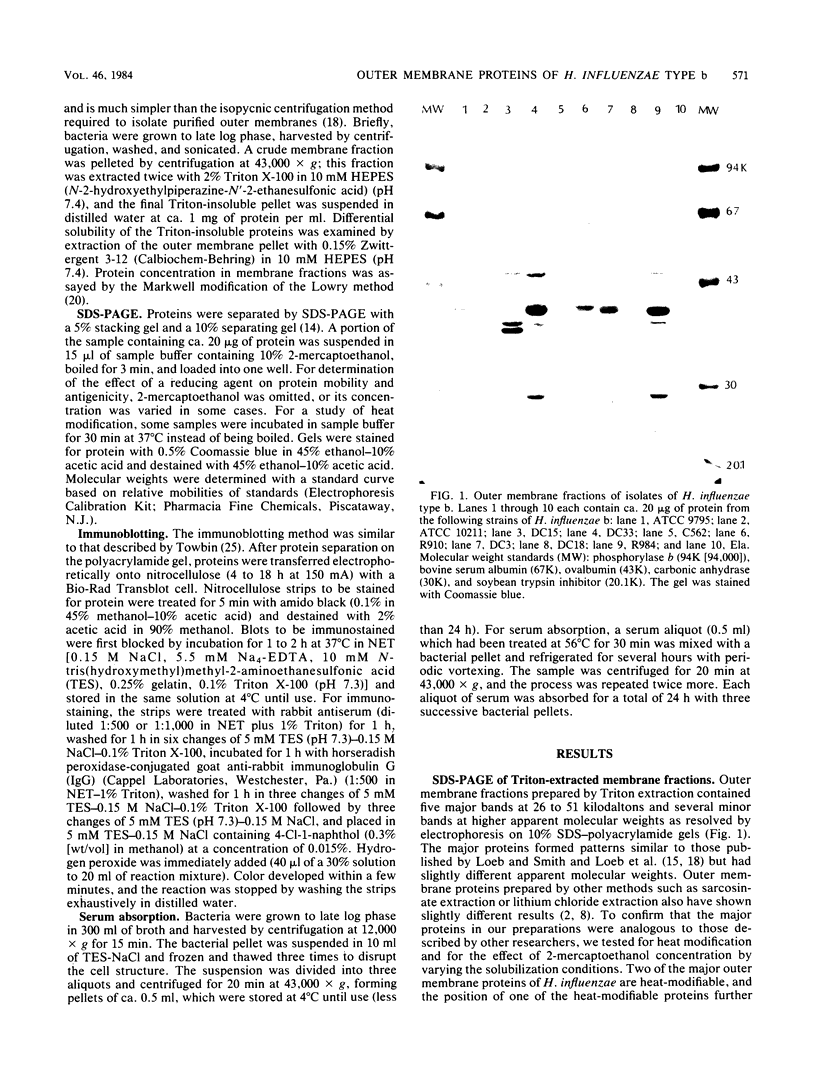
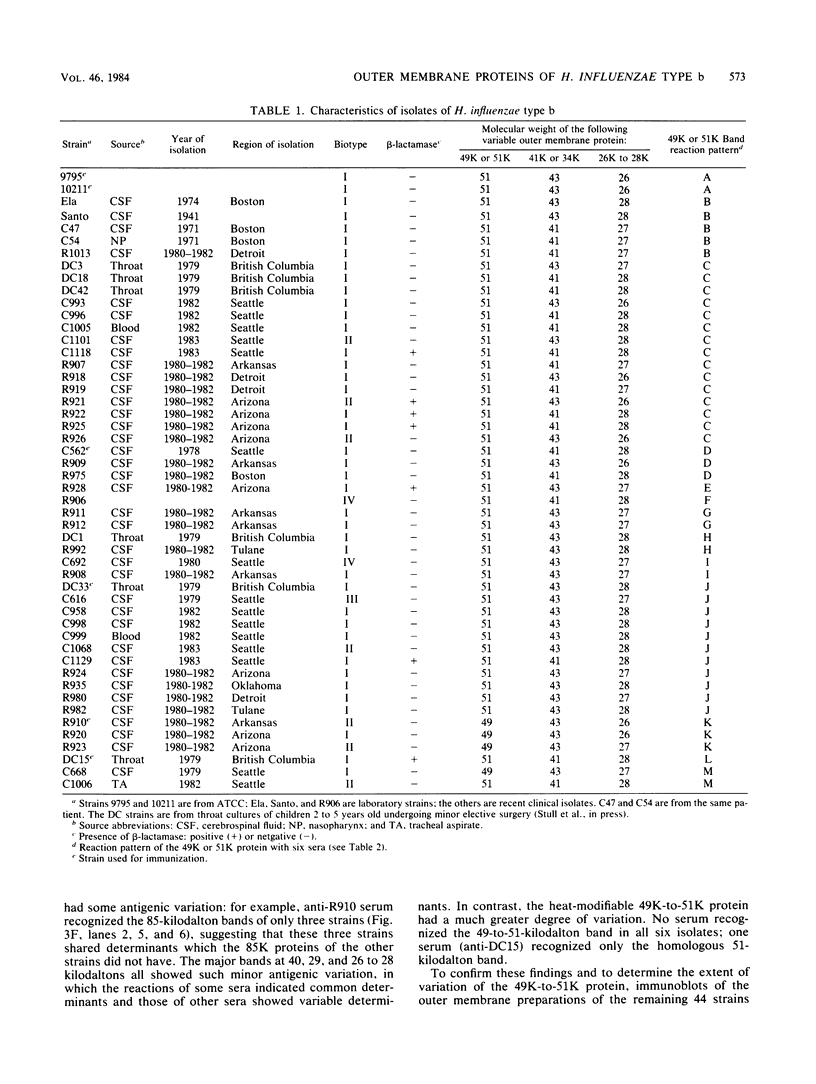
Antigenic variation of the outer membrane proteins among isolates of Haemophilus influenzae was examined by immunoblotting. Rabbit antisera were raised against six strains of H. influenzae type b and tested against outer membrane preparations of 50 isolates. The principal outer membrane band was not reactive on immunoblotting, so its antigenic heterogeneity could not be examined. Most of the other outer membrane proteins shared common determinants among all strains tested. Absorption of serum with heterologous bacteria removed antibody to nearly all proteins, confirming the extensive cross-reactivity among isolates. The greatest antigenic variation was seen in one major outer membrane band, a heat-modifiable, Zwittergent-soluble protein with a molecular weight of 49,000 to 51,000. One antiserum reacted with the 49,000-to-51,000-molecular-weight protein of the homologous isolate only; the remaining five antisera showed differing patterns of reactivity with heterologous 49,000-to-51,000-molecular-weight proteins. We were able to divide the 50 H. influenzae isolates into 13 antigenic groups based on their reaction patterns. The antigenic groupings may provide an epidemiological tool for studying the prevalence and transmission of strains of H. influenzae type b.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Flesher A., Shaw S., Harding A. L., Smith D. H. Phenotypic and genetic variation in the susceptibility of Haemophilus influenzae type b to antibodies to somatic antigens. J Clin Invest. 1980 Apr;65(4):885–891. doi: 10.1172/JCI109741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Burans J. P., Lynn M., Solotorovsky M. Induction of active immunity with membrane fractions from Haemophilus influenzae type b. Infect Immun. 1983 Jul;41(1):285–293. doi: 10.1128/iai.41.1.285-293.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filice G. A., Andrews J. S., Jr, Hudgins M. P., Fraser D. W. Spread of Haemophilus influenzae. Secondary illness in household contacts of patients with H influenzae meningitis. Am J Dis Child. 1978 Aug;132(8):757–759. [PubMed] [Google Scholar]
- Gulig P. A., Frisch C. F., Hansen E. J. A set of two monoclonal antibodies specific for the cell surface-exposed 39K major outer membrane protein of Haemophilus influenzae type b defines all strains of this pathogen. Infect Immun. 1983 Nov;42(2):516–524. doi: 10.1128/iai.42.2.516-524.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Robertson S. M., Gulig P. A., Frisch C. F., Haanes E. J. Immunoprotection of rats against Haemophilus influenzae type B disease mediated by monoclonal antibody against a haemophilus outer-membrane protein. Lancet. 1982 Feb 13;1(8268):366–368. doi: 10.1016/s0140-6736(82)91394-0. [DOI] [PubMed] [Google Scholar]
- Inzana T. J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983 Sep;148(3):492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- Kenny G. E. Heat-lability and organic solvent-solubility of mycoplasma antigens. Ann N Y Acad Sci. 1967 Jul 28;143(1):676–681. doi: 10.1111/j.1749-6632.1967.tb27713.x. [DOI] [PubMed] [Google Scholar]
- Kenny G. E. Immunogenicity of Mycoplasma pneumoniae. Infect Immun. 1971 Apr;3(4):510–515. doi: 10.1128/iai.3.4.510-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Human antibody response to individual outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1982 Sep;37(3):1032–1036. doi: 10.1128/iai.37.3.1032-1036.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Zachary A. L., Smith D. H. Isolation and partial characterization of outer and inner membranes from encapsulated Haemophilus influenzae type b. J Bacteriol. 1981 Jan;145(1):596–604. doi: 10.1128/jb.145.1.596-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Granoff D. M. Human antibody responses to lipopolysaccharide after meningitis due to Haemophilus influenzae type b. J Infect Dis. 1982 Feb;145(2):181–190. doi: 10.1093/infdis/145.2.181. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Zanen H. C. Characteristics of major outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Aug;155(2):878–885. doi: 10.1128/jb.155.2.878-885.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]