Abstract
The significance of collagen XVIII in the regulation of corneal reinnervation remains largely unknown. We used whole mount immunoconfocal microscopy to localize collagen XVIII to the nerve basement membrane of wild-type (WT) mouse corneas. Transmission electron microscopy showed corneal nerve disorganization in collagen XVIII knockout mice (col18a1−/−). Antibody 2H3-specific neurofilament colocalized with collagens XVIII and IV and laminin-2 in WT mouse corneas, but did not colocalize with collagen IV and laminin-2 in col18a1−/− mouse corneas. Following keratectomy, col18a1−/− mice displayed decreased corneal neurite extension compared to WT mice. Our data indicate that collagen XVIII may play an important role in corneal reinnervation after wounding.
Keywords: extracellular matrix, collagen XVIII
Introduction
Several extracellular matrix (ECM) components are involved in regulatory processes necessary for normal development and function of the nervous system [1–4]. These components include collagen IV, collagen XVIII, laminin, fibronectin, transforming growth factor (TGF)-β, basic fibroblast growth factor (bFGF), nidogen-1, and thrombospondin-4 [2]. The ECM not only plays a structural role in the nervous system, but is also involved in cell migration, nutritional homeostasis, and in maintaining the membrane potential [3,5]. Additionally, the ECM is specifically involved in growth and regeneration of the nervous system as well as neuronal cell death [6,7]. Because of the many functions performed by the ECM, alterations in its composition may play an important role in the development and progression of diseases of the nervous system.
Laminin-2 (composed of α2, β1 and γ1) is a major matrix component of the nerve basement membrane that is involved in nerve regeneration [1]. Disruption of the laminin γ1 gene in Schwann cells causes impaired axon regeneration following nerve injury [8]. Mutations in the nidogen-1 gene in mice (encoding a minor component of the basement membrane) leads to a loss of hind-limb motor control and spontaneous seizure-like symptoms [9]. Similarly, collagen XVIII, another component of the basement membrane, is involved in regulating the neuromuscular junction in C. elegans, and mutations in the collagen XVIII gene result in abnormal axon guidance [4]. Mice with mutations in the collagen XVIII gene display Knobloch syndrome, similar to vitreoretinal and macular degeneration, as well as occipital encephalocele [10]. These examples highlight the important role of the basement membrane in nervous system function and homeostasis.
The cornea is among the most densely innervated tissues of the human body, containing primarily sensory and some autonomic innervation. During development, sensory axons initially avoid the cornea and extend dorsally and ventrally to form a nerve ring around the cornea and pause in the limbus region of the eye. Subsequently, the axons grow radially into the cornea, eventually innervating its entire surface [11]. The molecular signals that control axon growth into the cornea and the significance of the ECM components of the nervous system basement membrane in the regulation of axon outgrowth into the cornea remain largely unknown. Lwigale et al. [12] recently demonstrated that lens-derived Sema3A mediates the initial repulsion of trigeminal sensory axons from the cornea, and is necessary for the proper formation of the nerve ring.
In this study we focus on collagen XVIII, one of the many components that comprise the corneal nerve basement membrane. While the role of collagen XVIII in ocular angiogenesis, lymphangiogenesis and wound healing has already been described [13–15], its function in corneal nerve regeneration has not been well characterized. In this study, we investigate corneal reinnervation following wounding in col18a1−/− mice to determine the role of collagen XVIII.
Materials and Methods
Animals
C57BL/6 mice were purchased from Charles River Laboratory. Col18a1−/− mice were generously provided by Dr. B. Olsen (Department of Oral and Developmental Biology, Harvard School of Dental Medicine, Boston, Massachusetts). The study was conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and approved by the Animal Care and Use Committee at the Massachusetts Eye and Ear Infirmary.
Whole-mount immunohistochemistry of the corneal nerve
Untreated mice were sacrificed at four to eight weeks of age. Whole corneas were fixed in a 4:1 mixture of 100% methanol and dimethyl sulfoxide for 2 h at room temperature, and then in 100% methanol at −20°C. The eye was treated with a methanol series using 70%, 50% and 30% methanol in phosphate-buffered saline (PBS) for 3 min each. Blocking was then performed using 1% bovine serum albumin (BSA) in PBS for 3 min. The primary antibody was applied overnight at 4°C. After washing, the secondary antibody was incubated for 4 h. Four radial incisions were made from the peripheral rim of the graft enabling it to be flattened on a glass slide and viewed using a confocal microscope (TCS 4D; Leica, Heidelberg, Germany). The antibodies used were anti-neurofilament (2H3; 1:5; University of Iowa Hybridoma Bank, Iowa City, IA), anti-collagen IV (1:50; Southern Biotechnology, Birmingham, AL), anti-collagen XVIII (anti-NC1-hinge domain; 1:50) [16], anti-laminin-2 alpha chain (1:50; Abcam, Cambridge, MA), and FITC, Cy5 or TRITC conjugated donkey anti-mouse -goat, or -rat (1:400; Jackson ImmunoResearch, West Grove, PA).
Immunohistochemistry of the cornea and trigeminal ganglion
After the mice were sacrificed, either the eye or the trigeminal ganglion was immediately frozen in OCT compound (Tissue-Tek, Sakura Finetek USA, Torrance, CA) in dry ice. Cryosections (8 µm) were made and fixed in 4% paraformaldehyde for 15 min at room temperature. After blocking with 1% bovine serum albumin (BSA) in PBS, sections were incubated for 2 h with primary antibodies (1:100 dilution). After washing with PBS, the sections were incubated with secondary antibodies for 1 h and subsequently viewed using a confocal microscope.
Transmission electron microscopy (TEM)
Corneal-scleral grafts were prepared for TEM in half-strength Karnovsky fixative (2% paraformaldehyde and 2.5% glutaraldehyde) and processed in 0.2 M cacodylate buffer (pH 7.4) overnight. The samples were then post-fixed in 1% osmium tetroxide, dehydrated in graded alcohols, embedded in epoxy resin (Epon 812; Epon-LKB Instrument, Gaithersburg, MD; and Araldite 506; Ernest F. Fullam, Latham, NY) and oven-dried at 60°C for 48 h. One µm thick sections were stained with toluidine blue for orientation. Subsequent ultra-thin sections were counterstained with 2% uranyl acetate lead citrate and analyzed using a TEM (Model 410; Philips, Eindhoven, the Netherlands).
Corneal reinnervation following keratectomy wounds
To assess nerve regeneration in the cornea, a DTAF-stained keratectomy model was used. DTAF was applied to aid in the recognition of the wound edge during subsequent immunohistochemistry experiments, as described by us previously [17].
Whole-mounted eyes were used to identify corneal reinnervation. Mice were anesthetized with an intramuscular injection of 1:1 mixture of ketamine (4 mg/kg body weight) and xylazine. For local anesthesia, 0.5% proparacaine eye drops were used. A 1.5 mm trephine was used to make an incision in the mid-stroma. Approximately 50 µl of 0.05% dichlorotriazinyl aminofluorescein (DTAF, Sigma-Aldrich, St. Louis, MO) was applied to stain the wound edge. The central part of the corneal stroma enclosed within the trephine mark was manually dissected. Mice were sacrificed at 28 days post keratectomy wounding and corneas were examined using immunohistochemistry. The reinnervating nerves, stained by anti-neurofilament antibodies, and the wound edges, stained by DTAF, were visualized using confocal microscopy.”
Results
Localization of collagen XVIII and collagen IV in corneal nerves
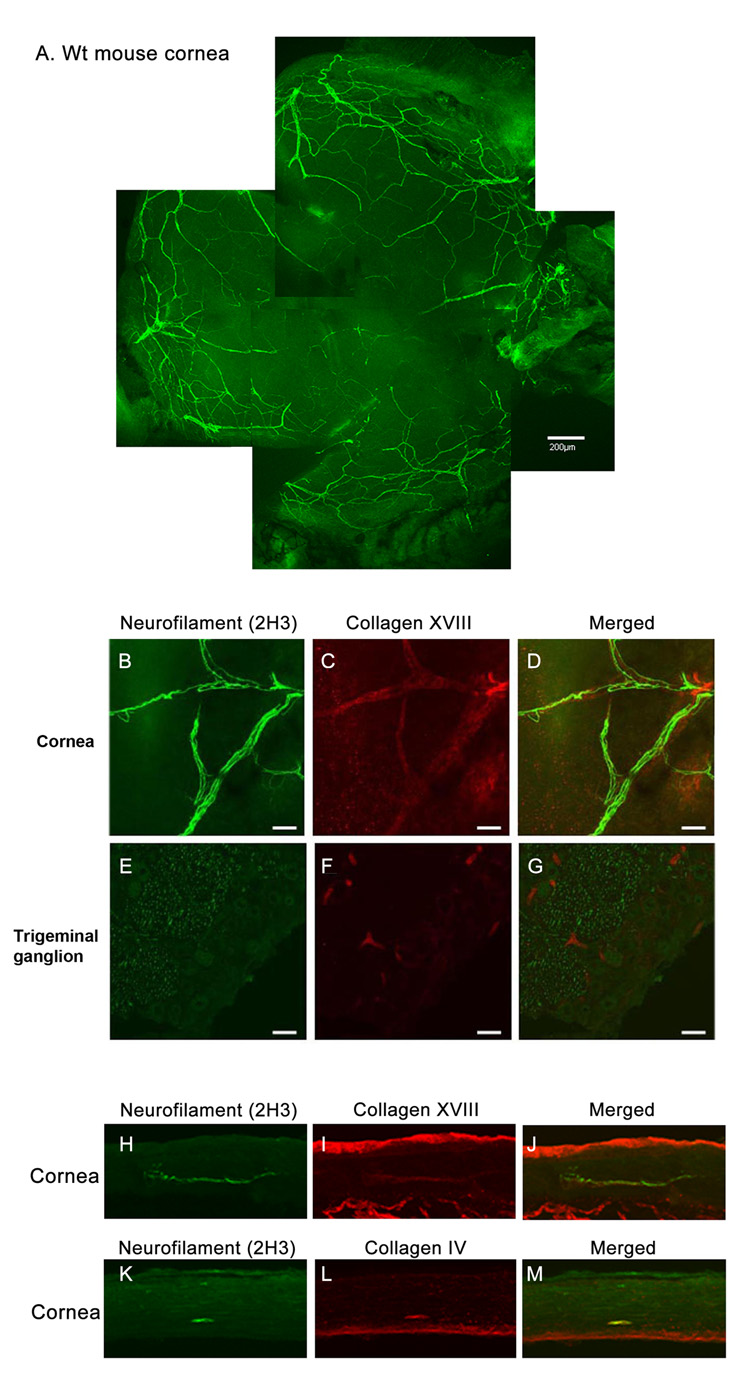
First, we examined the expression patterns of collagen XVIII and IV in corneal nerves using immunohistochemistry. Whole-mounts of mouse corneas showed immunolocalization of antibody 2H3-reactive neurofilament to stromal nerves entering peripheral stroma as 6–7 bundles before branching towards the central cornea (Fig. 1A). We also demonstrated a colocalization of collagen XVIII to the corneal nerve (neurofilament staining, Fig. 1B–D). There was no colocalization of collagen XVIII to neurofilament in the trigeminal ganglion (Figure 1E–G). In wild-type (WT) mouse corneal cross-sections, neurofilament (Fig. 1H, K) coimmunostained with collagen XVIII and collagen IV (Fig. 1I and L, respectively). Immunolocalization of collagen XVIII in the perilimbal conjunctival nerves was observed in the corneoscleral whole-mounts.
Figure 1. Localization of collagen XVIII and collagen IV in the corneal nerve.
A mouse cornea whole-mount stained with anti-neurofilament antibody 2H3 (A). In the corneal nerve, neurofilament was colabeled with collagen XVIII (B, C, D). No colocalization of neurofilament and collagen XVIII was present in the trigeminal ganglion (E–G; Bar = 40µm). In a cross-section, neurofilament coimmunostained with collagen XVIII (H–J) and collagen IV (K–M).
Transmission electron microscopy (TEM)
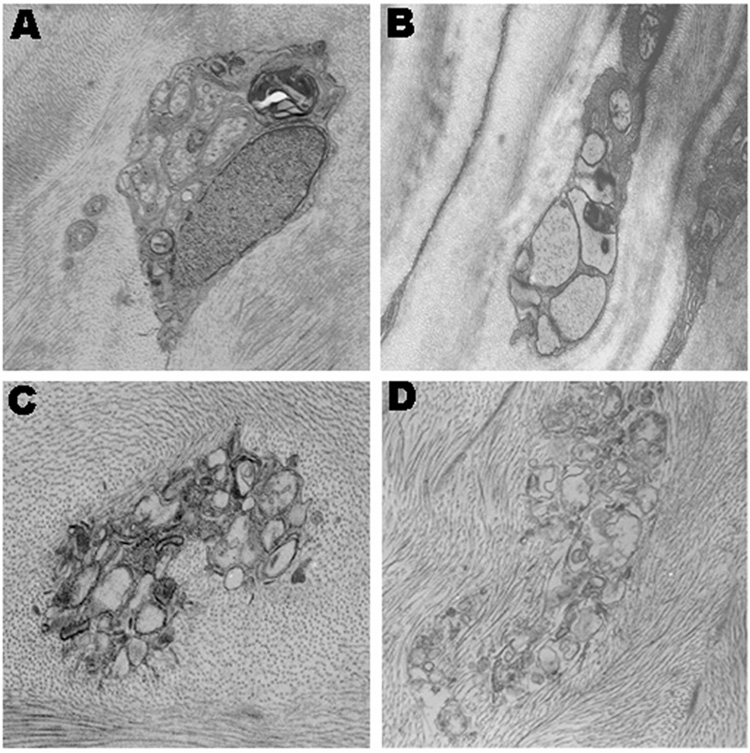
Transmission electron microscopy (TEM) of WT corneas showed evidence of well-organized nerve bundles surrounded by Schwann cells and basal lamina (Fig. 2A, B). The corneal nerves in the col18a1−/− mice appeared disorganized. The cytoplasm and nuclei of the Schwann cells appeared disrupted (Fig. 2C, D).
Figure 2. Transmission electron microscopy (TEM) of the mouse cornea.
Well-organized nerve bundles are surrounded by a Schwann cell in WT mice (A–B). Nerve bundles are disorganized in the col18a1−/− mice (C–D).
Abnormal basement membrane components of the cornea nerves in col18a1−/− mice
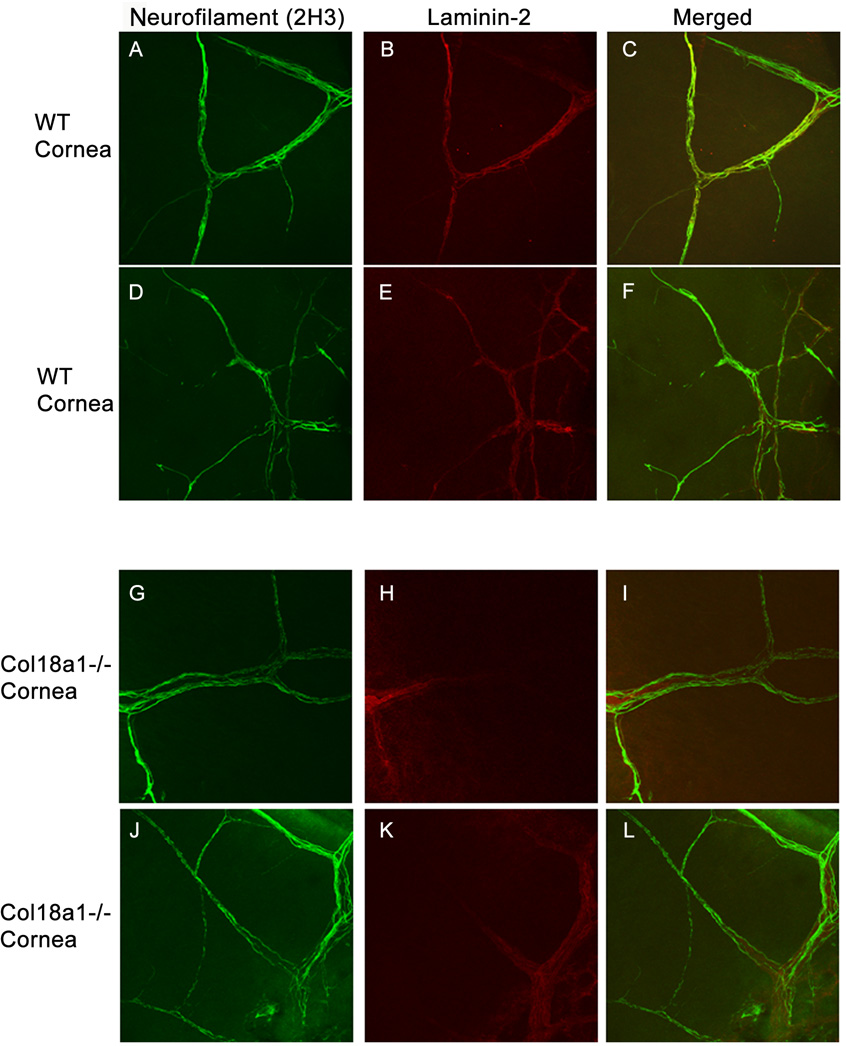
Immunostaining for the basement membrane marker laminin-2 was performed (Fig. 3B, E) and it colocalized with neurofilament (Fig. 3A, D) in the WT mouse cornea. Laminin-2 also colocalized with neurofilament in the bigger nerve bundles but not in the smaller nerve bundles in the col18a1−/− corneas (Fig. 3G–L). The distinction between bigger and smaller nerve bundles was based on thickness.
Figure 3. Abnormal laminin-2 staining in the cornea of col18a1−/−.
The cornea was whole-mount immunostained with anti-neurofilament antibody 2H3 (A, D, G, J) and anti-laminin-2 antibodies (B, E, H, K; merged images C, F, I, L, respectively). The corneal limbus is located on the left side of each picture and the central cornea is located on the right. Little to no laminin-2 colocalized with neurofilament in the col18a1−/− corneas (H, K I, L) relative to the WT corneas (B, E, C, F). Laminin-2 staining was only observed at the limbal area of col18a1−/− corneas (H, K), when compared with the WT cornea (B, E).
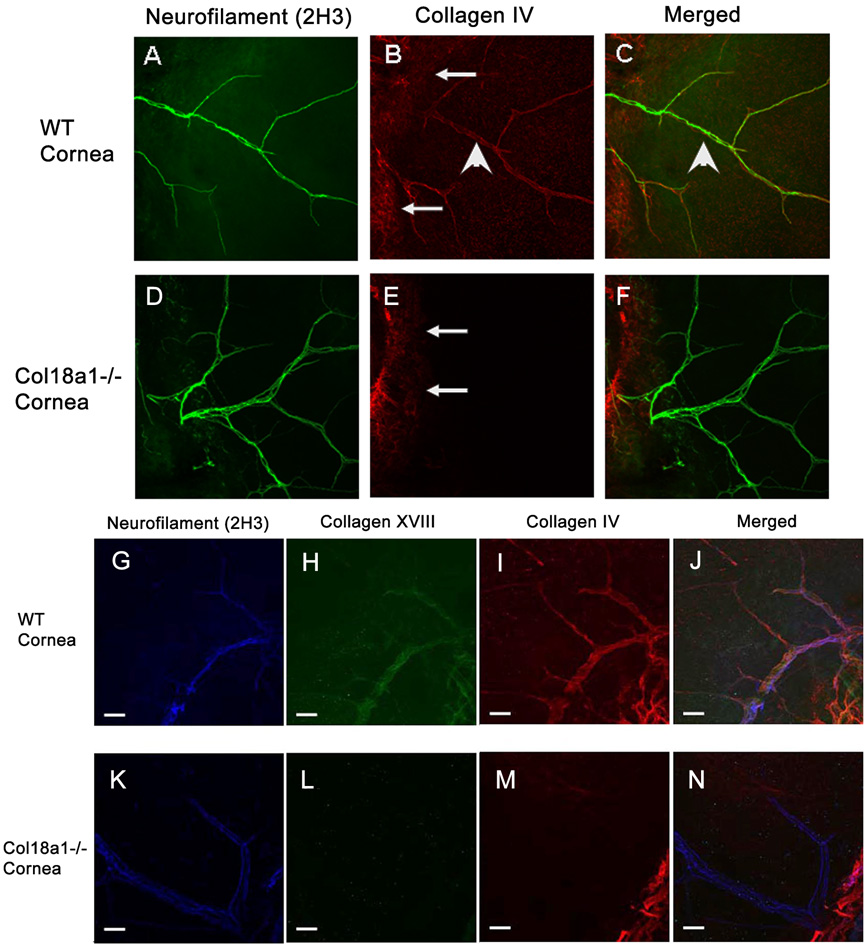
The expression of collagen IV was also examined in conjunction with neurofilament in WT and col18a1−/− mice. Collagen IV immunostained (Fig. 4B) and colocalized with neurofilament in WT mouse corneas (Fig. 4C). Little to no collagen IV colocalized with neurofiliment in col18a1−/− mouse corneas (Fig. 4F).
Figure 4. Abnormal composition of the corneal nerve basement membrane in col18a1−/−.
The whole-mount cornea was coimmunostained with anti-neurofilament (A, D) and anti-collagen IV antibodies (merged images C, F, respectively). Almost no collagen IV associated with neurofilament was detected in the col18a1−/− corneas (E) when compared with that of the WT corneas (B). The cornea was then triple stained and visualized using a confocal microscope [antibodies against collagen XVIII (H, L); neurofilament (G, K); collagen IV (I, M); merged (J, N)]. Collagen IV, collagen XVIII and neurofilament colocalized to the nerve basement membrane in WT mice (J). Triple immunostaining did not reveal colocalization of these three molecules in the col18a1−/− mouse corneas (N). The arrowheads point to neurofilament. The arrows point to stromal collagen IV that does not colocalize with the neurofilament.
We then carried out a triple immunostaining study using antibodies against neurofilament, collagen XVIII and collagen IV to determine colocalization of these basement membrane components in corneal nerves. This experiment revealed partial colocalization of these three molecules in WT mouse corneas (Fig. 4G–J) but not in col18a1−/− mouse corneas (Fig. 4K–N).
Delayed corneal innervation in col18a1−/− mice after keratectomy-induced wounding
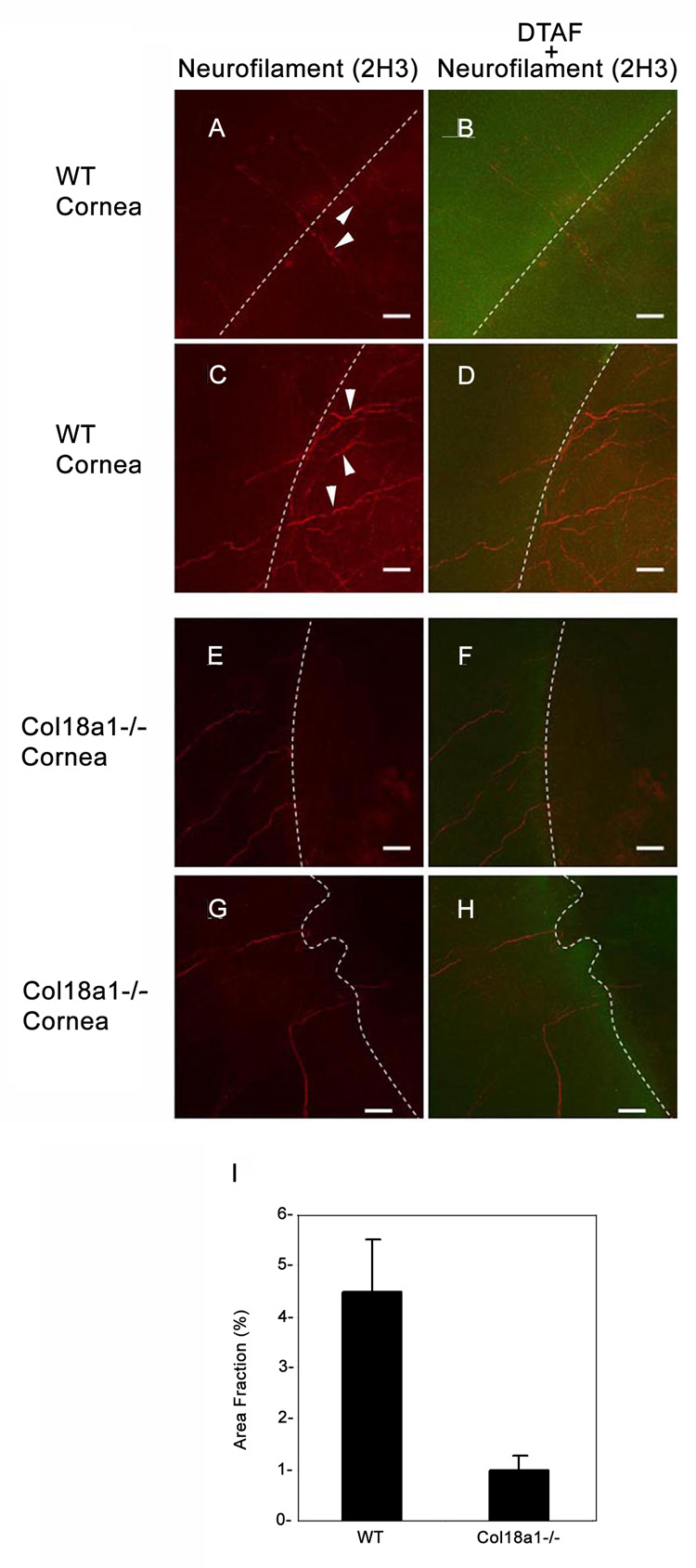
Using a DTAF-keratectomy model, we observed the reinnervation from the unwounded area (marked by DTAF staining) to the newly synthesized central corneal area (without DTAF) in WT mice (Figure 5A–D). The fraction of the area covered by neurofilament staining was significantly decreased at day 28 in col18a1−/− mice (1.03% ± 0.27%; Figure 5E–H) relative to WT mice (Figure 5A–D; 4.5% ± 1.01%).
Figure 5. Corneal reinnervation (day 28) after keratectomy was delayed in col18a1−/− mice.
The cornea wound edge was stained with DTAF (green; B, D, F and H) to allow a better comparison with the unwounded area in the peripheral cornea. White dotted lines represent the boundary of the wound edge between the DTAF-stained area and dark DTA-Funstained areas. Regenerating nerves (arrowheads), stained by anti-neurofilament antibodies (red; A and C) passed across the wound edge in WT mice. Regenerating nerves displayed delayed neurite extension into the wounded areas in col18a1−/− mouse corneas (E and G). Semi-quantitative analysis of neurite extension into the wounded corneal area was performed using the Image-J program (I). Four images from WT cornea and four images from the Col18a1−/− cornea were analyzed by adjusting the threshold for accurate representation of the borders of the neurons extending into the injured corneal area. The area fraction of the neurons compared to the entire injured area was calculated and graphed to compare the WT cornea with the col18a1−/− cornea (Bar = 40 µm).
Discussion
Corneal nerves are integral to cornea homeostasis due to their role in protecting the cornea from irritants, as well as their trophic properties, which are necessary to maintain a healthy ocular surface. Disruption of the corneal nerves has been shown to significantly impair corneal wound healing [18]. Penetrating keratoplasty severs corneal nerves, resulting in complete denervation of the transplanted cornea [19]. After corneal transplantation, corneal nerve regeneration proceeds from the peripheral recipient cornea, through the scar tissue, and into the donor cornea [20]. Many diseases, such as herpetic viral infections, cause corneal nerve dysfunction [21]. Moreover, many ophthalmic surgical procedures can potentially result in disruption of corneal nerve innervation, leading to severe conditions such as neurotrophic keratitis with corneal melting [22] . Consequently, the ability for corneal nerve regeneration is critical in the treatment and management of corneal disease.
The basement membrane plays a key role in nerve regeneration, functioning as a scaffold during axon regeneration [7]. Basement membranes are composed primarily of collagen IV, laminins, and heparin sulfate proteoglycans. Additional minor components of the basement membrane include collagen XV, collagen XVIII, BM4, and fibulin [23,24].
Collagen XVIII is a heparin sulfate-containing proteoglycan that is expressed in the epithelial and endothelial basement membranes [16,25]. It is an extracellular matrix protein, distinguished by its multiple interrupted triple helical regions, and belongs to a family of nonfibrillar collagen proteins called multiplexins. Collagen XVIII contains several domains that regulate cell proliferation and apoptosis, including the frizzled, thrombospondin (TSP)-1 and endostatin domains [26]. The anti-angiogenic properties of the unique C-terminal endostatin domain have been extensively characterized. Collagen XVIII has been shown to be a highly active endothelial-specific angiogenic inhibitor. Recently, Quelard et al. [26] demonstrated that the frizzled domain in collagen XVIII is involved in inhibiting Wnt/b-catenin signaling. Halfter et al. [27] showed that collagen XVIII is localized to the peripheral nerve in the cross-section of the trunk of E8 chicks. Several other reports have demonstrated that collagen XVIII may be involved in nervous system function. For example, collagen XVIII has been detected in the fetal brain, and deficiency of collagen XVIII isoforms may predispose individuals to epilepsy [28]. Furthermore, when the NC1 domain of the C. elegans collagen XVIII homologue Cle1 is deleted, viable fertile animals display multiple cell migration and axon guidance defects [4,29].
In this study, we investigated the role of collagen XVIII in corneal reinnervation following keratectomy-induced wounding using immunohistochemical analyses in a knockout mouse model. We were able to confirm the importance of collagen XVIII in the corneal nerves. First, we established the presence of collagen XVIII in the corneal nerve basement membrane. Collagen XVIII coimmunolocalized with antibody 2H3-reactive neurofilament in WT mice. In collagen XVIII knockout mice (col18a1−/−), the corneal nerve appeared disorganized relative to WT mice. Additionally, significant structural abnormalities were noted in the corneal nerve basement membrane, and nerve regeneration in col18a1−/− mice was significantly delayed relative to WT mice. Immunohistochemical analysis of col18a1−/− mice revealed that the basement membrane markers laminin-2 and collagen IV did not colocalize with neurofilament, indicating that the absence of collagen XVIII resulted in abnormal basement membrane formation. Lastly, the keratectomy model revealed that the collagen XVIII knockout mice had decreased corneal nerve regeneration capacity compared to the WT mice. Taken together, these data are consistent with the hypothesis that collagen XVIII and possibly other basement membrane components are important for proper corneal reinnervation. Using in vivo confocal microscopy, Auran et al investigated the centripetal movement of epithelial cells and corneal nerves in normal human corneas. They observed that neurite growth occurred by the addition of new membrane along the length of the axon rather than at a distal growth cone [30]. Since collagen XVIII is a component of the nerve basement membrane, it is likely that collagen XVIII may be important for the normal neural turnover associated with epithelial shedding. Additional investigations are required to confirm this possibility. Although the mechanism of the corneal nerve abnormality in col18a1−/− mice has not yet been determined and despite the absent information regarding differences in type XVIII collagen expression in various corneal nerve subtypes (e.g. substance P or calcinitonin gene related peptide producing nerves), it is very likely that the collagen XVIII plays an important role in corneal nerve regeneration.
Acknowledgements
We thank Dr. Bjorn Olsen for providing col18a1−/− mice and Patricia Pearson for TEM imaging.
Supported by NIH EY14048 (JHC), EY10101 (DTA), EY 001792 (DTA), an unrestricted departmental grant from Research to Prevent Blindness, the Bausch Lomb Japan/MEEI Research Fellowship Award, and the Japan Eye Bank Association Overseas Research Award (TS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Commercial relationships: None
References
- 1.Chen ZL, Strickland S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol. 2003;163:889–899. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer JM. Basement membranes. WormBook. 2005:1–15. doi: 10.1895/wormbook.1.16.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li F, et al. Cellular and nerve regeneration within a biosynthetic extracellular matrix for corneal transplantation. Proc Natl Acad Sci U S A. 2003;100:15346–15351. doi: 10.1073/pnas.2536767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackley BD, Kang SH, Crew JR, Suh C, Jin Y, Kramer JM. The basement membrane components nidogen and type XVIII collagen regulate organization of neuromuscular junctions in Caenorhabditis elegans. J Neurosci. 2003;23:3577–3587. doi: 10.1523/JNEUROSCI.23-09-03577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport. 2000;11:1991–1996. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong SJ, Wiberg M, Terenghi G, Kingham PJ. Laminin activates NF-kappaB in Schwann cells to enhance neurite outgrowth. Neurosci Lett. 2008;439:42–46. doi: 10.1016/j.neulet.2008.04.091. [DOI] [PubMed] [Google Scholar]
- 7.Fansa H, Keilhoff G. Comparison of different biogenic matrices seeded with cultured Schwann cells for bridging peripheral nerve defects. Neurol Res. 2004;26:167–173. doi: 10.1179/016164104225013842. [DOI] [PubMed] [Google Scholar]
- 8.Yu CQ, Rosenblatt MI. Transgenic corneal neurofluorescence in mice: a new model for in vivo investigation of nerve structure and regeneration. Invest Ophthalmol Vis Sci. 2007;48:1535–1542. doi: 10.1167/iovs.06-1192. [DOI] [PubMed] [Google Scholar]
- 9.Dong L, et al. Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1/nidogen-1. Lab Invest. 2002;82:1617–1630. doi: 10.1097/01.lab.0000042240.52093.0f. [DOI] [PubMed] [Google Scholar]
- 10.Utriainen A, et al. Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Hum Mol Genet. 2004;13:2089–2099. doi: 10.1093/hmg/ddh213. [DOI] [PubMed] [Google Scholar]
- 11.Bee JA. The development and pattern of innervation of the avian cornea. Dev Biol. 1982;92:5–15. doi: 10.1016/0012-1606(82)90145-2. [DOI] [PubMed] [Google Scholar]
- 12.Lwigale PY, Bronner-Fraser M. Lens-derived Semaphorin3A regulates sensory innervation of the cornea. Dev Biol. 2007;306:750–759. doi: 10.1016/j.ydbio.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Kojima T, Azar DT, Chang JH. Neostatin-7 regulates bFGF-induced corneal lymphangiogenesis. FEBS Lett. 2008;582:2515–2520. doi: 10.1016/j.febslet.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang JH, Javier JA, Chang GY, Oliveira HB, Azar DT. Functional characterization of neostatins, the MMP-derived, enzymatic cleavage products of type XVIII collagen. FEBS Lett. 2005;579:3601–3606. doi: 10.1016/j.febslet.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 15.Fukai N, et al. Lack of collagen XVIII/endostatin results in eye abnormalities. Embo J. 2002;21:1535–1544. doi: 10.1093/emboj/21.7.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin HC, Chang JH, Jain S, Gabison EE, Kure T, Kato T, Fukai N, Azar DT. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Invest Ophthalmol Vis Sci. 2001;42:2517–2524. [PubMed] [Google Scholar]
- 17.Chang SW, Benson A, Azar DT. Corneal light scattering with stromal reformation after laser in situ keratomileusis and photorefractive keratectomy. J Cataract Refract Surg. 1998;24:1064–1069. doi: 10.1016/s0886-3350(98)80099-0. [DOI] [PubMed] [Google Scholar]
- 18.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 19.Lagali NS, Griffith M, Fagerholm P, Merrett K, Huynh M, Munger R. Innervation of tissue-engineered recombinant human collagen-based corneal substitutes: a comparative in-vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.07-1354. [DOI] [PubMed] [Google Scholar]
- 20.Niederer RL, Perumal D, Sherwin T, McGhee CN. Corneal innervation and cellular changes after corneal transplantation: an in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2007;48:621–626. doi: 10.1167/iovs.06-0538. [DOI] [PubMed] [Google Scholar]
- 21.Dixit R, Tiwari V, Shukla D. Herpes simplex virus type 1 induces filopodia in differentiated P19 neural cells to facilitate viral spread. Neurosci Lett. 2008;440:113–118. doi: 10.1016/j.neulet.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye. 2003;17:989–995. doi: 10.1038/sj.eye.6700616. [DOI] [PubMed] [Google Scholar]
- 23.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 24.LeBleu VS, Macdonald B, Kalluri R. Structure and function of basement membranes. Exp Biol Med (Maywood) 2007;232:1121–1129. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- 25.Marneros AG, Olsen BR. Physiological role of collagen XVIII and endostatin. Faseb J. 2005;19:716–728. doi: 10.1096/fj.04-2134rev. [DOI] [PubMed] [Google Scholar]
- 26.Quelard D, et al. A cryptic frizzled module in cell surface collagen 18 inhibits Wnt/beta-catenin signaling. PLoS ONE 3. 2008:e1878. doi: 10.1371/journal.pone.0001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halfter W, Dong S, Schurer B, Cole GJ. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J Biol Chem. 1998;273:25404–25412. doi: 10.1074/jbc.273.39.25404. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki T, et al. Identification and mutational analysis of candidate genes for juvenile myoclonic epilepsy on 6p11-p12: LRRC1, GCLC, KIAA0057 and CLIC5. Epilepsy Res. 2002;50:265–275. doi: 10.1016/s0920-1211(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 29.Sertie AL, Sossi V, Camargo AA, Zatz M, Brahe C, Passos-Bueno MR. Collagen XVIII, containing an endogenous inhibitor of angiogenesis and tumor growth, plays a critical role in the maintenance of retinal structure and in neural tube closure (Knobloch syndrome) Hum Mol Genet. 2000;9:2051–2058. doi: 10.1093/hmg/9.13.2051. [DOI] [PubMed] [Google Scholar]
- 30.Auran JD, Koester CJ, Kleiman NJ, Rapaport R, Bomann JS, Wirotsko BM, Florakis GJ, Koniarek JP. Scanning slit confocal microscopic observation of cell morphology and movement within the normal human anterior cornea. Ophthalmology. 1995;102:33–41. doi: 10.1016/s0161-6420(95)31057-3. [DOI] [PubMed] [Google Scholar]