Abstract
In addition to the well-characterized GTP-dependent nuclear transport observed in permeabilized cells, we detected a mode of nuclear transport that was GTP-independent at elevated cytoplasmic calcium concentrations. Nuclear transport under these conditions was blocked by calmodulin inhibitors. Recombinant calmodulin restored ATP-dependent nuclear transport in the absence of cytosol. Calmodulin-dependent transport was inhibited by wheat germ agglutinin consistent with transport proceeding through nuclear pores. We propose that release of intracellular calcium stores upon cell activation inhibits GTP-dependent nuclear transport; the elevated cytosolic calcium then acts through calmodulin to stimulate the novel GTP-independent mode of import.
Protein transport across the nuclear pore is a highly regulated process that is just beginning to be understood (1, 2). Proteins destined for the nucleus contain a basic sequence of amino acids called a nuclear localization sequence (NLS; ref. 3). The NLS is recognized and bound in the cytoplasm by karyopherin-α,† which forms a complex with karyopherin-β and routes the substrate to the nuclear pore (reviewed in refs. 17 and 18). The substrate-karyopherin complex binds to RanBP2, a 360-kDa protein component of fibrils that extend from the surface of the nuclear pore into the cytoplasm of the cell. Ran/TC4 (a small GTP-binding protein of the Ras superfamily) and p10 bind to the transport complex, and the complex is delivered to the center of the pore in a mechanism that appears to involve GTP hydrolysis. At present, the mechanism of transit across the pore itself is unclear but it is known to require nucleotide triphosphate and is sensitive to incubation at 4°C. Soluble components of the transport machinery are then recycled to the cytoplasm for a new round of transport.
Indirect evidence suggests that the nuclear envelope must remain intact for active nuclear transport (19). In addition to the functional barrier that the nuclear envelope provides, recent evidence suggests that the calcium pools in the lumen of the nuclear envelope must also be intact for nuclear transport to occur. Greber and Gerace (20) have observed a requirement for intact intracellular calcium stores for active nuclear transport, as well as passive diffusion of 10-kDa dextrans across the pore. Nuclear import of microinjected fluorescent substrates in a low calcium buffer is reduced by pre-incubation of the cells with thapsigargin, which causes the release of calcium from intracellular stores. Stehno-Bittel et al. (21) also observed that there is an inhibition of diffusion of 10-kDa dextrans across the pore when isolated Xenopus laevis nuclei are treated with inositol trisphosphate to release calcium stores from the lumen of the nuclear envelope. In the only structural study of the effect of calcium on the nuclear pore, Jarnik and Aebi (22) found that the removal of calcium by chelation with EGTA led to an “open” appearance of the nuclear pores of X. laevis germinal vesicles as assessed by electron microscopy. The pores were restored to their “closed” appearance with intact “fish-baskets” when calcium was reintroduced to the germinal vesicles. The functional significance of this structural change is unknown.
We have used the digitonin-permeabilized cell system to demonstrate the involvement of calcium in nuclear transport and have found at least two distinct modes by which calcium can activate nuclear protein import. One mode of activation is GTP-dependent and requires intact intracellular calcium stores; a novel second mode of activation requires elevated cytoplasmic calcium and is mediated by calmodulin.
MATERIALS AND METHODS
Assay for Nuclear Import.
The import assay was modified from refs. 23–25. To initiate the assay, HeLa cells (ATCC CCL2) grown on 12-mm round coverslips were washed three times with 1 ml ice-cold buffer A [20 mM N-2-hydroxyethyl piperazine-N′-2-ethanesulfonic acid (Hepes), pH 7.3/110 mM potassium acetate/5 mM sodium acetate/2 mM magnesium acetate/2 mM dithiothreitol/1 μg/ml leupeptin/1 μg/ml aprotinin/1 μg/ml pepstatin] and permeabilized for 5 min in buffer A containing 40 μg/ml digitonin (Calbiochem) at 25°C. Under the conditions described here, more than 60% of the endogenous lactate dehydrogenase activity was released in the first 5 min of digitonin treatment (data not shown). The permeabilized cells were washed gently four times with 1 ml cold buffer A and mounted “face-up” in a humidified chamber pre-warmed to 37°C. Twenty-five microliters of complete import buffer was placed on each coverslip and incubated for 30 min at 37°C. Complete import buffer consisted of buffer A containing 20 units/ml creatine kinase, 1 mM magnesium ATP, 0.5 mM EGTA, 5 mM creatine phosphate, 100 nM B-phycoerythrin-peptide conjugate, and 1% untreated rabbit reticulocyte lysate (Promega). The cells were then fixed in buffer A containing 6.7% formaldehyde (EM grade 20%, Ladd Research Industries, Burlington, VT) at 25°C, mounted on 5 μl of 0.1% p-phenylenediamine/90% glycerol in phosphate-buffered saline (PBS) (Digene Diagnostics, Silver Spring, MD), and viewed immediately.
Image Analysis and Quantitation.
Microscopy was performed with either a Zeiss IM microscope or a Zeiss Axiovert 100TV microscope mounted with an MTI CCD72 video camera and an MTI GenIISys Image Intensifier. All images were captured with a 63× oil immersion objective, unless otherwise noted. Image analysis was performed on a Macintosh PowerPC 8100/80av computer using the public domain nih image program [developed at the National Institutes of Health and available from the Internet by anonymous file transfer protocol from zippy.nimh.nih.gov or on floppy disk from the National Technical Information Service (Springfield, VA), part number PB95-500195GEI]. To obtain relative nuclear import, the mean pixel intensity of the extracellular space was subtracted from the mean intensity of experimental nuclei. Background nuclear fluorescence (no calcium and no GTP added, unless otherwise stated) was subtracted and the values were normalized to positive controls (79 μM calcium and 1 mM GTP added, unless otherwise stated). At least 25 nuclei were measured for each experimental data point. The least-squares curve fit was performed using the kaleidagraph software package (Abelbeck Software) with the equation y = (axn/(b + xn)) + c, where y = relative nuclear import, x = −log[Ca2+]free, and the parameters a, b, and c are determined by the algorithm to yield the best fit. The parameter n was fixed at 8 for all plots that gave the best fit for the data.
Preparation of Cytosol Fractions.
For depletion of calcium, the untreated rabbit reticulocyte lysate was passed twice over a G-50 size-exclusion column (PD-10; Pharmacia) equilibrated in calcium- and magnesium-free PBS (Digene Diagnostics, Reading PA), and the excluded volume was concentrated to its original volume in a Centricon-10 microconcentrator (Amicon). The subsequent extract was used in the assay at a 100-fold dilution and calcium chloride was added to the transport buffer to a final free calcium concentration of 9.2 μM, unless otherwise noted. If GTP and calcium are not added back to the assay, the free calcium and GTP concentrations are estimated to be less than 1 nM and 0.14 μM, respectively (data not shown). Free calcium concentration was estimated by the algorithm of Fabiato and Fabiato (26) with a standard curve prepared with FURA-2 under the relevant assay conditions, as described (27).
RESULTS
GTP-Independent, Calcium-Stimulated Nuclear Transport.
Peptides corresponding to the NLS of the simian virus 40 large T antigen (28, 29) were covalently coupled to the highly fluorescent algal protein, B-phycoerythrin, to produce a nuclear-targeted reporter protein (30). In agreement with previous reports (23–25), the synthetic conjugate was specifically accumulated in the nuclei of digitonin-permeabilized HeLa, NRK, NIH 3T3, COS-1, and CV-1 cells in a manner that was dependent upon the addition of ATP, cytosol (rabbit reticulocyte lysate), and an active targeting peptide. The transport was inhibited by incubation at 4°C or by the presence of wheat germ agglutinin (data not shown). Furthermore, the assay supported the transport of B-phycoerythrin bearing either a nuclear targeting signal or the nucleolar targeting signal of the HIV-1 rev protein (ref. 31; unpublished work).
To examine the role of small molecules in nuclear transport, we fractionated rabbit reticulocyte lysate by G-50 size-exclusion chromatography. The resulting depleted extract supported nuclear transport when GTP and calcium were added back to the assay (Fig. 1A). At concentrations of calcium less than 1 nM, little transport was observed whether or not GTP was added. At calcium concentrations around 0.2 μM, transport was markedly stimulated by the presence of GTP. However, at calcium concentrations above 1 μM, nuclear transport was observed either in the presence or absence of GTP. Titration of the free calcium into the assay showed a concentration dependence on calcium with an effective half-maximal concentration (EC50) of 0.90 μM (Fig. 1B, ○). Addition of 1 mM GTP to the assay shifted the EC50 for calcium to 40 nM (Fig. 1B, •), suggesting an interdependence between the calcium-dependent and GTP-dependent steps of nuclear transport. Addition of 5 mM guanosine 5′-o-(3-thiotriphosphate) (GTPγS) to the assay to compete with any residual GTP had no effect upon the ability of the depleted extract to support nuclear transport in the presence of elevated calcium concentrations (Fig. 1B, □).
Figure 1.
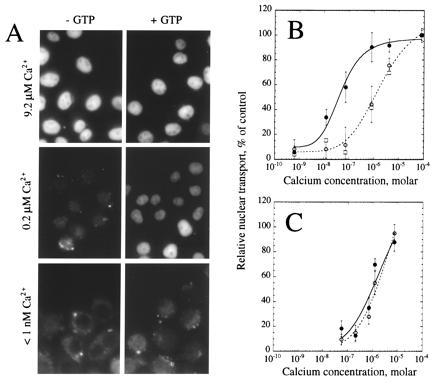
Calcium stimulation of nuclear transport in vitro. Nuclear transport in digitonin-permeabilized HeLa cells was assayed in the presence of a partially fractionated rabbit reticulocyte lysate. (A) Cells were assayed in the absence (Left) or presence (Right) of 1 mM GTP at buffered final free calcium concentrations of 9.2 μM (Top), 0.2 μM (Middle), or less than 1 nM (Bottom). (B) Relative nuclear import was quantified and normalized to control incubations containing 1 mM GTP and 79 μM calcium chloride. Assays were performed in the absence (○, broken line) or presence (•, solid line) of 1 mM GTP, or in the presence of 5 mM GTPγS (□). The data represent the average ± standard deviation (SD) of five (○ and •) or two (□; error bars not included on graph) independent experiments. (C) Prior to assay for nuclear import, thapsigargin was added to the medium on the cells to a final concentration of 10 μM and the cells were incubated for 10 min at 37°C. Thapsigargin was included in all subsequent incubations and washes at a final concentration of 10 μM. Cells were assayed for their ability to support nuclear import in the absence (○, broken line) or presence (•, solid line) of 1 mM GTP at varying calcium concentrations. The data represent the average ± SD of two independent experiments.
Thapsigargin-Sensitive GTP-Dependent Transport.
It has previously been reported that thapsigargin treatment inhibits nuclear transport when cytosolic calcium is buffered to 0.1 μM, presumably by depleting intracellular calcium stores (20). A titration of calcium into the assay in the presence of thapsigargin (Fig. 1C), both with (•) and without (○) GTP suggested that the GTP-dependent transport was inhibited by this agent. At calcium concentrations near 0.1 μM, total inhibition could be achieved with 10 μM thapsigargin (compare Fig. 1 B with C, •), consistent with the observations of Greber and Gerace (20). However, at higher calcium levels, transport occurred in a GTP-independent fashion, even in the presence of thapsigargin.
Inhibition by Calmodulin Antagonists.
Calcium-stimulated nuclear transport in the absence of added GTP or in the presence of thapsigargin required the addition of cytosol, which indicated that a cytoplasmic calcium-binding factor was stimulating nuclear transport. Therefore, we investigated the effects of antagonists of several modulators of cytoplasmic calcium metabolism on nuclear transport in vitro. Several antagonists of calcium metabolism [ryanodine, protopine, cyclopiazonic acid, 1-(β-(3-(4-methoxyphenyl)propoxy)-4-methoxyphenethyl)-1H-imidazole (SKF-96365), and 2,5-di-(t-butyl)-1,4-hydroquinone (BHQ)] had no apparent effect on nuclear transport in the assay (data not shown). Calmodulin antagonists, however, were effective at inhibiting nuclear transport in the assay (Table 1). The compounds W-7 and W-13 were significantly more effective at inhibiting nuclear transport than W-5 and W-12, respectively, consistent with the 5-fold higher binding affinity that these compounds have for calmodulin (32). A peptide corresponding to the calmodulin-binding domain of the plasma membrane calcium pump (calmodulin-binding domain; ref. 33) was also a potent inhibitor of nuclear transport, indicating that the effect of these inhibitors was likely to be specific for calmodulin. Interestingly, the inhibition of nuclear transport by calmodulin antagonists was reduced or not observed if 1 mM GTP was added to the assay (Table 1).
Table 1.
Inhibition of nuclear transport by calmodulin antagonists
| Antagonist | Relative transport (% of control)
|
|
|---|---|---|
| −GTP | +1 mM GTP | |
| Calmidazolium | −37.6 (10.5) | 118.0 (17.1) |
| Trifluoperazine | 19.2 (16.0) | 130.0 (17.4) |
| Mellitin | −41.3 (5.4) | 68.1 (14.0) |
| W-5 | 75.9 (15.2) | 118.3 (16.8) |
| W-7 | 15.8 (10.6) | 101.8 (34.1) |
| W-12 | 70.4 (21.2) | 117.9 (15.7) |
| W-13 | 9.9 (9.0) | 117.3 (25.3) |
| Calmodulin binding domain | 6.7 (4.3) | ND* |
Antagonists were added to a final concentration of 30 μM to assay incubations containing the depleted rabbit reticulocyte lysate, 79 μM calcium chloride, and either none or 1 mM GTP. Relative nuclear transport was estimated as described in Materials and Methods with the exceptions that a 4°C incubation was used as the negative control, and cytoplasmic fluorescence was subtracted from nuclear fluorescence. Thus, a negative value represents an increased cytoplasmic fluorescence relative to the negative control (4°C incubation). Data represent the mean (SD) for two independent experiments. *ND, no data.
Calmodulin-Activated Nuclear Transport.
Because the inhibitor profile suggested that calmodulin might play a role in nuclear transport, a highly purified recombinant bovine calmodulin was tested in the assay. The recombinant calmodulin fully restored nuclear transport to the assay in the absence of added cytosol (Fig. 2A). Accumulation of substrate into the nucleus was linear with time for up to 60 min (data not shown). Calmodulin-dependent transport was not observed in the absence of ATP (Fig. 2B), in the presence of wheat germ agglutinin (Fig. 2C), or when transport was assayed at 4°C (Fig. 2D). The observed import did not appear to represent opening of a simple diffusion channel at the pore, as the calmodulin-dependent import still required ATP and was cold-sensitive. Furthermore, dextrans of varying sizes did not show any increased permeability in the assay in the presence of recombinant calmodulin (data not shown). Transport did not occur when a B-phycoerythrin conjugate bearing a mutated NLS (29) was added (Fig. 2E), or when calmodulin was omitted from the assay (Fig. 2F). A titration of calcium into the assay in the presence of recombinant calmodulin and in the absence of added GTP mirrors the findings observed with depleted cytosol (Fig. 2G, ○). The addition of 1 mM GTP (Fig. 2G, •) or 5 mM GTPγS (Fig. 2G, □) to the assay had no effect upon the sensitivity of transport to calcium, in contrast to the effect of GTP seen with the cytosolic extract. To assess whether the observed transport effect was specific for calmodulin, we assayed nuclear transport in the presence of other “EF-hand” calcium-binding proteins. Addition of S100, parvalbumin, or troponin C to the assay did not support nuclear transport in the presence of micromolar concentrations of calcium (data not shown).
Figure 2.
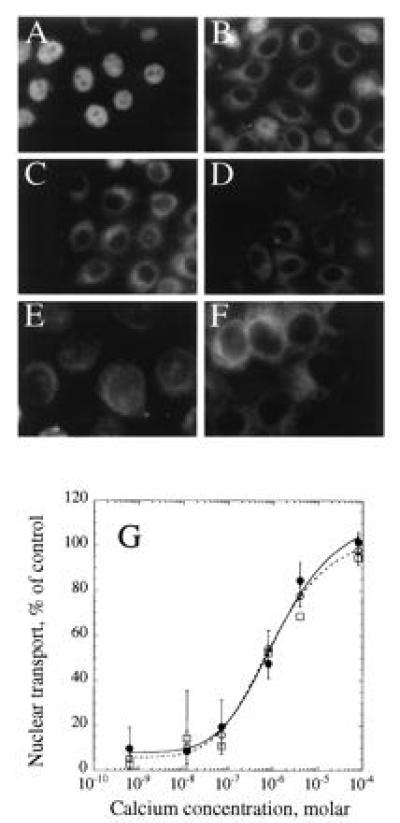
Calcium-dependent calmodulin stimulation of active nuclear import in vitro. HeLa cells were permeabilized with digitonin and assayed for nuclear transport in the absence of rabbit reticulocyte lysate, but in the presence of 30 μM calmodulin (Calbiochem, high purity) and 9.2 μM calcium (A). Incubations were also carried out in the absence of creatine kinase, creatine phosphate, or ATP, but in the presence of 6 units/μl hexokinase and 6 mM glucose (B), in the presence of 0.4 mg/ml wheat germ agglutinin (C), at 4°C (D), with B-phycoerythrin bearing an altered targeting peptide that is not sufficient to support transport to the nucleus (ref. 17, E), or in the absence of added calmodulin (F). Nuclear import was assayed at varying calcium concentrations (G) in the presence of 30 μM calmodulin and ATP in the absence (○, broken line) or presence (•, solid line) of 1 mM GTP, or in the presence of 5 mM GTPγS (□). Data represent the mean ± SD (not included for assays in the presence of GTPγS) of two independent experiments. Images in E and F were obtained with an 100× oil immersion objective; all other images were collected with a 63× objective.
Two Roles of Calcium in Nuclear Transport.
The data strongly suggested that calcium could activate nuclear transport in two distinct ways: one involving GTP and another involving cytoplasmic calcium and calmodulin. To test this model we selectively inhibited the calmodulin-dependent nuclear transport and titrated calcium into the assay in the absence and presence of GTP (Fig. 3, ○ and •, respectively). Calmidazolium inhibited nuclear transport in the absence of GTP, but had no observed effect upon transport in the presence of GTP, consistent with the hypothesis that the GTP-dependent transport does not use calmodulin and, therefore, is not sensitive to calmodulin inhibitors. We had previously observed that titration of calcium into the assay in thapsigargin-treated cells showed a loss of GTP-stimulated transport with an EC50 in the micromolar range with or without GTP present, consistent with a calmodulin-dependent mode of activation (Fig. 1C). Finally, titration of calcium into the assay in thapsigargin-treated cells in the presence of calmidazolium showed no nuclear transport in the absence or presence of GTP (Fig. 3, □ and ▪, respectively), consistent with the existence of two calcium-dependent modes of activation of nuclear protein import.
Figure 3.
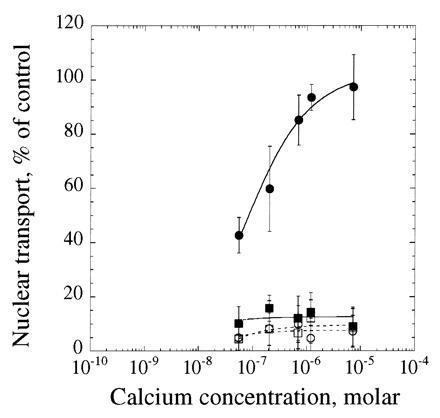
Inhibition of both calcium-dependent modes of activation of nuclear transport. Cells were pretreated without (circles) or with (squares) 10 μM thapsigargin as described in Fig. 1 and assayed in the presence of 30 μM calmidazolium and the depleted rabbit reticulocyte lysate. Samples were assayed for the ability to support nuclear transport in the absence (open symbols, broken lines) or presence (closed symbols, solid lines) of 1 mM GTP at various calcium concentrations. The data represent the mean ± SD of two independent experiments.
DISCUSSION
Model for Activation of Nuclear Transport.
The data are consistent with a model in which two distinct calcium-dependent modes of activation of nuclear transport exist within mammalian cells. The first mode of transport is GTP-dependent and requires calcium in the lumen of the nuclear envelope. The second mode of transport occurs when the intracellular stores of calcium are depleted and free cytoplasmic (or nucleoplasmic) calcium is elevated, activating calmodulin (Fig. 4).
Figure 4.
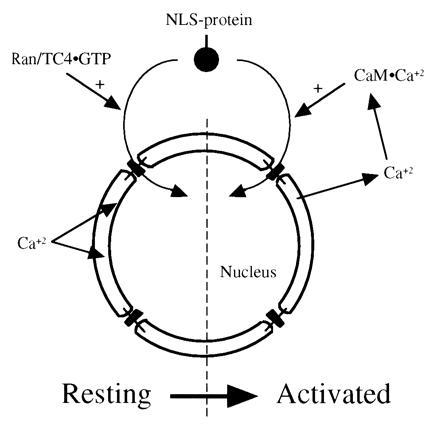
A model for the roles of calcium in GTP- and calmodulin-dependent nuclear protein import. Under resting cell conditions, the calcium concentration is high in the lumen of the nuclear envelope and endoplasmic reticulum and low in the cytoplasm. Nuclear transport of nucleophiles is stimulated by Ran/TC4 in the GTP-bound state (Left). When the cell is activated, calcium is released from the lumen of the nuclear envelope and endoplasmic reticulum. Evidence indicates that this release of lumenal calcium may inhibit the GTP-dependent activation of transport. However, the elevated cytoplasmic calcium interacts with calmodulin, and the activated calmodulin (CaM·Ca2+) stimulates nuclear import (Right).
The data presented here indicate that intracellular calcium stores play a role in GTP-dependent activation of nuclear transport. First, we observed that in the presence of a cytosolic extract, nuclear transport was stimulated by GTP over a range of calcium concentrations from approximately 10 nM to 1 μM. The half-maximal calcium concentration for the GTP-stimulated transport was approximately 40 nM, which is well below physiological free calcium concentrations in unactivated cells (34, 35). Second, over the range of calcium concentrations where GTP stimulates nuclear transport, GTPγS inhibits nuclear transport, consistent with previous observations (36, 37). Finally, thapsigargin inhibited the GTP-induced stimulation of nuclear transport in the assay, consistent with the observations of Greber and Gerace (20), and is likely to be due to the release of calcium from the lumen of the nuclear envelope (21).
GTP-independent activation of nuclear transport by calmodulin is also supported by several lines of evidence presented here. First, calcium was found to stimulate nuclear transport in the absence of GTP or in the presence of GTPγS with a half-maximal calcium concentration of 0.90 μM. Second, activation of nuclear transport at the elevated calcium concentrations was due to calcium in the cytoplasm and not the lumen of the nuclear envelope as evidenced by the insensitivity of transport to thapsigargin pretreatment at elevated calcium concentrations. Third, antagonists of calmodulin, but not other calcium metabolism antagonists, inhibited nuclear transport in vitro. Finally, recombinant calmodulin, and not other calcium-binding proteins of the EF-hand family, was able to support nuclear transport in the assay. Calmodulin-dependent transport required ATP and was not the result of diffusion. Transport proceeded through the nuclear pore as evidenced by the sensitivity of transport to the presence of wheat germ agglutinin. The calmodulin-dependent nuclear transport was sensitive to the calmodulin-binding domain peptide inhibitor (33), and showed a dose response curve to calcium similar to that observed in the presence of cytosol (data not shown).
The independence of the two modes of activation is also supported by several lines of evidence. First, nuclear transport in the presence of cytosol or recombinant calmodulin was not sensitive to GTPγS at elevated calcium levels. Second, thapsigargin had no effect on nuclear transport at elevated calcium concentrations. Third, nuclear transport in the presence of calmodulin was unaffected by the addition of GTP. And finally, transport in the presence of cytosol and GTP was unaffected by the addition of calmodulin antagonists.
It has been shown that karyopherins-α and -β, p10, and Ran/TC4 are required for the GTP-dependent pathway for nuclear protein import (reviewed in refs. 17 and 18), and the observations in our study are consistent with these findings: GTP-dependent transport was only observed in the presence of added cytosol. However, the calmodulin-dependent transport reported here does not appear to require additional soluble factors. It is possible that these factors may be present in sufficient quantities in our assay system such that at elevated calcium concentrations the amount of calmodulin present in the assay is limiting. There is precedence for nuclear import that does not require one or more of the known soluble factors (38–41).
Another distinction between the two modes of activation lies in the role that calcium plays in activation of transport. For GTP-dependent transport to occur, calcium is required in the lumen of the nuclear envelope (refs. 20 and 21, Fig. 1). Release of calcium from the nuclear envelope does not appear to be required for GTP-dependent transport because 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) and EGTA have no inhibitory effect upon transport unless thapsigargin, inositol trisphosphate, or A23187, a calcium ionophore, are added (ref. 20, data not shown). A role for calcium within the nuclear envelope is congruous with the high concentrations of free calcium within the lumen of the nuclear envelope and endoplasmic reticulum (42, 43), and with the requirement that several resident proteins of these organelles have calcium for structure and function (44, 45). Furthermore, an antibody raised against a peptide corresponding to the portion of a nuclear pore protein that extends into the lumen of the nuclear envelope inhibits nuclear transport (46), suggesting that the lumenal domains of nuclear pore proteins are important for the function of the pores.
In contrast to a role for calcium in the lumen of the nuclear envelope, calmodulin-dependent nuclear transport is insensitive to release of calcium from the intracellular stores. Calmodulin typically acts as a molecular switch that responds to elevated free calcium levels by binding to effector proteins to activate numerous cellular processes (34, 35). The identity of a calmodulin-responsive effector protein involved in nuclear transport is not yet known, but this protein may be the convergence point of the two modes of activation of transport. GTP-binding proteins also act as molecular switches, and Ran/TC4 may activate nuclear transport by binding to the effector protein, which is stimulated by calmodulin. Interestingly, calmodulin is present in the nucleoplasm as well as the cytoplasm (47) and appears to be able to cross the nuclear pore by a facilitated diffusion mechanism (48).
Physiological Significance.
The identification of a second calcium-dependent mode for activation of nuclear transport may shed light on an apparent contradiction in the literature. Release of calcium from the intracellular stores leads to an inhibition of active transport and passive diffusion across the pore in microinjected cells and isolated nuclei (20, 21). However, there are numerous events in cells that lead to the release of calcium from intracellular stores and result in transport of novel proteins to the nucleus, rather than an inhibition of transport. A variety of hormonal and mitogenic stimuli elicit release of intracellular calcium stores into the cytoplasm, as well as transport of a variety of transcription factors to the nucleus (34, 35). Calcium transients in and around the nucleus are also temporally associated with transport of cyclin to the nucleus at the onset of mitosis (49, 50). The presence of the observed calmodulin-dependent transport would allow nuclear transport to proceed under these conditions. Under resting cellular conditions, cytoplasmic calcium is low and lumenal calcium in the endoplasmic reticulum and nuclear envelope is high, enabling the GTP-dependent pathway and disabling the calmodulin-dependent pathway. Under conditions where calcium is released from intracellular stores, the GTP-dependent pathway would be inhibited, and the elevated cytoplasmic calcium would stimulate nuclear transport via calmodulin (Fig. 4).
Acknowledgments
We thank M. Ragano-Carriaccolo for constructive discussions and J. E. Hinshaw, A. R. Robbins, S. M. Sweitzer, D. W. Frank, and D. C. Love for critical reading of the manuscript.
Footnotes
Abbreviations: NLS, nuclear localization sequence; GTPγS, guanosine 5′-o-(3-thiotriphosphate); EC50, effective concentration for 50% of maximal transport.
Karyopherin-α (4) is used here to denote a functionally analogous class of proteins that includes the NLS-receptor (5), importin-60 (6), importin-α (7), Srp1p (8), Rch1p (6), NPI-1 (9), and NBP60 (10). Karyopherin-β (11, 12) is also known as p97 (13), Kap95p (14), importin-90 (6), and importin-β (7). p10 (11, 12) is also known as NTF2 (15) and pp15 (16).
References
- 1.Davis L I. Annu Rev Biochem. 1995;64:865–896. doi: 10.1146/annurev.bi.64.070195.004245. [DOI] [PubMed] [Google Scholar]
- 2.Pante N, Aebi U. J Struct Biol. 1994;113:179–189. doi: 10.1006/jsbi.1994.1052. [DOI] [PubMed] [Google Scholar]
- 3.Dingwall C, Laskey R A. Trends Biochem Sci. 1991;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- 4.Radu A, Blobel G, Moore M S. Proc Natl Acad Sci USA. 1995;92:1769–1773. doi: 10.1073/pnas.92.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stochaj U, Bossie M A, van Zee K, Whalen A M, Silver P A. J Cell Sci. 1993;104:89–95. doi: 10.1242/jcs.104.1.89. [DOI] [PubMed] [Google Scholar]
- 6.Görlich D, Kostka S, Kraft R, Dingwall C, Laskey R A, Hartmann E, Prehn S. Curr Biol. 1995;5:383–392. doi: 10.1016/s0960-9822(95)00079-0. [DOI] [PubMed] [Google Scholar]
- 7.Görlich D, Vogel F, Mills A D, Hartmann E, Laskey R A. Nature (London) 1995;377:246–248. doi: 10.1038/377246a0. [DOI] [PubMed] [Google Scholar]
- 8.Enenkel C, Blobel G, Rexach M. J Biol Chem. 1995;270:16499–16502. doi: 10.1074/jbc.270.28.16499. [DOI] [PubMed] [Google Scholar]
- 9.O’Neill R E, Palese P. Virology. 1995;206:116–125. doi: 10.1016/s0042-6822(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 10.Kawahire S, Tachibana T, Umemoto M, Yoneda Y, Imai N, Saito M, Ichimura T, Omata S, Horigome T. Exp Cell Res. 1996;222:385–394. doi: 10.1006/excr.1996.0048. [DOI] [PubMed] [Google Scholar]
- 11.Moroianu J, Blobel G, Radu A. Proc Natl Acad Sci USA. 1995;92:2008–2011. doi: 10.1073/pnas.92.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moroianu J, Hijikata M, Blobel G, Radu A. Proc Natl Acad Sci USA. 1995;92:6532–6536. doi: 10.1073/pnas.92.14.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adam E J H, Adam S A. J Cell Biol. 1994;125:547–555. doi: 10.1083/jcb.125.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iovine M K, Watkins J L, Wente S R. J Cell Biol. 1995;131:1699–1713. doi: 10.1083/jcb.131.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paschal B M, Gerace L. J Cell Biol. 1995;129:925–937. doi: 10.1083/jcb.129.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore M S, Blobel G. Proc Natl Acad Sci USA. 1994;91:10212–10216. doi: 10.1073/pnas.91.21.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Görlich D, Mattaj I W. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 18.Melchior F, Gerace L. Curr Opin Cell Biol. 1995;7:310–318. doi: 10.1016/0955-0674(95)80084-0. [DOI] [PubMed] [Google Scholar]
- 19.Newmeyer D D, Finlay D R, Forbes D J. J Cell Biol. 1986;103:2091–2102. doi: 10.1083/jcb.103.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greber U F, Gerace L. J Cell Biol. 1995;128:5–14. doi: 10.1083/jcb.128.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stehno-Bittel L, Perez-Terzic C, Clapham D E. Science. 1995;270:1835–1838. doi: 10.1126/science.270.5243.1835. [DOI] [PubMed] [Google Scholar]
- 22.Jarnik M, Aebi U. J Struct Biol. 1991;107:291–308. doi: 10.1016/1047-8477(91)90054-z. [DOI] [PubMed] [Google Scholar]
- 23.Adam S A, Marr R S, Gerace L. J Cell Biol. 1990;111:807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam S A, Marr R S, Gerace L. Methods Cell Biol. 1991;35:469–482. doi: 10.1016/s0091-679x(08)60584-1. [DOI] [PubMed] [Google Scholar]
- 25.Adam S A, Marr R S, Gerace L. Methods Enzymol. 1992;219:97–110. doi: 10.1016/0076-6879(92)19013-v. [DOI] [PubMed] [Google Scholar]
- 26.Fabiato A, Fabiato F. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- 27.Bers D M, Patton C W, Nuccitelli R. In: A Practical Guide to the Study of Calcium in Living Cells. Nuccitelli R, editor. San Diego: Academic; 1994. pp. 3–29. [Google Scholar]
- 28.Lanford R E, Feldherr C M, White R M, Dunham R G, Kanda P. Exp Cell Res. 1990;186:32–38. doi: 10.1016/0014-4827(90)90206-p. [DOI] [PubMed] [Google Scholar]
- 29.Goldfarb D S, Gariépy J, Schoolnik G, Kornberg R D. Nature (London) 1986;322:641–644. doi: 10.1038/322641a0. [DOI] [PubMed] [Google Scholar]
- 30.Wolff B, Willingham M C, Hanover J A. Exp Cell Res. 1988;178:318–334. doi: 10.1016/0014-4827(88)90402-8. [DOI] [PubMed] [Google Scholar]
- 31.Sweitzer T D, Hanover J A. FASEB J. 1994;8:A1459. (abstr.). [Google Scholar]
- 32.Hidaka H, Tanaka T. Methods Enzymol. 1983;102:185–194. doi: 10.1016/s0076-6879(83)02019-4. [DOI] [PubMed] [Google Scholar]
- 33.Yazawa M, Vorherr T, James P, Carafoli E, Yagi K. Biochemistry. 1992;31:3171–3176. doi: 10.1021/bi00127a018. [DOI] [PubMed] [Google Scholar]
- 34.Vogel H J. Biochem Cell Biol. 1994;72:357–376. [PubMed] [Google Scholar]
- 35.Carafoli E. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- 36.Melchior F, Paschal B, Evans J, Gerace L. J Cell Biol. 1993;123:1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore M S, Blobel G. Nature (London) 1993;365:661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- 38.Görlich D, Henklein P, Laskey R A, Hartmann E. EMBO J. 1996;15:1810–1817. [PMC free article] [PubMed] [Google Scholar]
- 39.Weis K, Ryder U, Lamond A I. EMBO J. 1996;15:1818–1825. [PMC free article] [PubMed] [Google Scholar]
- 40.Moroianu J, Blobel G, Radu A. Proc Natl Acad Sci USA. 1996;93:6572–6576. doi: 10.1073/pnas.93.13.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kendall J M, Badminton M N, Dormer R L, Campbell A K. Anal Biochem. 1994;221:173–181. doi: 10.1006/abio.1994.1394. [DOI] [PubMed] [Google Scholar]
- 43.Clementi E, Martino G, Grimaldi L M, Brambilla E, Meldolesi J. Eur J Immunol. 1994;24:1365–1371. doi: 10.1002/eji.1830240619. [DOI] [PubMed] [Google Scholar]
- 44.Ou W-J, Bergeron J J M, Li Y, Kang C Y, Thomas D Y. J Biol Chem. 1995;270:18051–18059. doi: 10.1074/jbc.270.30.18051. [DOI] [PubMed] [Google Scholar]
- 45.Nigam S K, Goldberg A L, Ho S, Rohde M F, Bush K T, Sherman M Y. J Biol Chem. 1994;269:1744–1749. [PubMed] [Google Scholar]
- 46.Greber U F, Gerace L. J Cell Biol. 1992;116:15–30. doi: 10.1083/jcb.116.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bachs O, Agell N, Carafoli E. Biochim Biophys Acta. 1992;1113:259–270. doi: 10.1016/0304-4157(92)90041-8. [DOI] [PubMed] [Google Scholar]
- 48.Pruschy M, Ju Y, Spitz L, Carafoli E, Goldfarb D S. J Cell Biol. 1994;127:1527–1536. doi: 10.1083/jcb.127.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilchrist J S, Czubryt M P, Pierce G N. Mol Cell Biochem. 1994;135:79–88. doi: 10.1007/BF00925963. [DOI] [PubMed] [Google Scholar]
- 50.Whitaker M, Patel R. Development (Cambridge, UK) 1990;108:525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]


