Abstract
In the mature central nervous system (CNS) regulated secretion of ATP from astrocytes is thought to play a significant role in cell signaling. Whether such a mechanism is also operative in the developing nervous system and, if so, during which stage of development, has not been investigated. We have tackled this question using cells derived from reconstituted neurospheres, as well as brain explants of embryonic mice. Here, we show that in both models of neural cell development, astrocyte progenitors are competent for the regulated secretion of ATP-containing vesicles. We further document that this secretion is dependent on cytosolic Ca2+ and the v-SNARE system, and takes place by exocytosis. Interference with ATP secretion alters spontaneous Ca2+ oscillations and migration of neural progenitors. These data indicate that astrocyte progenitors acquire early in development the competence for regulated secretion of ATP, and that this event is implicated in the regulation of at least two cell functions, which are critical for the proper morphogenesis and functional maturation of the CNS.
Keywords: purinergic signaling, glia, neural progenitors, evanescent field microscopy, neurosphere, astrocyte
INTRODUCTION
A critical process necessary in the development and repair of the central nervous system (CNS) is the migration of neural precursor cells to their final destination. Besides the well-established roles of cell adhesion and extracellular molecules, other signaling mechanisms have been identified, among which are the intracellular Ca2+ fluctuations resulting from ion channel and membrane receptor activation (Komuro and Rakic, 1998; Kumada and Komuro, 2004). It has been suggested that glial cells participate in this signaling process by releasing Ca2+-mobilizing transmitters, such as glutamate and ATP (Coco et al., 2003; Parpura et al., 1995; Queiroz et al., 1997).
ATP is an important neurotransmitter and modulator that activates purinergic receptors (P2Rs), a family of seven ionotropic (P2X1-7) and eight metabotropic (P2Y1,2,4,6,11-14) receptors. P2Rs are abundant in both developing and mature CNS where they participate in neural stem cell proliferation (Mishra et al., 2006; Ryu et al., 2003), neural progenitor migration (Agresti et al., 2005; Scemes et al., 2003), apoptosis (Abbracchio, 1997; Lin et al., 2003; Virginio et al., 1999) and cell differentiation (Neary and Zhu, 1994).
Although spontaneous Ca2+ waves and oscillations have been reported to occur during CNS development (Aguado et al., 2002; Kumada and Komuro, 2004; Meister et al., 1991; Parri and Crunelli, 2001; Parri et al., 2001; Yuste et al., 1992; Weissman et al., 2004), very little is known about the signaling mechanisms involved in such endogenous activity. Using an in vitro model of neural cell development, we have previously provided evidence that neural progenitor cells display spontaneous Ca2+ oscillations that are highly dependent on activation of P2Y1Rs, and suggested that these progenitors have the ability to release ATP, which then influences cell migration by paracrine/autocrine signaling (Scemes et al., 2003). Work performed on slice preparations of embryonic rat brain, also showed that spontaneous Ca2+ waves spreading between ventricular zone radial glial cells were dependent on ATP-activated P2Y1 receptors (Weissman et al., 2004).
An issue that is still debated, however, regards the mechanism by which ATP is released from mature glial cells, as there is little available information regarding the pathway by which this nucleotide is released from progenitor and immature cells. In mature astrocytes, substantial evidence supports regulated exocytosis as the main pathway of gliotransmitter release under physiological conditions (Coco et al., 2003; Mothet et al., 2005; Parpura et al., 1994), while diffusion through ion channels is likely to be involved under non-physiological conditions (Evanko et al., 2004; Parpura et al., 2004; Suadicani et al., 2006). To evaluate whether, and if so, at which stage of development regulated secretion of ATP is functionally competent, we used in vitro differentiating neurospheres (Duval et al., 2002; Scemes et al., 2003), as well as explants of embryonic mouse forebrains. Here we show that neural progenitors of the astrocytic lineage release ATP by regulated exocytosis and that this process is involved in the modulation of spontaneous Ca2+ oscillations and cell migration. Our study supports the notion that astrocyte purinergic signaling plays important roles in the early organization of the CNS.
MATERIALS AND METHODS
Neurospheres and Neural Progenitor Cultures
Neurospheres were prepared using a modification of a method previously described (Duval et al., 2002; Scemes et al., 2003). Briefly, neural progenitor cells were obtained by aspiration of forebrain tissue from 14-day-old (E14) wild-type C57Bl6 mouse embryos (time-pregnant females obtained from Charles River; the AECOM Animal Care and Use Committee approved all experimental procedures performed) and mechanically dissociated in ice-cold Hanks balanced solution (HBSS Ca2+ and Mg2+-free). Viable cells were transferred to tissue culture dishes containing DMEM-F12 (Gibco, Invitrogen, Carlsbad, CA) supplemented with 5% B27 (Gibco), 1% antibiotics and 20 ng/mL human recombinant epidermal growth factor (EGF; Sigma, St. Louis, MO), and allowed to grow into floating neurospheres. Culture medium was changed two to three times a week, and neurospheres were mechanically dissociated into smaller neurospheres once a week. Neurosphere cultures were maintained for no longer than two months. Following the growth of neural cells into floating neurospheres, in vitro cell differentiation was induced by withdrawing EGF from the culture medium and plating the neurospheres on poly-D-lysine- (10 μg/mL; Sigma) and fibronectin- (10 μg/mL; Sigma) coated glass bottom microwells (MatTek, Ashland, MA). One to five day cultured adherent neurospheres were used to evaluate pathways of ATP release. To this end, we focused on the monolayers of immuno-identified progenitors that had emigrated out of the neurospheres, and surrounded the organoid (Fig. 1S in Supplemental Material). Explants of E14 forebrain were used in some experiments aimed to evaluate the contribution of ATP secretion on cell migration. For that, freshly isolated round fragments (70-200 μm diameter) of forebrain tissues were plated on polylysine- and fibronectin-coated MatTek dishes, and maintained in serum-free DMEM-F12 medium during experimentation (see also supplemental material).
Immunocytochemistry
Adherent cultures of progenitors were fixed in 4% paraformaldehyde for 5-10 min. Following several washes in 1X PBS and a 30-min incubation with 1X PBS containing 0.4% Triton-X and 10% goat serum, cells were incubated for 24-48 h at 4°C with the following primary monoclonal antibodies: anti-nestin (1:500; Chemicon, Temecula, CA), anti-neuronal class III β-tubulin (1:500; Stemcell Technologies, Vancouver, British Columbia), anti-A2B5 (1:500; R&D Systems), anti-O4 (1:500; Chemicon, Temecula, CA), anti-Cd44S (1:200; Chemicon, Temecula, CA), anti-synaptobrevin-2 (1:500; Synaptic Systems). Polyclonal anti-GFAP antibodies (1:200) were from Sigma. Relevant secondary monoclonal or polyclonal secondary antibodies, tagged with either Alexa Fluor 488 nm or 594 nm (Molecular Probes) were added to the cells, at 1:2000 dilution, for 1-2 h at room temperature. Epifluorescence images were acquired using an inverted microscope (Eclipse TE2000-S; Nikon, Japan Nikon) equipped with CCD cameras (Orca-ER or SPOT-RT; Hamamatsu, Japan) using Metamorph software (Universal Imaging Systems, Downingtown, PA). Confocal images were acquired using an upright confocal microscope (Olympus, Melville, NY) equipped with an argon/krypton laser. Images were captured using the Fluoview software provided by the manufacturer and collected as 1024 × 1024 pixels, using the sequential module with a pinhole size of 80 μm. For live-immunostaining, adherent cultures of progenitors were incubated in serum-free DMEM-F12 containing monoclonal anti-Cd44S (1:200) or anti-A2B5 (1:200) antibodies for 30 min, at 37°C. After several washes with DMEM-F12, the cultures were incubated with Alexa fluor 594-tagged secondary antibodies, diluted 1:1,000, for 30 min, at 37°C, and then used to identify the type of cells displaying spontaneous Ca2+ oscillations and exocytic events (see below).
Intracellular Calcium Transients
Spontaneous intracellular Ca2+ transients were measured in adherent, immuno-identified progenitors loaded for 30 min at 37°C with 5 μM Fluo-3-AM (Molecular Probe) in DMEM-F12. Cells were then washed with Dulbecco's phosphate buffered saline (DPBS, pH 7.4; Cellgro, Herndon, VA) and imaged on an epifluorescence microscope (Eclipse TE2000-S; Nikon, Japan), equipped with a CCD camera (Orca-ER; Hamamatsu, Japan). Fluo-3 fluorescence intensity emitted at one excitation wavelength (488 nm) was continuously acquired at a rate of 1.0 Hz using combined systems of filters and shutter (Lambda DG-4 Diaphot, Sutter Instruments, Burlingame, CA) driven by a computer through Metafluor software (Universal Imaging Systems, Downingtown, PA). Fluo-3 fluorescence intensity (F) obtained from regions of interest were normalized to initial values (F0) and expressed as relative changes (F/F0).
Total Internal Reflected Fluorescence Microscopy
For measurements of exocytosis, adherent progenitors plated in fibronectin-polylysine-coated MatTek dishes, were loaded for 5-10 min at 37°C with 5 μM quinacrine (Sigma). After several washes with DPBS, changes in the intensity of quinacrine fluorescence emitted at 512 nm when excited at 488 nm were acquired at 0.6 s intervals for 1-2 min. The fluorescent vesicles located within the evanescence field were analyzed using Metamorph software (Universal Imaging). To monitor vesicular ATP, cells were incubated for 5 h at 37°C with 50 μM Mant-ATP [2′(3′)-O-(N-methylanthraniloyl)-adenosine-5′-O-triphosphate; BioLog Life Science] and imaged by TIRF microscopy 3.5 h after washout of this fluorescent ATP analog. Total internal reflected fluorescence (TIRF) images were acquired by either laser TIRF or white light TIRF. The laser system consisted of an inverted microscope (Zeiss Axiovert 100M) modified for through-the-objective evanescent field illumination, equipped with an APO100× oil immersion objective (1.45 NA; 0.17 WD), filter set (488 nm), and a CCD camera (ORCA-ER, Hamamatsu, Japan), driven by OpenLab software on a Macintosh computer. The white-light system (Nikon) consisted of a special arc shape diaphragm placed in the path of a lens-collimated light generated by an arc lamp and mounted on an inverted TE-2000 Nikon microscope equipped with filter sets (488 nm and 594 nm), a 100× (1.45 NA) oil immersion objective, a CCD camera (Orca-ER, Japan) coupled to a PC computer through Metamorph/Metafluor software.
Exocytosis
The time course and the number of spontaneous and evoked exocytic events, defined as the quasi-instantaneous disappearance within the evanescent field of the fluorescence of quinacrine-loaded vesicles, were measured by TIRF microscopy for 1-2 min. The evaluations compared loaded vesicles in the absence or presence of one of the following drugs: 5-10 μM Ca2+ ionophore A-23187; 10-50 μMCa2+ chelator Bapta-AM for 1-3 h; 300 nM tetanus-toxin (TnTx) for 24 h, and 5 lμM v-ATPase inhibitor bafilomycin-A1 (Baf-A1) for 1 h. In addition, we also monitored progenitor cells which were transiently transfected with a dominant-negative domain of synaptobrevin-2 (see below). Evaluation of quinacrine fluorescence changes during exocytosis was performed by measuring fluorescence intensity within regions of interest (ROI) placed on vesicles present in the evanescent field. ROIs of different sizes were drawn manually to circle each quinacrine vesicle (diameter 168.8 ± 8.31 nm, N = 26), using Metamorph software. Changes in quinacrine fluorescence intensity were displayed either as absolute fluorescence intensity values or as fraction of initial values (F/F0).
Measurements of ATP
The amount of ATP released in the bathing solution by adherent progenitor cells was measured for 2 min in 200 μL DPBS in the absence and presence of 10 μM A23187, from both untreated cells and cells treated with TnTx (300 nM/24 h), bafilomycin-A1 (5 μM/1 h), and Bapta-AM (50 μM/3 h), as well as from cells transfected with dn-syb2 (see below). The amount of ATP was measured using the luciferin/luciferase assay (Molecular Probes, Invitrogen). Fifty microliters of a buffered solution containing luciferin (50 μM) and luciferase (1.25 μg/mL) were placed in triplicates in a 96-well plate luminometer (Harta Lumi 812; kindly borrowed from Dr. R. Burk, Department of Pediatrics, Albert Einstein College of Medicine) for background luminescence subtraction. Reactions were started by adding 5 μL of the experimental samples. The amount of ATP in the samples was calculated from standard curves generated from a series of ATP concentrations (50-5 μM; diluted in the same solutions used for the experimental measurements) and normalized to the total amount of protein, using the BCA assay (Pierce).
Transfection of the Dominant-Negative Domain of Synaptobrevin-2 (dn-syb2)
Plasmid containing the cDNA corresponding to aminoacids 1-96 of the rat v-SNARE protein synaptobrevin-2 (Accession no. M24105.1)—a motif that prevents SNARE complex formation and regulated exocytosis in astrocytes (Zhang et al., 2004)—was amplified from a pRV9-synaptobrevin II SNARE-IRES-EGFP construct (kindly provided by Dr. Phillip G. Haydon, Department of Neuroscience, University of Pennsylvania, PA) using rat synaptobrevin-2 specific primers. The PCR product was inserted into pcDNA 3.1-CT-GFP TOPO vector (Invitrogen, Carlsbad, CA) and sequence verified. Floating neurospheres, forebrain explants, or adherent progenitors were transfected with 8 μg/mL dn-syb2 cDNA, using lipofectamine-2000 reagent (Invitrogen). These conditions resulted in the transfection of about 80% cells (Fig. 2S of Supplemental Material; Fig. 7 in Scemes et al., 2003). After 18-19 h, cells were washed and maintained in DMEM-F12 until the experiment, which took place 24-48 h after the transfection.
Outgrowth Index
To evaluate the migration of neural progenitor cells, non-transfected and dn-syb2-transfected floating neurospheres were plated on fibronectin/poly-D-lysine coated glass-bottomed microwells containing DMEM-F12. After 6, 20, and 30 h of culture, the outgrowth index (OI) of migrating cells was calculated as the ratio between the distance of the nucleus of the foremost cells to the center of the sphere and the diameter of the sphere core (Scemes et al., 2003).
Statistical Analysis
The ANOVA analysis of variance followed by Newnam-Keuls' test was used.
RESULTS
Spontaneous Ca2+ Oscillation and ATP Release from Astrocyte Progenitors
In this study we used progenitor cells derived from day 14 mouse embryo (E14) forebrains. When first grown to form floating spheroid bodies—referred as to neurospheres—and then plated on a fibronectin substrate and cultured in the absence of growth factors, these undifferentiated cells progressively become astrocytes, oligodendrocytes, neuron progenitors and mature cells. To identify and quantitate these various cell types, we performed immunocytochemical studies using cell type specific markers. One day after adhesion of neurospheres to fibronectin-coated dishes, about 83% of the cells that had emigrated out of the neurospheres were positive for the cell adhesion glycoprotein Cd44S, a marker of astrocyte progenitors (Liu and Rao, 2004; Liu et al., 2002; Rao and Mayer-Proschel, 1997), 0.5% expressed the intermediate filament glial fibrilary acid protein GFAP and 97% were immunoreactive for the intermediate filament protein nestin. These proportions changed over time. Thus, after one week after adhesion, about 80-85% emigrated cells were positive for GFAP, a marker of differentiated astrocytes, 12% expressed the oligodendrocyte ganglioside O4 and 6-8% were positive for the neuronal marker IIIβ-tubulin (Fig. 1S in supplemental material). These results indicate that the great majority of precursor cells derived from adherent neurospheres are glial progenitors mainly of the astrocytic lineage.
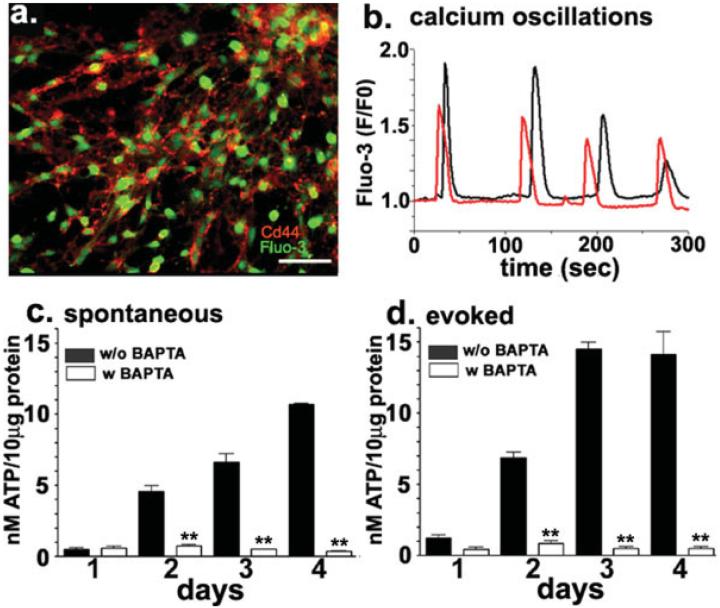
To verify whether these astrocyte progenitors were the same cells that were previously reported to display spontaneous intracellular Ca2+ oscillations (Scemes et al., 2003), Fluo-3-AM loaded cells emigrated from 1-4 day-adherent neurospheres were immunostained with anti-Cd44S antibodies (Fig. 1a). As shown in Fig. 1b Cd44S positive cells displayed intracellular Ca2+ oscillations with frequencies of 14.61 ± 0.88 mHz (N = 161 cells) and amplitudes of 1.34 ± 0.03 fold (N = 231 events). These data indicate that, in vitro, Cd44S positive astrocyte progenitors display spontaneous intracellular Ca2+ oscillations.
Fig. 1.
Spontaneous Ca2+ oscillations and Ca2+-dependent secretion of ATP from astrocyte progenitors. (a) Epifluorescence images of live-immunostained progenitors derived from 4-day adherent neurospheres loaded with the calcium indicator Fluo-3-AM (green) showing the expression of Cd44S (red) in astrocytic precursors. Bar: 10 μm. (b) Examples of spontaneous intracellular Ca2+ oscillations recorded from two Cd44S expressing cells derived from 4-day adherent neurospheres. (c, d) Bar histograms showing the amount of ATP secreted from 1-4 day-adherent progenitors; (c) spontaneous and (d) 10 μM A23187-induced ATP release were measured from control cells (black bars) and from cells loaded with 50 μM Bapta-AM (white bars). Application of the Ca2+ ionophore (d) induced a significant increase in the amount of ATP secreted compared with that released by non-stimulated cells (c) and Bapta greatly reduced both spontaneous and evoked ATP release. ATP measurements were performed using the luciferin-luciferase assay and luminescence values were transformed into [ATP] according to calibration curves performed in the presence and absence of the agents mentioned above, and the values were normalized to the total amount of protein. (**P < 0.001; ANOVA analysis of variance followed by Newman-Keuls' paired test).
Our previous study showing that apyrase and P2R antagonists greatly prevented the spontaneous Ca2+ oscillations of neural progenitors (Scemes et al., 2003), indicated that these events were likely dependent on the release of ATP and on the action of the nucleotide on purinergic receptors. To evaluate whether astrocyte progenitor cells were able to release ATP, we measured the amount of this triphosphate nucleotide in the solution bathing progenitor cells using a luciferin-luciferase assay. One-to-four day-adherent progenitors spontaneously released ATP, in levels that increased with culture time (Fig. 1c). Addition of the Ca2+ ionophore A23187 (10 μM) potentiated this release (Fig. 1d; P < 0.0001 ANOVA analysis of variance) and both spontaneous and A23187-evoked ATP release were prevented in cells preloaded with the Ca2+ chelator Bapta-AM (50 μM; Figs. 1c,d). These data indicate that astrocyte progenitors do release ATP, and that this release occurs via a Ca2+-dependent mechanism.
Ca2+-Dependent Release of Vesicular ATP from Astrocyte Progenitors
Given that it has been previously proposed that the release of ATP from mature astrocytes may occur via regulated exocytosis (Coco et al., 2003), we tested whether the Ca2+-dependent release of ATP from astrocyte progenitors also involved a similar process. For that, we experimentally altered specific steps of the storage and release of the transmitter, using TIRF microscopy to evaluate exocytic events and bioluminescence techniques to measure ATP release.
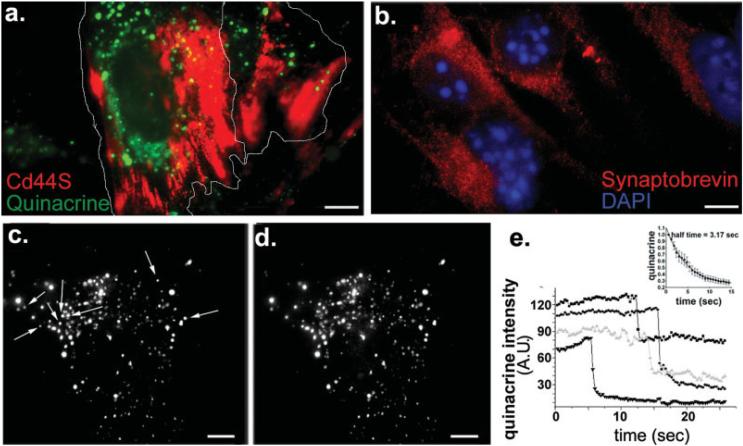
Live immunostaining for Cd44S followed by loading with 5 μM quinacrine, a dye that stains high levels of ATP bound to peptides within acidic, dense core vesicles (Belai and Burnstock, 2000; Bodin and Burnstock, 2001), indicated that astrocyte progenitors contained quinacrine-loaded vesicles (Fig. 2a). Immunocytochemistry further showed the presence of native v-SNARE protein synaptobrevin-2 in these progenitor cells (Fig. 2b). To follow the fate of the quinacrine-loaded vesicles, time-lapse TIRF imaging was performed on 3-4 day progenitors. Analysis of quinacrine fluorescence intensity revealed abrupt decreases in fluorescence intensity of some of the loaded vesicles present in the evanescent field (Figs. 2c-e), suggesting loss of quinacrine from vesicles through exocytic events. On average, the half-time of quinacrine diffusion from vesicles that underwent spontaneous exocytosis was 3.2 s (inset in Fig. 2e). To investigate whether the loss of quinacrine fluorescence involved a Ca2+-dependent mechanism, quinacrine-loaded cells were stimulated with 5-10 μM A23187 to monitor by TIRF microscopy the time course of quinacrine release (Fig. 3a; see also Fig. 3S in supplemental material). Bath application of this Ca2+-ionophore, which raised intracellular Ca2+ levels in Fluo-3-AM loaded progenitors (Fig. 3b), also induced a five-fold increase in quinacrine fluorescence, indicating the rapid recruitment of loaded vesicles into the membrane region that was monitored within the evanescent field (Fig. 3a). This change was followed by a more gradual decrease in fluorescence (half-time of quinacrine diffusion of 3.1 s), consistent with the secretion of quinacrine from loaded vesicles (Fig. 3c). These results support the idea that the loss of quinacrine from vesicles of astrocyte progenitors involves a Ca2+-dependent mechanism, most likely regulated exocytosis. Moreover, because quinacrine is known to bind to adenine nucleotides and to accumulate in purinergic nerve terminals (Alund and Olson, 1980; Bock, 1980; Crowe and Burnstock, 1982; Olson and Alund, 1979; Unsworth and Johnson, 1990), our results suggest that astrocyte progenitors store ATP in a vesicular compartment.
Fig. 2.
Quinacrine-containing compartments in astrocyte progenitors. (a) Total internal reflected fluorescence (TIRF) view of quinacrine-containing vesicles (green) in 3-4 day-adherent progenitors (the contour of cells is outlined), immunostained for Cd44S (red). (b) Epifluorescence image showing immunoreactivity for the vSNARE protein synaptobrevin-2. Note the vesicular-like distribution of the endogenous synaptobrevin-2. (c, d) TIRF images of quinacrine-loaded progenitors derived from 3 day-adherent neurospheres. Arrows in (c) indicate some of the quinacrine-containing vesicles that are absent in image (d), which was acquired 1 min later. (e) The intensity of quinacrine fluorescence of vesicles illustrated in panel c-d abruptly decreased at various time points, indicating the spontaneous vesicular release of the tracer. Inset: Time course of the decay of quinacrine fluorescence during the diffusion of quinacrine from vesicles that underwent spontaneous exocytosis. The mean± SEM half-time values of quinacrine diffusion (3.2 s; N = 14 vesicles) was obtained by fitting the equation Y = Ymax exp(-kt), where Y is the change in fluorescence intensity over time (t), Ymax is the maximal fluorescence intensity, and k is the rate constant (half-time is 0.69/k). The absolute values of fluorescence intensity changes were followed from regions of interest comprising quinacrine-positive vesicles present in the first TIRF image (t = 0). The TIRF image displayed in panel a was obtained using a Nikon White Light TIRF system, whereas those displayed in panels c-d were obtained with a laser-TIRF system. Bars: 10 μm.
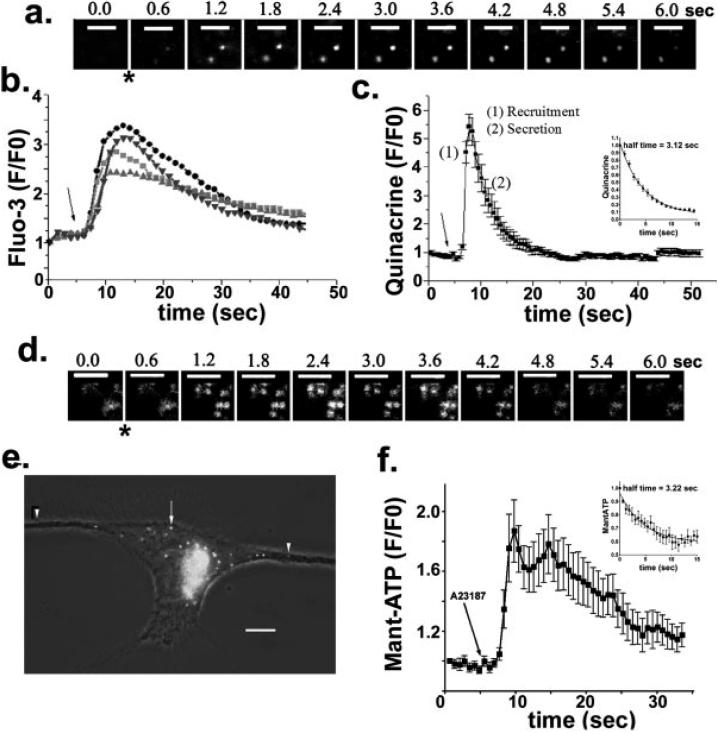
Fig. 3.
Ca2+-dependent vesicular release of quinacrine and Mant-ATP from astrocyte progenitors. (a) Sequential White Light TIRF images of quinacrine-loaded vesicles showing the time course of exocytotic events induced by bath application (*) of A23187 (10 μM) in 3-day progenitor. Bars: 4 μm. (b, c) Time courses of relative fluorescence intensity (F/F0) changes of Fluo-3 (b) and quinacrine (c) induced by exposure (arrows) of 3 day-adherent progenitors to A23187 (10 μM). After addition of A23187 there is an increase in quinacrine fluorescence because of the recruitment (1) of vesicles at the cell periphery, observed in the evanescent field, followed by a decrease in quinacrine fluorescence as the dye is released (2) from loaded vesicles. Quinacrine fluorescence intensity changes were evaluated from regions of interest with a constant position throughout the series of TIRF images. (d) Sequential 488 nm laser-based TIRF images of Mant-ATP-loaded vesicles showing the time course of exocytosis induced by A23187 (*). Bars: 4 μm. (e) Live epifluorescence image showing vesicles containing Mant-ATP in a 2 day-adherent progenitor cell. Note the presence of small (arrowheads) and large (arrow) vesicles. Image acquired with a SPOT-RT camera attached to an inverted Nikon microscope equipped with a ×100 oil immersion objective (N.A. 1.4) and FITC filter set. Bar: 8.5 μm. (f) Time course of changes in Mant-ATP fluorescence intensity induced by bath application (arrow) of 10 μM A23187, and recorded from 3-day progenitors using 488 nm laser-based TIRF microscopy. Changes in Mant-ATP fluorescence intensity were evaluated in regions of interest that retained a constant position throughout the series of TIRF images. Insets in parts c and f show the time course (mean ± SEM) of quinacrine and Mant-ATP fluorescence, respectively, during the diffusion of the dyes from vesicles that underwent regulated exocytosis. Note the similar half-time values obtained for the diffusion of quinacrine (3.1 s; N = 19 vesicles) and Mant-ATP (3.2 s; N = 10 vesicles). The half-time was obtained by fitting the equation Y = Ymax exp(-kt), where Y is the change in fluorescence intensity over time (t), Ymax is the maximal fluorescence intensity, and k is the rate constant (half-time is 0.69/k).
To obtain direct evidence for such storage of ATP in a vesicular compartment, progenitor cells were incubated for 5 h with 50 μM Mant-ATP, a fluorescent analogue of ATP. After washout of the probe and a 3.5 h incubation to allow for its compartmentalization, TIRF and epifluorescence microscopy showed Mant-ATP-containing vesicles in astrocyte progenitors (Figs. 3d,e). Similarly to the changes in quinacrine fluorescence (Fig. 3c), the kinetics of the changes in Mant-ATP fluorescence were abrupt, as assessed by TIRF after stimulation with A23187 (Figs. 3e,f). The half-time of Mant-ATP diffusion (3.2 s) was similar to that of quinacrine (insets in Figs. 3c,f). These data are consistent with the view that ATP is accumulated in a vesicular compartment, which is recruited and then released by exocytosis during stimulation with the Ca2+ ionophore.
v-SNARE-Dependent Vesicular ATP Release from Astrocyte Progenitors
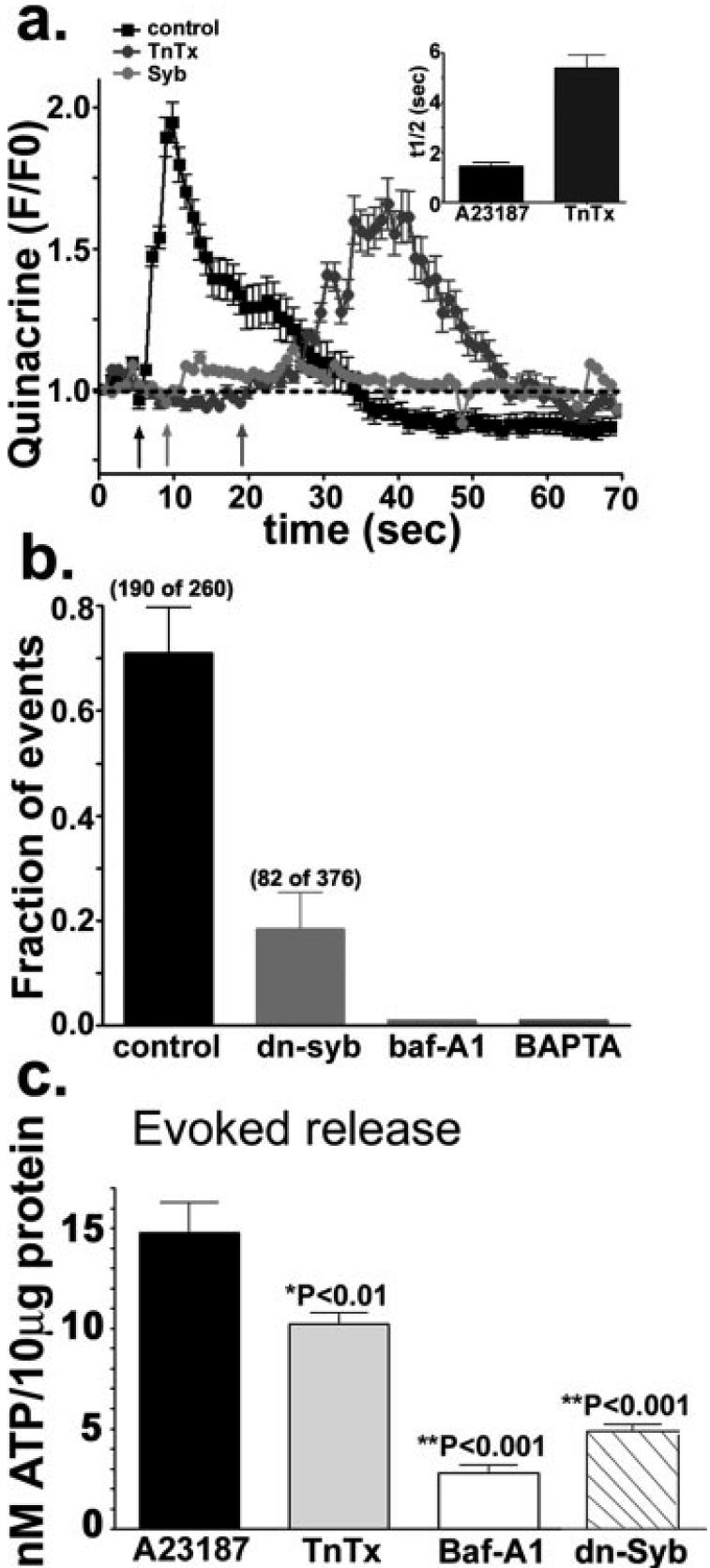
To examine v-SNARE-dependent exocytosis of ATP from astrocyte progenitors, we analyzed exocytic events and quantified the amount of ATP released under conditions known to interfere with the storage and release of transmitter from vesicles. To this end, we treated quinacrine-loaded cells with the v-H+-ATPase inhibitor bafilomycin-A1 (5 μM, 1 h), a blocker that prevents the development of the proton gradient which drives the accumulation of transmitters into synaptic vesicles (Bowman et al., 1988). We also used the clostridium holotoxin tetanus toxin (300 nM, 24 h) that cleaves the v-SNARE protein synaptobrevin-2 (Schiavo et al., 1992), and transfected progenitors with a domain of synaptobrevin-2 (dn-syb2) that exerts a dominant-negative effect on the release process (Zhang et al., 2004). All these conditions altered exocytosis (Figs. 4a,b), and reduced the amount of ATP released (Fig. 4c). Exposure of astrocyte progenitors to bafilomycin-A1 resulted in the absence of quinacrine-loaded vesicles and consequently no exocytosis was recorded by TIRF microscopy (Fig. 4b). The Ca2+ ionophore-evoked quinacrine exocytosis, which was prevented by pre-loading the cells with Bapta-AM (Fig. 4b), was not blocked by TnTx treatment (Fig. 4a). However, under this latter condition, the half-time of quinacrine release from vesicles that still underwent exocytosis was increased to 5.4 ± 0.5 s (N = 25 events), i.e., was about three times as long as that of untreated, control cultures (1.5 ± 0.2 s, N = 21 events) (Fig. 4a). The modest effects of TnTx on exocytosis is most likely due to the fact that astrocytes lack clostridium toxin receptors (Ahnert-Hilger and Bigalke, 1995) necessary for the appropriate internalization of this proteolytic toxin. To bypass this problem, we transfected progenitors with a dominant-negative form of synaptobrevin2 (dn-syb2), which has been shown to impair vesicular release of transmitter from mature astrocytes (Zhang et al., 2004). Forced expression of dn-syb2 decreased the fraction of vesicles that underwent exocytosis from 0.71 ± 0.09 (N = 3 experiments) in controls to 0.18 ± 0.07 (N = 3 experiments) in dn-syb2 transfected cells (Fig. 4b). To confirm that these treatments did not interfere with other physiological processes, we measured intracellular Ca2+ transients induced by bath application of a purinergic receptor agonist. As expected, Bapta-AM prevented the A23187- and the agonist-induced intracellular Ca2+ transients, whereas bafilomycin-A1, TnTx and dn-syb2 did not alter the responses induced by 100 nM P2Y1R agonist MeSATP (Fig. 4S in supplemental material).
Fig. 4.
vSNARE-dependent release of quinacrine and ATP. (a) Time course of quinacrine fluorescence intensity (F/F0) changes induced by 10 μM A23187 (arrows) in 3-4 day-adherent untreated (black squares), TnTx-treated (300 nM; dark gray circles) and dn-syb2-transfected (light gray circles) progenitor cells. Inset: Bar histograms show the mean ± SEM values of the half-time (t1/2) of the rising phase of quinacrine fluorescence intensity, defined as the interval between the emergence of quinacrine vesicles into the evanescent field and the moment at which their fluorescence intensity started decreasing, in control and TnTx-treated progenitors. (b) Bar histograms show that the fraction of exocytic events recorded from progenitors transfected with the dominant-negative domain of synaptobrevin-2 (dn-syb) was greatly reduced and that these events were absent in progenitor cells treated with either bafilomycin-A1 (5 lμM; baf-A1) or a calcium chelator (50 lμM; Bapta-AM). (c) The amount of ATP released from 4-day progenitors exposed to the Ca2+-ionophore was significantly reduced by both TnTx (300 nM) and bafilomycin-A1 (5 μM; Baf-A1), and by transfecting the cells with the dominant-negative domain of synaptobrevin-2 (dn-Syb). Values are mean ± SEM. *P < 0.01; **P < 0.001; ANOVA followed by Newman-Keuls' paired test.
To test whether the Ca2+- and the SNARE-dependent exocytosis of quinacrine-loaded vesicles were related to the ATP secretion, we measured the amount of ATP present in the bathing solution of progenitors subjected to the conditions described above. Exposure of progenitors to TnTx or transfection of these cells with dn-syb2 significantly reduced the amount of A23187-evoked ATP release from 4-day progenitors (Fig. 4c). Moreover, a significant reduction of ATP release was also observed when cells were exposed to the v-H+-ATPase inhibitor-bafilomycin-A1. Transfection of progenitor cells with empty vector did not alter the amount of A23187-evoked ATP (14.58 ± 1.66 nM ATP/10 μg protein; N = 6) compared with that of untransfected cells (14.10 ± 1.63 nM ATP/10 μg protein; N = 3). Together, these results show that a regulated, Ca2+- and v-SNARE-dependent ATP secretion occurs in the early developmental stages of astrocyte progenitors.
Regulated Exocytosis of ATP Modulates Spontaneous Calcium Oscillations and the Migration of Progenitors
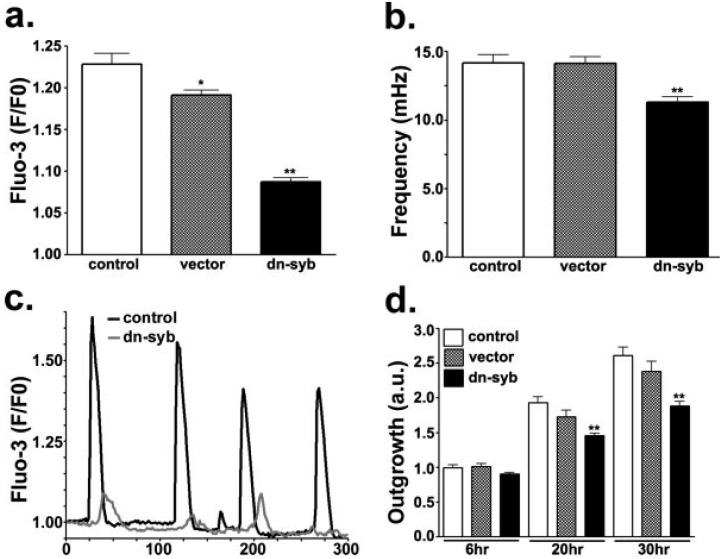
To test whether the ATP secretion and spontaneous Ca2+ oscillations of astrocyte progenitors are regulated in parallel, 3-5 day-adherent progenitors were transfected with dn-syb2, and spontaneous calcium oscillations were recorded from Fluo-3-AM loaded Cd44S positive cells. As shown in Fig. 5, dn-syb2 transfectants exhibited spontaneous Ca2+ oscillations of significantly lower amplitude (1.087 ± 0.005 fold, N = 1,006 events; Fig. 5a) and frequency (11.30 ± 0.39 mHz, N = 343 cells; Fig. 5b) than both non-transfected (1.23 ± 0.01 fold, N = 633 events; 14.16 ± 0.60 mHz, N = 247 cells) and mock-transfected (1.19 ± 0.006 fold, N = 1,060 events; 14.13 ± 0.47 mHz, N = 252 cells) cells. These results, together with the observation that expression of dn-syb2 impairs the release of ATP (Fig. 4c), indicate that spontaneous calcium oscillations and regulated secretion of ATP are co-regulated in astrocyte progenitors.
Fig. 5.
Regulated secretion of ATP modulates spontaneous Ca2+ oscillations and cell migration. (a, b) Bar histograms show the mean ± SEM values of the amplitude (a) and frequency (b) of spontaneous Ca2+ oscillations recorded from control, 2 day-adherent progenitors (white bars), progenitors transfected with an empty vector (gray bars) and progenitors transfected with a dominant-negative domain of synaptobrevin-2 (dn-syb; black bars). Note the slight reduction in frequency (b) and the dramatic decrease in the amplitude (a) of spontaneous Ca2+ oscillations in the dn-syb transfectants. (c) Spontaneous Ca2+ oscillations recorded from Fluo-3-AM-loaded control progenitors (black trace) and progenitors transfected with the dn-syb2 (gray trace). (d) Bar histograms show the mean ± SEM values of outgrowth index (OI) obtained from progenitors derived from control neurospheres (white bars), neurospheres transfected with an empty vector (gray bars) and neurospheres transfected for dn-syb2 (black bars). Meaurements were performed 6-30 h after adhesion of neurospheres to fibronectin-coated dishes. Note that the OI of dn-syb transfectants was significantly decreased compared with that of both control and empty vector-transfected cells. *P < 0.01; **P < 0.001; ANOVA followed by Newman-Keuls' paired test.
To evaluate whether the impairment in ATP secretion and spontaneous Ca2+ oscillations influence the emigration of progenitor cells from neurospheres, floating neurospheres were transfected with dn-syb2 and an OI (Scemes et al., 2003) measured at 6, 20, and 30 h after adhesion to fibronectin-coated dishes. Compared with untransfected and mock-transfected cells, dn-syb2 transfectants featured a decreased OI (Fig. 5d). Thus, after 20 h of adhesion, the value of this index in dn-syb2 transfectants (1.46 ± 0.04; N = 66 neuropheres) was significantly smaller (P < 0.001) than that measured from both non-tranfected (1.93 ± 0.09; N = 60 neuro-spheres) and mock-transfected cells (1.73 ± 0.093; N = 25 neurospheres). This difference was maintained after 30 h of adhesion, at which time point the OI of dn-syb2 transfectants was 1.89 ± 0.06 (N = 61 neurospheres) compared with 2.61 ± 0.12 (N = 44 neurospheres) and 2.34 ± 0.14 (N = 28 neurospheres) in non-transfected and in mock-transfected, respectively.
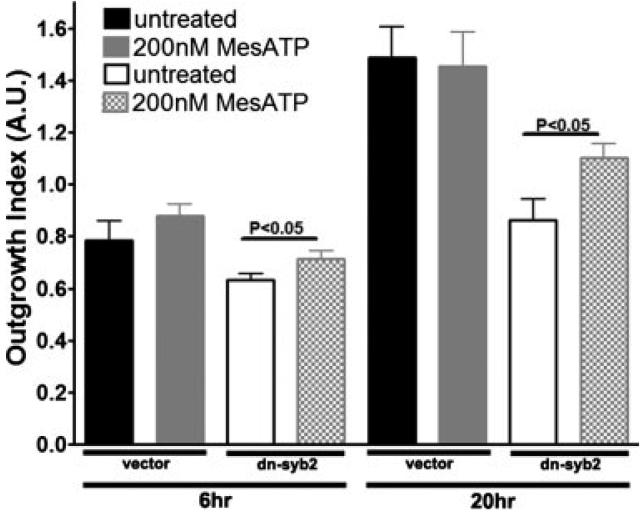
The contribution of exocytosis of ATP to progenitor cell migration is further supported by experiments in which dn-syb2 transfected cells were supplemented with 200 nM MeSATP, a P2Y1R agonist previously shown to mediate progenitor cell migration (Scemes et al., 2003). As shown in Fig. 6, addition of the P2Y1R agonist to dn-syb2 transfected progenitors significantly increased their OI compared with untreated and dn-syb2 transfected cells, and had no effect on the OI of mock transfected progenitors. These results indicate that proper secretion of ATP and/or spontaneous Ca2+ activity significantly contribute to the migration of differentiating progenitor cells out of the undifferentiated cell population that forms the bulk of neurospheres. Evidence that such a process may also occur under in vivo conditions is provided in Fig. 5S (supplemental material). In this figure, astrocyte progenitors, obtained from explants of E14 mouse forebrains, are shown to release quinacrine and ATP in a Ca2+-dependent manner. Similarly to what was observed for neurosphere cultures, interference with the formation of SNARE complexes through transfection of dn-syb2 in freshly prepared forebrain explants, reduced progenitor cell migration (Fig. 5S, supplemental material).
Fig. 6.
P2Y1 receptor activation increases migration of dn-syb2-expressing progenitor cells. Bar histograms show the mean ± SEM values of outgrowth index (OI) from progenitors derived from mock (empty-vector) and dn-syb2 transfected neurospheres before (black and white bars, respectively) and after exposure to 200 nM MeSATP (gray and cross-hatched bars, respectively). Measurements were performed 6-30 h after adhesion of neurospheres to fibronectin-coated dishes. Note the significant OI increase in dn-syb2 transfectants treated with MeSATP compared with untreated dn-syb2 transfectants. *P < 0.05; t-test.
DISCUSSION
ATP is an important neurotransmitter and neuromodulator that mediates a broad range of events including cell proliferation (Ryu et al., 2003) and migration (Agresti et al., 2005; Scemes et al., 2003), and serves as a signal to initiate brain repair mechanisms (Neary et al., 1996). The mechanism by which ATP is released from glial cells is still not well resolved. Two main pathways of ATP release from astrocytes have been suggested, regulated secretion/exocytosis (Abdipranoto et al., 2003; Coco et al., 2003) and the transfer from the cytoplasm to the extracellular space, the latter occurring through membrane transporters (Abdipranoto et al., 2003; Darby et al., 2003) and ion channels including the ionotropic P2X receptors (Anderson et al., 2004; Ballerini et al., 1996; Suadicani et al., 2006), connexin hemichannels (Anderson et al., 2004; Cotrina et al., 1998; Stout et al., 2002; but see Spray et al., 2006), and pannexins (Bao et al., 2004; Locovei et al., 2006). Given that a variety of different conditions, including mechanical stress, agonist stimulation, osmotic shock and low extracellular Ca2+ levels induce the release of ATP from astrocytes (Neary et al., 2003; Queiroz et al., 1997, 1999; Wang et al., 2002), it is most likely that distinct pathways mediate ATP release from mature astrocytes under physiological and pathological conditions.
Evidence that the regulated secretion of vesicular ATP from astrocytes, first reported in culture (Abdipranoto et al., 2003; Coco et al., 2003), is of physiological significance in the adult CNS was recently provided in acute hippocampal slices isolated from transgenic mice expressing a dominant-negative SNARE domain in the astrocytic population (Pascual et al., 2005). In this system, the SNARE-dependent release of ATP from astrocytes leads to the suppression of excitatory synaptic transmission, thereby regulating the degree of synaptic plasticity (Pascual et al., 2005). These exciting findings indicate that purinergic signaling originating in astrocytes plays a crucial role in the mature CNS.
Given the potential relevance of astrocyte purinergic signaling to CNS function, we investigated whether and in which stage of astrocyte development regulated exocytosis is competent and evaluated the biological significance of this signaling pathway in the early development of the CNS. Here, we have approached these questions using a neurosphere model, which provides for the in vitro differentiation of neuronal, oligodendrocytic and astrocytic progenitors. We now show that astrocyte progenitors are competent for the regulated secretion of ATP. We further document that, like mature astrocytes (Coco et al., 2003), astrocyte progenitors release most of the nucleotide by exocytosis of ATP-containing vesicles, which is dependent on cytosolic Ca2+ levels and the v-SNARE complex. The size of these exocytic vesicles (about 170 nm in diameter) is in the same range (about 115 nm) of that reported for the dense-core vesicles containing secretogranin II and ATP in mature astrocytes (Calegari et al., 1999; Coco et al., 2003), and much larger than that (30 nm) of the electro-lucent vesicles containing glutamate (Bezzi et al., 2004; but see Chen et al., 2005; Kang et al., 1998; for review see Montana et al., 2006). Interestingly, however, synaptobrevin-2 has been reported to be mainly associated with electron-lucent vesicles containing glutamate or D-serine (Anlauf and Derouiche, 2005; Crippa et al., 2006; Montana et al., 2004; Mothet et al., 2005; Oliet and Mothet, 2006), and absent from secretogranin II fractions containing dense-core vesicles (Coco et al., 2003). Although our results indicate that vesicular ATP release from astrocytes progenitors is dependent on the functional integrity of synaptobrevin-2, suggesting that this SNARE protein is associated with this vesicular pool, further molecular biological, biochemical and electron-microscopy experiments are now needed to unambiguously demonstrate this association.
In the present study, we provide evidence for an autocrine/paracrine mechanism, whereby alterations in the regulated secretion of ATP are associated to alterations in the amplitude and frequency of intracellular Ca2+ oscillations and in the rate of migration of the progenitor cells. Given the importance of these two events for the proper differentiation, morphogenesis and cross-talk of neural networks, the data imply a significant role of the ATP-dependent signaling in the structural and functional development of the CNS. Accordingly, expression of a dominant-negative domain of synaptobrevin-2, which interferes with the SNARE complex, resulted in a decreased secretion of ATP from astrocyte progenitors. This decreased ATP secretion was associated with alterations in their spontaneous Ca2+ oscillations, and in their rate of emigration from the neurosphere, that mostly comprises undifferentiated, nestin positive cells (Duval et al., 2002; Scemes et al., 2003).
These data have at least two implications. First, by showing that a molecular change expected to selectively alter the exocytotic machinery resulted in alterations of secretion-independent events, the data support the primordial role of the exocytotic ATP release. Thus, although we cannot rule out the possibility that alternative mechanisms, such as those provided by ion channel activation (Anderson et al., 2004; Ballerini et al., 1996; Bao et al., 2004; Cotrina et al., 1998; Locovei et al., 2006; Stout et al., 2002; Suadicani et al., 2006) may also marginally contribute to the release of ATP, our experiments clearly demonstrate that, all together, these putative mechanisms could not compensate for the loss of the regulated secretion of the nucleotide. In turn, this implies that the diffusional loss of cytosolic ATP is presumably less important than regulated secretion, at least for promoting the emigration of neural cell progenitors from the neurosphere core. Second, our findings also imply that, in astrocyte progenitors, a close relationship exists between the control of ATP secretion and that of cytosolic Ca2+. The finding that a decrease in v-SNARE expression, which does not directly affect Ca2+ mobilization induced by bath application of P2R agonists, affected the spontaneous oscillations of the cation in astrocyte progenitors, suggests that changes in ATP secretion drive those in Ca2+ pattern, in this cell population. This conclusion is also in agreement with our observation that a sizable number of ATP-containing vesicles were recruited and exocytosed at time points that during stimulation preceded the maximum Ca2+ elevation. Thus, the most likely scenario accounting for the available data favors the view that ATP is the primary signal that controls Ca2+ oscillations and cell migration. In this perspective, our study implicates a central role of astrocyte progenitors during the early development of the CNS, specifically through an ATP-dependent signaling that modulates the migration of progenitor cells.
Supplementary Material
ACKNOWLEDGMENTS
We appreciate the discussions and helpful comments provided by Dr. Peter Mabie and Dr. David C. Spray. We have greatly appreciated the valuable assistance of Dr. Bernhard Wehrle-Haller with the initial experiments using laser-based evanescent field microscopy, and the technical assistance of Ms. Marcia Urban-Maldonado with the dn-syb construct.
Grant sponsor: NMSS; Grant number: PPO-1019; Grant sponsor: NIH; Grant number: NS-52245; Grant sponsor: SNSF; Grant number: 310000-109402; Grant sponsor: JDRF; Grant number: 1-2007-158.
Footnotes
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/0894-1491/suppmat
REFERENCES
- Abbracchio MP. ATP and brain function. In: Jacobson KA, Jarvis MF, editors. Purinergic approaches in experimental therapeutics. Willey-Liss; New York: 1997. pp. 383–404. [Google Scholar]
- Abdipranoto A, Liu GJ, Werry EL, Bennett MR. Mechanisms of secretion of ATP from cortical astrocytes triggered by uridine triphosphate. Neuroreport. 2003;14:2177–2181. doi: 10.1097/00001756-200312020-00009. [DOI] [PubMed] [Google Scholar]
- Agresti C, Meomartini ME, Amadio S, Ambrosini E, Serafini B, Franchini L, Volonte C, Aloisi F, Visentin S. Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia. 2005;50:132–144. doi: 10.1002/glia.20160. [DOI] [PubMed] [Google Scholar]
- Aguado F, Espinosa-Parrilla JF, Carmona MA, Sorian E. Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. J Neurosci. 2002;22:9430–9444. doi: 10.1523/JNEUROSCI.22-21-09430.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnert-Hilger G, Bigalke H. Molecular aspects of tetanus and botulin neurotoxin poisoning. Prog Neurobiol. 1995;46:83–96. doi: 10.1016/0301-0082(95)00003-e. [DOI] [PubMed] [Google Scholar]
- Alund M, Olson L. Release of 14C quinacrine from peripheral and central nerves. J Auton Nerv Syst. 1980;2:281–294. doi: 10.1016/0165-1838(80)90017-x. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Berger JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem. 2004;88:246–256. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- Anlauf E, Derouiche A. Astrocytic exocytosis vesicles and glutamate: A high-resolution immunofluorescence study. Glia. 2005;49:96–106. doi: 10.1002/glia.20094. [DOI] [PubMed] [Google Scholar]
- Ballerini P, Rathbone MP, Di Iorio P, Renzetti A, Giuliani P, D'Alimonte I, Trubiani O, Caciagli F, Ciccarelli R. Rat astroglial P2Z (P2X7) receptors regulate intracellular calcium and purine release. Neuroreport. 1996;7:2533–2537. doi: 10.1097/00001756-199611040-00026. [DOI] [PubMed] [Google Scholar]
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Belai A, Burnstock G. Pattern of distribution and co-localization of NOS, ATP in the myenteric plexus of human fetal stomach and intestine. Neuroreport. 2000;11:5–8. doi: 10.1097/00001756-200001170-00002. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Bock P. Identification of paraneurons by labeling with quinacrine (Atebrin) Arch Histol Jpn. 1980;43:35–44. doi: 10.1679/aohc1950.43.35. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Bowman EJ, Siebers A, Altendorf K. Bafilomycins: A class of inhibitors of membrane ATPases from microorganisms, animal cells and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calegari F, Coco S, Taverna E, Bassetti M, Verderio C, Corradi N, Matteoli M, Rosa P. A regulated secretory pathway in cultured hippocampal astrocytes. J Biol Chem. 1999;274:22539–22547. doi: 10.1074/jbc.274.32.22539. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang I, Zhou Y, Zheng LH, Zhou Z. “Kiss-and-run” glutamate secretion in cultured freshly isolated rat hippocampal astrocytes. J Neurosci. 2005;25:9236–9243. doi: 10.1523/JNEUROSCI.1640-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa D, Schenk U, Francolini M, Rosa P, Verderio C, Zonta M, Pozzan T, Matteoli M, Carmognoto G. Synaptobrevin-2-expressing vesicles in rat astrocytes: Insights into molecular characterization, dynamics and exocytosis. J Physiol. 2006;570:567–582. doi: 10.1113/jphysiol.2005.094052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe R, Burnstock G. Fluorescence histochemical localisation of quinacrine-positive neurones in the guinea pig and rabbit atrium. Cardiovasc Res. 1982;16:384–390. doi: 10.1093/cvr/16.7.384. [DOI] [PubMed] [Google Scholar]
- Darby M, Kuzmiski JB, Panenka W, Feighan D, MacVicar BA. ATP released from astrocytes during swelling activates chloride channels. J Neurophysiol. 2003;89:1870–1877. doi: 10.1152/jn.00510.2002. [DOI] [PubMed] [Google Scholar]
- Duval N, Gomes D, Calaora V, Calabrese A, Meda P, Bruzzone R. Cell coupling and Cx43 expression in embryonic mouse neural progenitor cells. J Cell Sci. 2002;115:3241–3251. doi: 10.1242/jcs.115.16.3241. [DOI] [PubMed] [Google Scholar]
- Evanko DS, Zhang Q, Zorec R, Haydon PG. Defining pathways of loss and secretion of chemical messengers from astrocytes. Glia. 2004;47:233–240. doi: 10.1002/glia.20050. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2+ fluctuations. J Neurobiol. 1998;37:110–130. [PubMed] [Google Scholar]
- Kumada T, Komuro H. Completion of neuronal migration regulated by loss of Ca2+ transients. Proc Natl Acad Sci USA. 2004;101:8479–8484. doi: 10.1073/pnas.0401000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Yang J, Liu S, Takano T, Wang X, Gao Q, Willecke K, Nedergaard M. Connexin mediates gap junction-independent resistance to cellular injury. J Neurosci. 2003;23:430–441. doi: 10.1523/JNEUROSCI.23-02-00430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rao MS. Glial progenitors in the CNS, possible lineage relationships among them. Biol Cell. 2004;96:279–290. doi: 10.1016/j.biolcel.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu Y, Lee JC, Xue H, Pevny LH, Kaprielian Z, Rao MS. Oligodendrocyte and astrocyte development in rodents: An in situ and immunohistological analysis during embryonic development. Glia. 2002;40:25–43. doi: 10.1002/glia.10111. [DOI] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Braun N, Shukla V, Fullgrabe M, Schomerus C, Korf HW, Gachet C, Ikehara Y, Sevigny J, Robson SC, Zimmermann H. Extracellular nucleotide signaling in adult neural stem cells: Synergism with growth factor-mediated cellular proliferation. Development. 2006;133:675–684. doi: 10.1242/dev.02233. [DOI] [PubMed] [Google Scholar]
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura P. Vesicular transmitter release from astrocytes. Glia. 2006;54:700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura P. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet J-P, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci USA. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Willoughby KA, Ellis EF. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP, P2 purinergic receptors. J Neurosci. 2003;23:2348–2356. doi: 10.1523/JNEUROSCI.23-06-02348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Zhu Q. Signaling by ATP receptors in astrocytes. Neuroreport. 1994;5:1617–1620. doi: 10.1097/00001756-199408150-00019. [DOI] [PubMed] [Google Scholar]
- Neary JT, Zhu Q, Kang Y, Dash PK. Extracellular ATP induces formation of AP-1 complexes in astrocytes via P2 purinoceptors. Neuroreport. 1996;7:2893–2896. doi: 10.1097/00001756-199611250-00017. [DOI] [PubMed] [Google Scholar]
- Oliet SHR, Mothet J-P. Molecular determinants of D-serine-mediated gliotransmission: From release to function. Glia. 2006;54:726–737. doi: 10.1002/glia.20356. [DOI] [PubMed] [Google Scholar]
- Olson L, Alund M. Quinacrine-binding nerves: Presence in the mouse ano-coccygeus muscle disappearance after muscle transsection. Med Biol. 1979;57:182–186. [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signaling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parpura V, Fang Y, Basarsky T, Jahn R, Haydon PG. Expression of synaptobrevin II, cellubrevin and syntaxin but not SNAP-25 in cultured astrocytes. FEBS Lett. 1995;377:489–492. doi: 10.1016/0014-5793(95)01401-2. [DOI] [PubMed] [Google Scholar]
- Parpura V, Scemes E, Spray DC. Mechanisms of glutamate release from astrocytes: Gap junction “hemichannels”, purinergic receptors and exocytotic release. Neurochem Int. 2004;45:259–264. doi: 10.1016/j.neuint.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Parri HR, Crunelli V. Pacemaker calcium oscillations in thalamic astrocytes in situ. Neuroreport. 2001;12:3897–3900. doi: 10.1097/00001756-200112210-00008. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Queiroz G, Gebicke-Haerter PJ, Schobert A, Starke K, von Kugelgen I. Release of ATP from cultured rat astrocytes elicited by glutamate receptor activation. Neuroscience. 1997;78:1203–1208. doi: 10.1016/s0306-4522(96)00637-9. [DOI] [PubMed] [Google Scholar]
- Queiroz G, Meyer DK, Meyer A, Starke K, von Kugelgen I. A study of the mechanism of the release of ATP from rat cortical astroglial cells evoked by activation of glutamate receptors. Neuroscience. 1999;91:1171–1181. doi: 10.1016/s0306-4522(98)00644-7. [DOI] [PubMed] [Google Scholar]
- Rao MS, Mayer-Proschel M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Dev Biol. 1997;188:48–63. doi: 10.1006/dbio.1997.8597. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Choi HB, Hatori K, Heisel RL, Pelech SL, McLarnon JG, Kim SU. Adenosine triphosphate induces proliferation of human neural stem cells: Role of calcium and p70 ribosomal protein S6 kinase. J Neurosci Res. 2003;72:352–362. doi: 10.1002/jnr.10507. [DOI] [PubMed] [Google Scholar]
- Scemes E, Duval N, Meda P. Reduced expression of P2Y1 receptors in connexin43-null mice alters calcium signaling and migration of neural progenitor cells. J Neurosci. 2003;23:11444–11452. doi: 10.1523/JNEUROSCI.23-36-11444.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulin B, Rossetto O, Polverino de Laureto P, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: A critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth CD, Johnson RG., Jr ATP compartmentation in neuroendocrine secretory vesicles. Ann NY Acad Sci. 1990;603:353–363. doi: 10.1111/j.1749-6632.1990.tb37685.x. [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, North RA, Surprenant A. Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol. 1999;519:335–346. doi: 10.1111/j.1469-7793.1999.0335m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CM, Chang YY, Kuo JS, Sun SH. Activation of P2X7 receptors induced [3H]GABA release from the RBA-2 type-2 astrocyte cell line through a Cl-/HCO3--dependent mechanism. Glia. 2002;37:8–18. doi: 10.1002/glia.10004. [DOI] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Yuste R, Peinado A, Katz LC. Neuronal domains in developing neocortex. Science. 1992;257:665–669. doi: 10.1126/science.1496379. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Pangr T, Kreft M, Kran M, Li N, Sul JY, Halassa M, Van Bockstaele E, Zore RC, Haydon PG. Fusion-related release of glutamate from astrocytes. J Biol Chem. 2004;279:12724–12733. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.