Abstract
Signal transduction pathways guided by cellular receptors commonly exhibit low-level constitutive signaling in a continuous, ligand-independent manner. The dynamic equilibrium of positive and negative regulators establishes such a tonic signal. Ligand-independent signaling by the precursors of mature antigen receptors regulates development of B and T lymphocytes. Here we describe a basal signal that controls gene expression profiles in the Jurkat T cell line and mouse thymocytes. Using DNA microarrays and Northern blots to analyze unstimulated cells, we demonstrate that expression of a cluster of genes, including RAG-1 and RAG-2, is repressed by constitutive signals requiring the adapter molecules LAT and SLP-76. This TCR-like pathway results in constitutive low-level activity of Erk and Abl kinases. Inhibition of Abl by the drug STI-571 or inhibition of signaling events upstream of Erk increases RAG-1 expression. Our data suggest that physiologic gene expression programs depend upon tonic activity of signaling pathways independent of receptor ligation.
In the absence of basal signaling, RAG activity is high at a time during T cell development when it is otherwise normally suppressed
Introduction
Considerable evidence supports the notion that in most signal transduction systems regulated by cellular receptors some basal level of signaling occurs continuously in a ligand-independent manner, although the flux through such systems may vary considerably. The basal tone or the steady-state level of signaling in unstimulated cells is the result of an equilibrium of positive and negative regulators within a signaling pathway. This dynamic equilibrium is often revealed when the functions of negative regulators of signal transduction are impaired. For instance, inactivation of tyrosine phosphatase function by inhibitors (e.g., by pervanadate) frequently leads to an increased level of tyrosine phosphorylation of cellular proteins, in a ligand-independent manner. Recent studies in the yeast mating pathway have shown that inactivation of regulators of G-protein signaling (RGS proteins) can induce constitutive activation of downstream signaling pathways even in the absence of receptor expression (Siekhaus and Drubin 2003). Thus, the balanced actions of positive and negative regulators of signal transduction set the steady-state equilibrium. Receptor stimulation then perturbs the equilibrium state in various ways to initiate cellular responses. The steady-state level of signaling in the unstimulated state may itself have functional consequences, for instance, to maintain certain differentiated cellular properties or functions.
In the immune system, signal transduction pathways that are regulated by antigen receptors are functionally important for the appropriate development of properly selected T and B lymphocytes as well as in controlling responses to antigen by more mature cells. Ligand-independent signaling by the pre-T and pre-B cell antigen receptors (pre-TCR and pre-BCR, respectively) promotes the developmental progression of immature cells (Irving et al. 1998; Saint-Ruf et al. 2000; Fuentes-Panana and Monroe 2001; Aifantis et al. 2002). Upregulation of receptor expression may account for the unique property of these unligated receptors to initiate the signaling events that are required for maturation to the next stage. An alternative explanation for induction of signaling events by unligated receptors may be receptor clustering and localization to lipid rafts. However, unique cellular compartmentalization of the receptor does not seem to explain the requirement for cell surface expression of the mature BCR for B cell survival, as observed in a hapten-specific receptor system (Lam et al. 1997).This suggests that basal signaling tone by the unligated BCR that can interact, perhaps by chance, with cellular machinery may be sufficient to send signals downstream that are required for B cells' survival. The precise nature of the signaling events that set the basal steady-state level of signaling at any stage of differentiation has not been studied in detail. However, the mechanisms by which the TCR and BCR initiate signaling in response to ligand have been well studied. The mechanisms involved in transmitting the ligand-occupied state of the receptor are likely to also contribute to the basal state of signaling since these same effectors must be regulated before and after receptor ligation. The magnitude and qualitative properties of the signals generated, however, are likely to differ substantially.
The TCR consists of the antigen-binding TCRα? and TCRβ chains associated with the signal-transducing subunits CD3γ, CD3δ, CD3ɛ, and TCRζ chains (Weiss and Littman 1994). TCR stimulation (with or without CD4 or CD8 coreceptors) results in the activation of Lck and Fyn, Src protein tyrosine kinases (PTKs) that phosphorylate immunoreceptor tyrosine-based activation motifs (ITAMs) present in the cytoplasmic tail of TCRζ and CD3 chains. The Syk PTKs, Syk and ZAP-70, are recruited to the doubly phosphorylated tyrosines in the ITAM and are subsequently tyrosine-phosphorylated themselves, resulting in their activation (Weiss and Littman 1994; Kane et al. 2000). Activated Src and Syk family kinases phosphorylate various substrates, including the adapter LAT (linker for activation in T cells) (Zhang et al. 1998). LAT is palmitoylated, a modification that ensures its proper localization and function in the glycolipid-enriched microdomains (GEMs) (Lin et al. 1999). Phosphorylation of multiple tyrosine residues in LAT creates distinct docking sites for many signaling proteins, including phospholipase Cγ1 (PLCγ1), SLP-76 (via Gads), Grb2, Grap, Gads, the p85 regulatory subunit of phosphoinositide 3′-kinase (PI3K), Vav, and Cbl (reviewed in Tomlinson et al. 2000)). A pivotal role for LAT in TCR signaling was demonstrated in studies using Jurkat T cells deficient in LAT, which display severely impaired PLCγ1 phosphorylation, calcium influx, and MAP kinase (MAPK) activation, as well as impaired activation of transcription factors AP-1, NFAT, and NF-κB upon TCR engagement (Finco et al. 1998). A similar, though less severe, phenotype was observed in SLP-76-deficient Jurkat T cells (Yablonski et al. 1998). These results point to a requirement for the LAT/SLP-76 module to transmit a signal(s) initiated by engagement of the TCR to the downstream calcium and MAPK pathways.
The thymic developmental blocks observed in mice deficient in LAT or SLP-76 highlight the physiologic importance of the signaling events mediated by these proteins (Clements et al. 1998; Pivniouk et al. 1998; Zhang et al. 1999). Mice rendered deficient in these proteins display an early block in thymocyte development at the CD44−CD25+ double-negative (DN) (CD4− and CD8−) stage due to a defect in ligand-independent pre-TCR signaling (see below). Similarly, thymocytes of mice lacking Rag-1 or Rag-2 are arrested at the same stage, thus linking the genes responsible for recombination and subsequent expression of the TCR with the downstream signaling pathways (Mombaerts et al. 1992; Shinkai et al. 1992).
Physiological Rag (in this manuscript we refer to both Rag-1 and Rag-2 as Rag) expression is strictly regulated, allowing sequential rearrangements of the TCRβ and TCRα genes (Schatz et al. 1992). Rag gene expression can first be detected when DN thymocytes become positive for CD25 and start to rearrange the TCRβ locus. Successful β rearrangement results in surface expression of the pre-TCR, made up of the β chain heterodimerized with a surrogate for the α chain (pre-Tα) and complexed with the signal transducing CD3 and ζ chains. Low-level surface expression of the pre-TCR by itself can signal independently of the extracellular portions of the TCRβ and pre-Tα chains leading to increased activities of downstream signaling pathways (Irving et al. 1998). Perhaps this reflects the unique localization of the pre-TCR receptor to GEMs (Aifantis et al. 2002), although other studies suggest that GEM localization may not be a unique property of the pre-TCR compared to the αβTCR on thymocytes (Haks et al. 2003). For the thymocyte, this results in increased survival, proliferation, progression to the double-postive (DP) (CD4+ and CD8+) stage, and TCRβ allelic exclusion. This transition is at all times accompanied by termination of Rag gene expression, although the exact mechanism is unclear (Nagaoka et al. 2000). It is apparent that such termination is as important as the induction of Rag gene expression, as evidenced by altered thymocyte development in mice with transgenic, aberrant Rag expression (Wayne et al. 1994). DP thymocytes undergo a second wave of Rag gene expression, required to rearrange the newly accessible TCRα locus (Wilson et al. 1994), but it remains unclear which signals induce reexpression of Rag genes. Productive rearrangement of the TCRα locus leads to expression of the mature αβTCR on the surface. In contrast to pre-TCR β-chain selection in DN thymocytes, Rag expression and rearrangement in DP cells are only terminated after appropriate ligation of the TCR during positive selection by self-peptide and major histocompatibility complex (MHC) molecules (Nagaoka et al. 2000). Positively selected thymocytes proceed to the single-positive stage (SP) (CD4+ or CD8+), the direct precursors of peripheral T cells.
Rag-1 and Rag-2 are unusual genes in that they have remained linked and conserved throughout evolution, are orientated in opposite directions, and are exclusively coexpressed in lymphoid cells (Schatz et al. 1989, 1992; Oettinger et al. 1990). Two crucial regulatory regions in the Rag-1 and Rag-2 loci were mapped in studies of transgenic mice carrying bacterial artificial chromosomes encompassing different portions of the Rag locus (Yu et al. 1999). DN thymocytes of these mice required 10 kb of genomic sequence 5′ of Rag-2 essential for normal expression of both Rag-1 and Rag-2, whereas DP cells needed an 110 kb region upstream of Rag-2 for both genes. Interestingly, Rag gene expression occurs in two waves. This characteristic rules out transcriptional regulation by permanent, heritable silencing after the first window of Rag gene expression (Fisher and Merkenschlager 2002). Instead, it suggests a requirement for transient repression by uncharacterized signaling pathways until Rag gene expression is no longer needed and the genes can be heritably silenced, accompanied by locus translocation to centromeric DNA, as has been described for SP thymocytes (Brown et al. 1999).
Basal signaling events in DN and DP thymocytes could contribute to the differentiated state of the thymocyte and to the regulation of Rag gene expression. The exact nature of basal signaling in thymocytes is unknown, but it is probably mechanistically similar to that which is regulated by the pre-TCR and mature TCR. In unstimulated Jurkat T cells, basal signaling activities can be visualized, for instance, by pervanadate treatment or expression of truncated signaling molecules that block signal transduction. We used this model cell line to initiate our studies to examine the potential constitutive signaling pathways in thymocytes. We first analyzed the basal signaling pathway that is downstream of the TCR using the Jurkat T cell line and TCR signaling pathway mutant clones, which were derived from wild-type Jurkat cells. We hypothesized that these well-characterized mutants should have perturbations in their basal signaling machinery. We compared gene expression patterns in wild-type Jurkat T cells with those in these somatic mutants. Our identification of RAG genes as targets of a basal signaling pathway defective in some Jurkat mutants led us to explore the potential function of this pathway in regulating RAG gene expression in Jurkat cells and in thymocytes, by using chemical inhibitors to block basal signaling. We found that, in both Jurkat cells and in thymocytes, constitutive signaling represses expression of a group of genes that includes the RAG genes. Normal RAG gene expression depends on the adapter protein LAT, which transduces a signal to two pathways that induce low-level kinase activity of Erk and Abl.
Results
Evidence for Transcriptional Regulation by Tonic Activity of a TCR-Like Signaling Pathway in Unstimulated Jurkat T Cells
The Jurkat T cell leukemic line E6–1 expresses high levels of mature TCR and low levels of CD4, but no CD8 or MHC class II. These cells therefore do not cross-present antigen or respond to administration of superantigen alone (Fraser et al. 1992). In this cell line, phospho-TCRζ can be trapped and upregulated in resting cells that overexpress the tandem SH2 domains of ZAP-70 in the absence of TCR stimulation (Qian et al. 1996). The tandem SH2 domains of ZAP-70 presumably compete with endogenous phosphatases that oppose the action of Lck and dephosphorylate TCRζ under basal conditions. This lends support to the existence of a constitutive but tonically regulated signaling pathway in unstimulated Jurkat cells. Therefore, we postulated that the same tonic signaling pathway might regulate a gene expression program in Jurkat cells and that a similar pathway might exist in thymocytes or T cells.
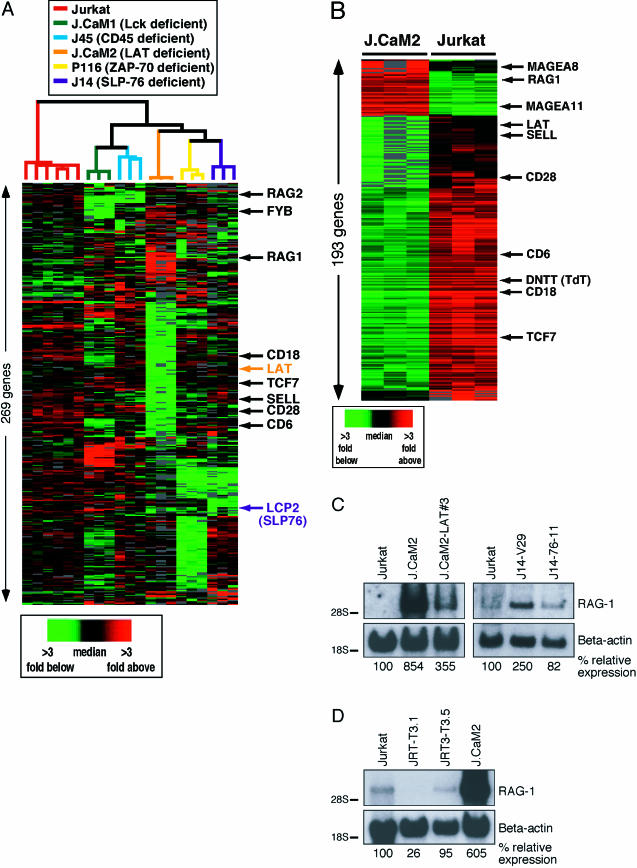
We employed a panel of Jurkat T cell mutants lacking specific TCR signaling proteins to study the transcriptional consequences of this constitutive signaling pathway, with the notion that the loss of key components, even in unstimulated cells, might have consequences on the expression of a set of genes. Using DNA microarrays, we compared gene expression profiles of Jurkat-derived T cell lines deficient for Lck (J.CaM1) (Straus and Weiss 1992), LAT (J.CaM2) (Finco et al. 1998), SLP-76 (J14) (Yablonski et al. 1998), ZAP-70 (P116) (Williams et al. 1998), and CD45 (J45) (Koretzky et al. 1991) to those of wild-type Jurkat T cells. We set out to analyze aberrant gene expression caused by the lack of constitutive signaling in the mutant lines. Confirming original reports, LAT and SLP-76 (LCP2) mRNA levels were reduced in J.CaM2 and J14 (Finco et al. 1998; Yablonski et al. 1998) (Figure 1A). In addition, all mutant lines demonstrated many unique alterations in expression levels of a large number of genes compared to wild-type Jurkat cells, even though they are all derived from the parental Jurkat T cell line. It is possible that altered gene expression resulting from random mutations caused by chemical or radiation mutagenesis, unrelated to the TCR signaling defect, complicated this analysis (Figure 1A).
Figure 1. Gene Expression Analysis of Resting Jurkat T Cells and Derived Signaling Mutants.
(A) Basal gene expression of wild-type Jurkat T cells compared to Lck-deficient J.CaM1, CD45 (PTPRC)-deficient J45, LAT-deficient J.CaM2, ZAP-70-deficient P116, and SLP-76 (LCP2)-deficient J14 signaling mutants. High-quality data were analyzed using the Statistical Analysis of Microarrays software package (Tusher et al. 2001). After selecting high-quality data, array elements that were 2.5-fold above or below the median on at least two microarrays were included (337 cDNA elements). These elements are displayed in hierarchical cluster format where rows represent genes and columns represent experimental samples. Colored pixels capture the magnitude of the response for any gene. Shades of red and green represent fold above and fold below the median, respectively. Black pixels reflect the median and gray pixels represent missing data. Data in 337 rows correspond to 321 unique cDNA clones, representing 269 unique genes. Jurkat T cells were analyzed in six individual samples and each mutant cell line in triplicate, and the sample dendrogram was generated by hierarchically clustering the arrays using the elements shown. To interactively explore the data, go to http://microarray-pubs.stanford.edu/cgi-bin/tonicsignal/fig1a/gx?n=fig1a. Abbreviations: FYB, Fyn-binding protein; SELL, CD62L.
(B) Basal gene expression differences between unstimulated LAT deficient (J.CaM2) and wild-type Jurkat T cells. Data presented in the 273 rows correspond to 251 unique cDNA clones and represent the 193 unique genes most differentially expressed between the two lines that were selected (false discovery rate, <1%). Elements were further filtered for being at least 2-fold different between the lines and for technically adequate measurements in at least five of six samples analyzed. These elements are displayed as in (A). Abbreviations: MAGEA-8, melanoma antigen, family A, 8; MAGEA-11, melanoma antigen, family A, 11. To interactively explore the data, go to http://microarray-pubs.stanford.edu/cgi-bin/tonicsignal/fig1b/gx?n=fig1b.
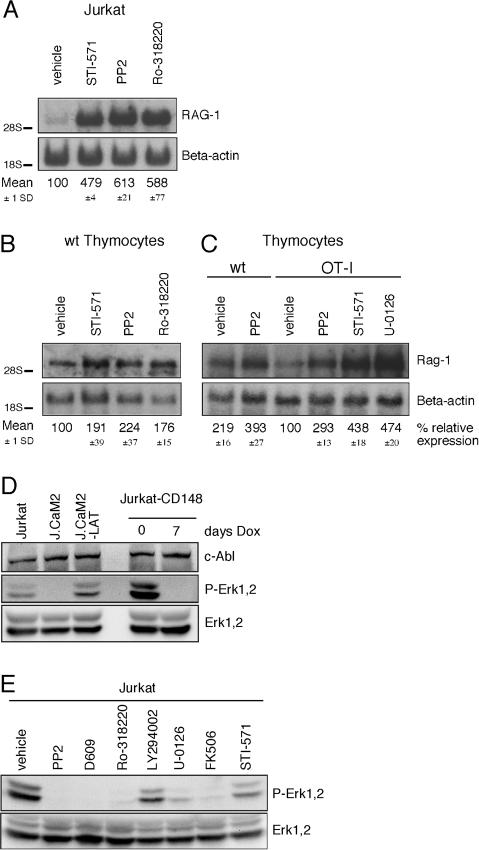
(C) Northern blot analysis of RAG-1 gene expression in Jurkat, J.CaM2, J14, and cDNA-reconstituted cell lines J.CaM2-LAT and J14-76-11. RNA levels are indicated by β-actin hybridization. All Northern blots presented in this study are representative examples of three independent experiments, unless mentioned otherwise.
(D) Resting Jurkat, TCRα-deficient (J.RT-T3.1), TCRβ-deficient (J.RT3-T3.5), and LAT-deficient (J.CaM2) cell lines were analyzed for RAG-1 and β-actin mRNA expression by Northern blot analysis.
We reasoned that the effects of disrupting a low-level, basal signal would be most evident in a cell line with an absolute block in TCR signaling and thus initially focused on J.CaM2. Signaling events like calcium mobilization or activation of MAPKs are severely impaired in LAT-deficient J.CaM2 even when the TCR is heavily cross-linked (Finco et al. 1998) (data not shown). We mathematically isolated the differences in gene expression profiles between J.CaM2 and Jurkat and identified a large cluster of genes expressed at lower levels in J.CaM2 than in wild-type Jurkat T cells (left three columns in green in Figure 1B). This cluster encompassed many cell surface markers, including CD18 (β2 integrin), CD62L (L-selectin), CD28, and TCRα. We validated these observations by fluorescence-activated cell sorting (FACS) analysis and noticed that the expression levels of other cell surface markers like CD2, CD5, and CD7 were also reduced. Stable reconstitution of J.CaM2 with LAT cDNA (J.CaM2-LAT) (Finco et al. 1998), however, did not result in reexpression of these markers as analyzed by FACS or Northern blot analysis (data not shown). Expression levels of two nuclear proteins, TCF7 (Tcf-1) and BIN2, were also reduced in J.CaM2, but again not restored in J.CaM2-LAT, as evidenced by Northern blotting (data not shown). We concluded that the reduced expression levels of these genes could not be explained by a lack of LAT expression. Instead, one or more additional key transcriptional regulators may be defective in the J.CaM2 line.
LAT-deficient cells also displayed a distinct cluster of genes, including RAG-1 and RAG-2, expressed at higher levels than wild-type Jurkat (left three columns in red in Figure 1B). These findings were shared with SLP-76-deficient cells (Figure 1A). The elevated expression levels of RAG-1 and RAG-2 particularly interested us since it has been well established that induction of the TCR signaling pathway is able to terminate Rag gene expression (Nagaoka et al. 2000). We therefore focused on this cluster of “derepressed” genes observed in the J.CaM2 cells. We hypothesized that normal RAG gene expression in resting cells is held in check by a basal signal that requires both LAT and SLP-76.
To test this hypothesis and to confirm the results obtained in the DNA microarray experiments, we isolated RNA from several cell lines and determined RAG-1 expression levels by Northern blot analysis. Wild-type Jurkat T cells express very low but detectable levels of RAG-1 mRNA (Figure 1C, lane 4). The RAG locus is thus accessible to transcription factors, making this cell line a fortuitous model for our experiments. RAG-1 expression was markedly increased in J.CaM2 and more moderately in J14 cells compared to that in wild-type Jurkat (Figure 1C). The reduced effect of missing SLP-76 in J14 cells on RAG expression could reflect the leaky TCR-signaling phenotype of this mutant (Yablonski et al. 1998). Importantly, expression levels were reduced in both mutant cell lines when the repective mutations were complemented with cDNAs encoding full-length LAT (J.CaM2-LAT) (Finco et al. 1998) or SLP-76 (J14–76-11) (Yablonski et al. 1998). The elevated RAG-1 expression in the mutants was therefore not an aberrant characteristic caused by accidental mutations at other loci, but rather a result of the deficiency of the adapters LAT or SLP-76. As on the microarray, RAG-1 expression levels analyzed by Northern blot analysis were not increased in J.CaM1 (data not shown). Despite the Lck deficiency, J.CaM1-deficient cells flux calcium when the TCR is heavily cross-linked or in response to anti-CD3 antibodies (Straus and Weiss 1992). Expression of Fyn in these cells is a likely explanation for this signal. In a similar fashion, a low signal may be capable to control RAG-1 expression in J.CaM2 cells.
We also made use of mutant Jurkat lines to test whether surface αβTCR expression was required for normal RAG-1 expression, as observed in wild-type Jurkat cells. TCRα-deficient (J.RT-T3.1) or TCRβ-deficient (J.RT3-T3.5) Jurkat T cells (Ohashi et al. 1985) were generated in a screen for αβTCR-negative cell lines (Weiss and Stobo 1984). We repeated previously published FACS analyses and functional experiments on these lines. The TCRα- and TCRβ-deficient Jurkat lines express very low, if any, CD3ɛ on the surface, but no αβTCR (Weiss and Stobo 1984; data not shown). As a result, these cells do not flux calcium, produce IL-2, or demonstrate NFAT transcriptional activity in response to TCR stimulation (Ohashi et al. 1985; data not shown).The TCRα- or TCRβ-deficient Jurkat T cells did not display elevated RAG-1 expression levels such as we observed in LAT-deficient cells (Figure 1D). Surface expression of the αβTCR is therefore dispensable for maintenance of normal levels of RAG-1 transcripts. Importantly, these results imply that the tonic signal regulating RAG gene expression is not generated by (stimulation of) the extracellular portion of the αβTCR, but rather by an intrinsically active signaling pathway. At least one of these mutants, JRT-T3.1, has been reported to express surface TCRζ (Ono et al. 1995); we can therefore not completely exclude the possibility that signaling components of the TCR play a role in regulating this pathway.
Requirement for LAT in Tonic Repression of RAG-1 Transcription Is Bypassed by Direct Activation of PKC or MAPK Pathways
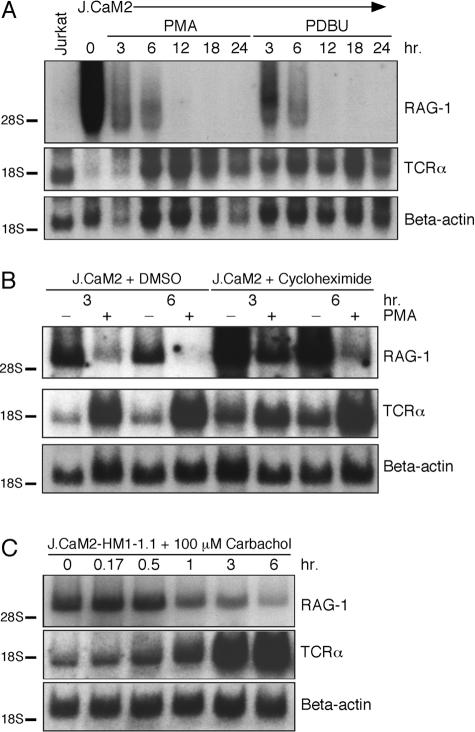
Rag-1 and Rag-2 are expressed in DP thymocytes and turned off during subsequent thymic maturation. This process can be mimicked in thymocytes in vitro by cross-linking the TCR, which leads to activation of PLCγ and subsequent generation of the second messengers diacylglycerol (DAG) and inositol trisphosphate (IP3) (Turka et al. 1991). The same effect is obtained when DAG and IP3 generation are replaced by stimulation with synthetic analogues phorbol myristate acetate (PMA) and ionomycin (Turka et al. 1991). PMA and ionomycin stimulation also bypasses LAT and SLP-76 deficiencies in transcriptional responses in the Jurkat line (Finco et al. 1998; Yablonski et al. 1998). Stimulation of J.CaM2 by PMA or another phorbol ester, phorbol-12,13-dibutyrate (PDBu), resulted in complete downregulation of the elevated RAG-1 transcripts within 12 h (Figure 2A). Induction of a calcium flux by ionomycin, however, did not substantially diminish RAG-1 gene expression in J.CaM2 cells (data not shown). PMA or PDBu stimulation induced a similar decrease in J14 cells (data not shown). The same kinetics of decreased RAG-1 mRNA levels were observed when transcription was blocked in J.CaM2 cells by actinomycin D, demonstrating the instability of RAG-1 mRNA (data not shown). In contrast, transcripts of TCRα were induced following PMA stimulation of J.CaM2 (Figure 2A), as has been described for wild-type Jurkat T cells (Lindsten et al. 1988). The reduction of RAG-1 levels by PMA stimulation was still observed when protein synthesis was blocked with cycloheximide (Figure 2B). The cycloheximide and actinomycin D experiments suggest that signals through the PKC/MAPK pathways are able to repress RAG-1 gene transcription in a relatively direct fashion, leading to loss of detectable RAG-1 mRNA. The fact that RAG-1 expression is elevated in these cells, but can be reduced by a PMA signal, suggests that the LAT/SLP-76 module is essential for a constitutive, suppressive signal that operates, in part, through the downstream signaling pathways. The MAPK pathway can also be activated through seven-transmembrane receptor signaling (Crespo et al. 1994). In J.CaM2 cells, this can be achieved by carbachol treatment of cells that stably express the human muscarinic receptor (J.CaM2-HM1-1.1) (Goldsmith et al. 1989). Carbachol stimulation decreased RAG-1 and increased TCRα expression to the same extent as observed with PMA (Figure 2C). Thus, pharmacologic and physiologic activation of the PKC and MAPK pathways can lead to inhibition of RAG-1 gene expression.
Figure 2. Stimuli Bypassing LAT Deficiency in J.CaM2 Reduce Elevated RAG-1 Expression.
(A) LAT-deficient J.CaM2 cells were stimulated with PMA or PDBu and analyzed for RAG-1 mRNA expression by Northern blot analysis. In (A), (B), and (C), the same blot was hybridized with a fragment encoding the constant region of TCRα and with β-actin to control for stimulation and loading.
(B) Analysis of PMA-induced reduction of RAG-1 expression levels in absence of protein synthesis blocked by cycloheximide treatment of J.CaM2 cells. Control hybridizations were carried out by hybridization with the constant region of TCRα and with β-actin.
(C) Analysis of RAG-1 transcripts in J.CaM2-HM1-1.1 cells stimulated for various time intervals with carbachol to trigger the human muscarinic (HM) receptor subtype I on the surface of these cells.
Normal Expression of a Gene Cluster Including RAG-1 and RAG-2 Relies on a Signaling Competent LAT Molecule
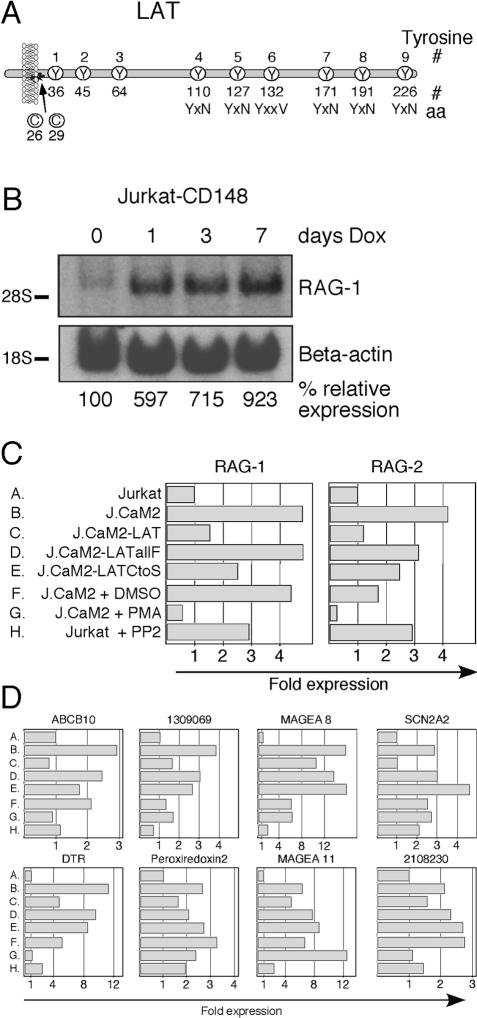
We next examined whether signaling via LAT is essential for the tonic suppression of RAG-1 expression. Phosphorylation of conserved tyrosines in LAT (Figure 3A) is required for the recruitment of downstream signaling effectors and adaptors. It is technically very difficult to detect basally phosphorylated LAT in resting cells since the antibody that immunoprecipitates LAT recognizes phosphorylated LAT very poorly (data not shown). However, we have previously demonstrated that these phosphorylated tyrosine residues in LAT can be substrates for the phosphatase CD148. Doxycyclin-inducible expression of CD148 in Jurkat T cells rather selectively inhibits TCR-stimulated phosphorylation of LAT and PLCγ1; the latter effect may be secondary to the effect on LAT (Baker et al. 2001). Induction of CD148 expression for 1, 3, or 7 d resulted in increasingly high RAG-1 levels, suggesting that basal phosphorylation of LAT in unstimulated cells is crucial for proper RAG-1 expression (Figure 3B). To further address the importance of signaling via LAT, we generated two new LAT mutant J.CaM2 cell lines. The first (J.CaM2-LATallF) stably expresses normal levels of a LAT molecule with all tyrosine residues replaced by phenylalanine residues. The second (J.CaM2-LATCtoS) stably expresses a LAT molecule in which sites for palmitoylation, cysteines 26 and 29, were both replaced by serine residues, displacing LAT from localization to the GEMs (data not shown). Both cell lines demonstrated the same impaired TCR-induced phosphorylation of Erk as we had previously observed in transient assays (Lin et al. 1999; Lin and Weiss 2001). We used DNA microarrays to compare global gene expression patterns in Jurkat, J.CaM2, J.CaM2 reconstituted with wild-type LAT (J.CaM2-LAT), J.CaM2-LATallF, and J.CaM2-LATCtoS cells. These samples were also compared to J.CaM2 treated with dimethyl sulphoxide (DMSO) as a control, J.CaM2 stimulated with PMA that bypasses the requirement for LAT, and Jurkat treated with the Src kinase inhibitor PP2. Figure 3C shows the relative expression level of RAG-1 and RAG-2 in the different samples compared to expression in Jurkat control cells (expression = 1). Expression of both RAG genes was elevated in the absence of the adapter LAT and reduced in J.CaM2 reconstituted with wild-type LAT cDNA (Figure 3Ca–c). By contrast, reconstitution with a signaling-defective LAT molecule without tyrosine residues, and thus without docking sites for other signaling proteins, did not result in a reduction of RAG gene expression (Figure 3Cd). Reconstitution with a LAT molecule that does not get palmitoylated resulted only in partial reduction, suggesting that there is a partial, constitutive signal generated by LAT molecules independent of GEM localization (Figure 3Ce). These results clearly indicate that proper localization and signaling through the adapter LAT are required for normal suppression of RAG gene expression in resting cells. Src and Syk family kinases initiate signaling events upstream of LAT, leading to its tyrosine phosphorylation. Since a potent and selective Syk kinase inhibitor is not available, we used the Src kinase selective inhibitor PP2 to inhibit Lck and Fyn function in Jurkat cells. Addition of a related inhibitor, PP1, has been reported to displace ZAP-70 from the cortical membrane of Jurkat T cells (Huby et al. 1998), presumably resulting in reduced phosphorylation of LAT. Treatment of unstimulated Jurkat cells with PP2 increased expression of RAG-1 and RAG-2 to a level similar to that in J.CaM2 cells, suggesting that basal Lck and/or Fyn kinase activity is required for constitutive repression of these genes (Figure 3Ch).
Figure 3. Proper Expression of RAG-1 and RAG-2 Requires a Signaling-Competent LAT Molecule.
(A) Graphic representation of human LAT. Conserved tyrosine residues in mammals are numbered 1 through 9, with the corresponding amino acid numbering below. Cysteine residues 26 and 29 are required for palmitoylation. Adapted from Lin and Weiss (2001).
(B) RAG-1 expression by Northern blotting in Jurkat T cells induced to express the phosphatase CD148 for increasing periods of time.
(C) RAG-1 and RAG-2 expression by DNA array analysis. Expression of the two genes was set at 1 in wild-type Jurkat cells and compared to expression in J.CaM2 cells, in J.CaM2 reconstituted with wild-type LAT (J.CaM2-LAT), or with signaling-mutant LAT molecules (J.CaM2-LATallF and J.CaM2-LATCtoS). In addition, expression profiles were compared to J.CaM2 treated with DMSO, J.CaM2 stimulated with PMA (25 ng/ml for 24 h), and Jurkat cells incubated with PP2 (20μM for 24 h).
(D) Expression profiles of genes with increased expression in J.CaM2 and a similar expression behavior as RAG-1 and RAG-2. Abbreviations: ABCB10, ATP-binding cassette, subfamily B, member 10; DTR, diphteria toxin receptor; 1309069, similar to Rattus norvegicus nuclear-encoded mitochandrial elongation factor G; SCN2A2, sodium channel, voltage-gated, type II, α2 polypeptide; 2108230, unknown human expressed sequence tag.
To investigate whether constitutive signaling through LAT controls other genes in addition to RAG-1 and RAG-2, we plotted the expression profiles of genes whose expression was elevated in J.CaM2 relative to Jurkat cells and diminished by restoration of LAT function in J.CaM2-LAT (Figure 3D). Roughly 20 genes demonstrated such an expression profile. As for the RAG genes, elevated levels of some but not of all of these genes could be reduced by PMA stimulation of J.CaM2 or induced by PP2 treatment of wild-type Jurkat. Although at this moment these genes have no obvious function in lymphocyte biology or gene rearrangement, these data suggest that expression patterns of several other genes can be influenced by basal signaling through components of the TCR signaling pathway in resting cells. It will be interesting to investigate what role this group of genes may play in T cell maturation.
Chemical Inhibitors Map the Tonic Suppression of RAG Gene Expression to a TCR-Like Signaling Pathway
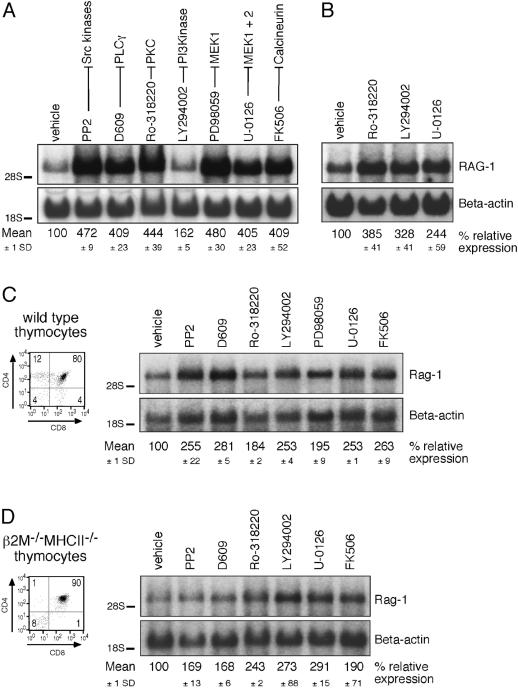
To further trace specific molecular components of this tonic signaling pathway, we treated Jurkat cells for 24 h with chemical inhibitors to various TCR-regulatable signaling molecules. Resting Jurkat T cells were incubated with the following inhibitors (molecular target indicated in parenthesis): PP2 (Src kinases), D609 (PLCγ?), Ro-318220 (novel PKC family members), LY294002 (PI3K), PD98059 (MEK-1), U-0126 (MEK-1 and MEK-2), FK506 (calcineurin), and DMSO (vehicle control) (see Figure 4A). Addition of DMSO did not alter the RAG-1 expression level. Inhibition of many of these signaling molecules in wild-type Jurkat T cells induced marked increases in RAG-1 gene expression (Figure 4A). All these inhibitors except LY294002 yielded an approximately 4-fold induction of expression. The effects of the inhibitors shown were specific since no change in RAG-1 expression was observed when Jurkat cells were treated with inhibitors specific for protein kinase A (PKA) (H89) and the mammalian target of rapamycin (mTOR) (rapamycin) (data not shown).
Figure 4. Chemical Inhibitors Map the Constitutive Signal to a TCR-Like Pathway.
(A) Northern blot analysis for RAG-1 expression in Jurkat T cells treated with the indicated inhibitors. Cells were incubated for 24 h prior to RNA isolation. The relative expression level of RAG-1 was calculated from three independent experiments, and the mean expression and standard deviation (SD) are indicated.
(B) Northern blot analysis for RAG-1 mRNA in Jurkat expressing the phosphatase PTEN treated with indicated inhibitors. (B) and (D) are representative of two independent experiments.
(C) Basal signaling was blocked in wild-type thymocytes by treating with the indicated inhibitors for 20 h. CD4 and CD8 FACS profiles of thymocytes were determined and expression of Rag-1 and β-actin was analyzed by Northern blotting.
(D) Northern blot analysis for Rag-1 expression in β2M−/−/MHCII−/− thymocytes treated for 20 h with the enzyme inhibitors.
Jurkat T cells are deficient for the phosphatases PTEN (3′-phosphatase and tensin homolog deleted on chromosome 10) and SHIP (SH2-containing inositol polyphosphate 5′-phosphatase) (Astoul et al. 2001). As a result, there is more active inositol phospholipid signaling in Jurkat T cells, and PI3K inhibitors elicit little effect. Induction of PTEN expression using a doxycyclin-inducible expression system (Xu et al. 2002) produced no appreciable change in basal RAG-1 expression. Subsequent treatment of the PTEN-expressing cell with LY294002, however, led to a level of induction of RAG-1 expression similar to that observed with the other inhibitors (Figure 4B). This would suggest that the high basal level of phosphatidylinositol 3,4,5-trisphosphate (PIP3) in Jurkat cells is not responsible for RAG-1 gene repression and that the LY294002 could not modulate the levels of PIP3 in PTEN-deficient Jurkat cells, consistent with our previous findings (Kane et al. 2002).
The effects of selective inhibitors, together with the results of our previous experiments with mutant cell lines, point to a tonic signaling pathway that closely resembles a TCR-inducible pathway, with Src family kinase activity as the most upstream component and MAPK as the most downstream component regulating RAG-1 and RAG-2 expression in Jurkat cells (for a model, see Figure 8). The same inhibitors yielded very similar results in two mouse thymocyte cell lines, SCB29 and DPK (data not shown). The SCB29 cell line is derived from a Scid mouse and expresses a pre-TCR (Groettrup et al. 1992). DPK is a CD4+CD8+ thymocyte line expressing a transgene-encoded TCR (Kaye and Ellenberger 1992). Since TCR signaling has been studied in great detail in Jurkat T cells, we proceeded to use this model for our experiments and verified our results in primary thymocytes.
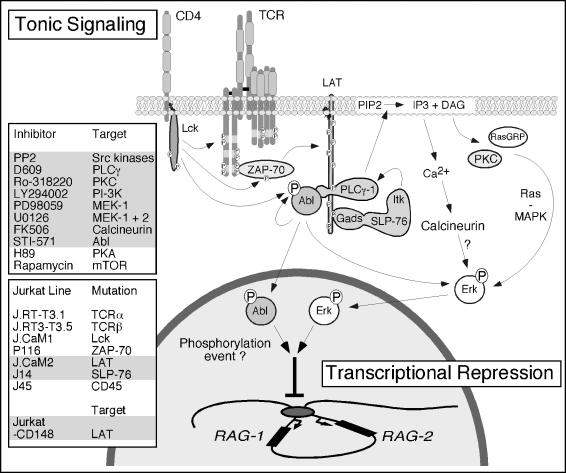
Figure 8. Model of the Constitutive Signaling Pathway That Provides a Basal Repression of RAG Gene Transcription.
Constitutive signaling in resting thymoytes and our model Jurkat T cell line represses RAG gene expression. Chemical inhibition of signaling molecules, genetic modifications, or induced expression of genes that resulted in elevated RAG expression are summarized in the gray-shaded boxes. The tonic signal and therefore normal expression of RAG genes rely strongly on signaling through the adapter LAT. The effects of basal phosphorylation of LAT are twofold: (1) recruitment and activation of PLCγ generates low levels of second messengers that signal through calcium and Ras pathways, culminating in Erk kinase activity; and (2) phosphorylation of tyrosine residue 6 of LAT, likely establishing a LAT–PLCγ–Abl complex, is required for low-level kinase activity of Abl. Abl and Erk kinase activities deliver unique repressive signals to control RAG gene expression.
A Similar Tonic Signaling Mechanism Represses Rag-1 Expression in Thymocytes of Wild-Type and β2M−/−/MHCII−/− Mice
We noticed that primary murine thymocytes were more sensitive to apoptosis induced by prolonged exposure to chemical inhibitors. Nevertheless, we were able to determine the effects of the signaling inhibitors on Rag-1 expression in wild-type thymocytes by adding a general caspase inhibitor (Z-DEVD-FMK) to our incubations in order to block apoptosis. Addition of Z-DEVD-FMK by itself had no effect on Rag-1 gene expression, but did improve survival without affecting the relative proportions of the different thymocyte subsets (determined by CD4, CD8, CD25, and CD44 staining; data not shown). As observed in cell lines, inhibition of components of the downstream TCR signaling pathway in total thymocytes significantly increased Rag-1 gene expression (Figure 4C). The inhibitors delineated the same pathway as in the Jurkat T cell and the thymocyte cell lines, including Src kinases, PLCγ1, PKC, PI3K, MEK-1, and calcineurin. The magnitude of induced Rag-1 expression was more moderate in thymocytes than the level observed in our model cell line. The fact that thymocytes in the process of receptor rearrangement already express Rag-1 may account for the smaller induction over the existing level. We therefore treated thymocytes from TCR transgenic OT-I mice with inhibitors in the same manner. OT-I transgenic mice have a thymus containing substantial numbers of DP thymocytes. In absence of the peptide from chicken ovalbumin, these DP thymocytes are positively selected on self-antigens and effectively downregulate Rag expression (McGargill et al. 2000). As a result, the level of Rag-1 expression in thymocytes of these animals is lower than that in wild-type littermate thymi (Figure 5C, lanes 1 and 3). Treatment of OT-1 thymocytes with PP2 or U-0126 led to a strong induction in Rag-1 expression, comparable to the effects observed in Jurkat T cells (Figure 5C). These data argue that detection of constitutive Rag repression in wild-type thymocytes is hindered by normal Rag expression during rearrangement.
Figure 5. Erk and Abl Kinases Transduce Repressive Signals That Control RAG Gene Expression.
(A) Northern blot analysis for RAG-1 gene expression in STI-571-treated Jurkat T cells and comparison to PP2- or Ro-318220-treated samples.
(B) Wild-type thymocytes were treated with the indicated inhibitors for 20 h and analyzed for Rag-1 expression.
(C) Northern blot analysis of Rag-1 expression in inhibitor-treated TCR transgenic OT-I thymocytes or wild-type thymocytes of littermate controls. The relative expression level of Rag-1 was calculated from two independent experiments, and the mean expression and standard deviation (SD) are indicated.
(D) Western blot analysis using RIPA lysates of the indicated cell lines. Protein levels of phosphorylated Erk-1 and Erk-2, Erk-1 and Erk-2, and c-Abl were determined in 4 × 106 resting cells per sample.
(E) Analysis of phospho-Erk-1 and Erk-2 levels in Jurkat T cells treated for 24 h with the indicated inhibitors prior to RIPA lysis. Equal loading is indicated by Erk-1 and Erk-2 levels determined by stripping and reprobing the same blot.
To eliminate the possibility that we were studying inhibition of a signal induced by an MHC–peptide interaction with the TCR on the surface of the thymocyte, we repeated the same experiment in β2M−/−/MHCII−/− thymocytes (Grusby et al. 1993), which cannot encounter MHC–peptide interactions and as a result are blocked at the DP stage. The pattern of effects of the inhibitors on Rag-1 gene expression in these mutant thymocytes was very similar to that observed in the wild-type thymocytes (see Figure 4D). The signal repressing Rag-1 gene expression is thus not initiated by MHC–peptide stimulation, but instead appears to be transmitted through a basal signaling pathway that resembles the TCR-inducible signaling pathway. We utilized the same panel of inhibitors to determine whether the same constitutive repression of Rag-1 expression occurs in peripheral T cells. To prevent stimulation by antibody binding, these cells were purified from lymph node and spleen through negative selection of other cell populations. In several independent experiments, we did not observe an increase in Rag-1 expression in the inhibitor-treated peripheral T cells (data not shown).
Parallel Signaling Pathways Dependent on LAT Maintain Basal Phosphorylation of Erk and Abl Kinases
The inhibitor studies suggest that a constitutive signaling pathway may use components very similar to those involved in conventional TCR signaling triggered upon receptor ligation. We wanted to confirm and identify the downstream effector molecules that transduce the repressive signal. The MAPK pathway was clearly implicated; however, recent studies suggested that Abl kinase activity might also be involved.
Human c-Abl and its homologue Abl-related gene (Arg) are nonreceptor tyrosine kinases with structural homology to Src kinases (reviewed in Van Etten 1999). Kinase activity of c-Abl in normal cells is suppressed by autoinhibition, depending on 80 N-terminal residues that are lost in translocations leading to BCR–Abl fusion proteins and development of chronic myelogenous leukemia (Pluk et al. 2002). The Abl inhibitor STI-571/Gleevec/Imatinib (STI-571) recognizes c-Abl and Arg but not Src, displaces ATP from Abl, and traps the kinase in an inactive conformation (Schindler et al. 2000). STI-571 has also been reported to inhibit the receptor tyrosine kinases c-Kit and platelet-derived growth factor (PDGF) receptor at higher doses (Schindler et al. 2000). Activation of c-Abl requires the Src family kinases (Plattner et al. 1999). Abl kinases have been linked to regulation of RAG gene expression in studies of pre-B cells transformed by temperature-sensitive mutants of the Abelson virus (Chen et al. 1994). Very recently, Muljo and Schlissel (2003) demonstrated that treatment of Abelson virus-transformed pro-B cell lines expressing active v-Abl with STI-571 activated transcription of Rag-1 and Rag-2. Jurkat T cells express moderate levels of c-Abl and low levels of Arg kinases, but are negative for c-Kit and PDGF receptor (Taylor et al. 2001; Bianchi et al. 2002; data not shown).
These findings prompted us to examine whether Abl kinases might play a role in constitutive repression of RAG genes. Indeed, inhibition of Abl kinase activity by STI-571 in Jurkat T cells induced RAG-1 expression to a level similar to that induced by the inhibitors PP2 and Ro-318220 (Figure 5A). Similarly, STI-571 induced elevated levels of Rag-1 in wild-type thymocytes (Figure 5B). This effect was more pronounced in TCR transgenic OT-1 thymocytes (Figure 5C).Abl has been reported to induce MAPK signaling in response to stress (Kharbanda et al. 1995). To address whether STI-571 functions to elevate RAG-1 expression indirectly by reducing a MAPK signal or whether there is a distinct Abl pathway, we carefully analyzed the specific phosphorylation status of Erk and Abl. Specific phosphorylation on threonine 202/tyrosine 204 residues reflects the kinase activities of Erk-1 and Erk-2. Active Abl is phosphorylated on tyrosine and serine residues (Pluk et al. 2002). Phosphorylation of tyrosine 245 and tyrosine 412 in c-Abl are crucial regulatory events required for its kinase activity (Brasher and Van Etten 2000). Phosphorylation of tyrosine 245 reflects disruption of the inactive conformation and can be detected with a phosphorylation-specific antibody.
In radioimmunoprecipitation assay (RIPA) lysates of resting J.CaM2 cells, we consistently observed an absence of basal phosphorylated Erk-1 and Erk-2 proteins, signals that were easily detected in Jurkat or J.CaM2-LAT (Figure 5D). Induced expression of the phosphatase CD148 for 7 d also ablated this constitutive phospho-Erk signal (Figure 5D, lane 5). Expression levels of c-Abl and the Erk-1 and Erk-2 kinases were equal in all samples (upper and lower panels of Figure 5D).
Treatment of wild-type Jurkat T cells with chemical inhibitors of signaling components revealed that inhibition of Src family kinases, PLCγ, PKC, MEK-1 and MEK-2, and calcineurin (using PP2, D609, Ro-318220, U-0126, and FK506, respectively) efficiently decreased the amount of phosphorylated Erk-1 and Erk-2, the reciprocal effect of that on RAG-1 expression (Figure 5E). Inhibition of calcineurin has been reported to induce expression of the MAPK phosphatase 1 and inhibit Erk signaling in neuronal cells (Zawadzka and Kaminska 2003). A similar mechanism may account for these effects on Jurkat T cells and thymocytes. As seen for other assays, the PI3K inhibitor LY294002 only has limited effect on phospho-Erk-1 and phospho-Erk-2 levels in Jurkat T cells. To our surprise, treatment with STI-571 only slightly decreased phospho-Erk (Figure 5E, lane 8), raising the possibility that Abl and Erk kinases may act in separate, basally active pathways to repress RAG-1 expression.
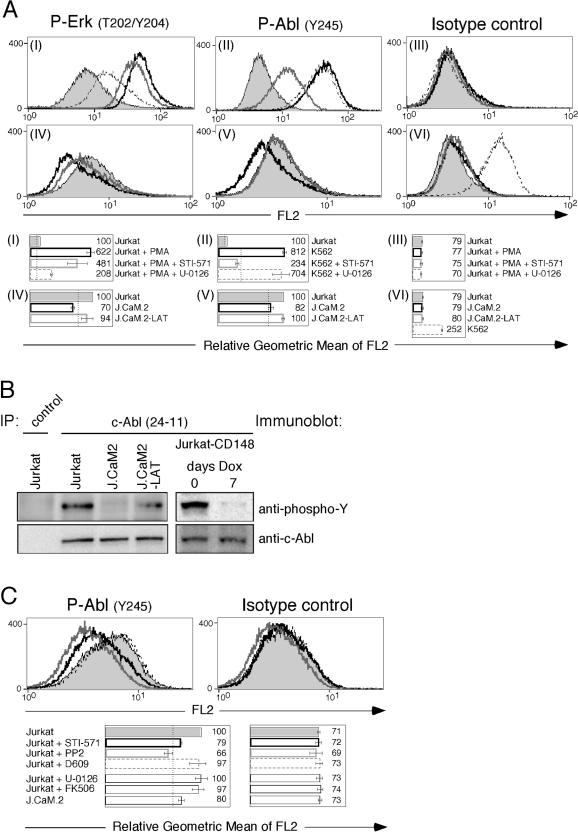
To resolve this question, we analyzed these low-level, constitutive phospho-c-Abl and phospho-Erk signals by intracellular FACS staining (we were unable to use the site-specific phospho-Y245 for c-Abl in Western blot analysis; data not shown). Panels I–III of Figure 6A demonstrate the feasibility of this approach. PMA stimulation of Jurkat T cells for 10 min resulted in 6-fold induction of phospho-Erk signal compared to unstimulated Jurkat (arbitrarily set at 100 in the accompanying bar graph of Figure 6A, panel I). As shown in the same panel, 24-h administration of a MEK-1 and MEK-2 inhibitor (U-0126) effectively blunted the phospho-Erk response, whereas the c-Abl inhibitor had a detectable but consistently smaller effect. The STI-571 inhibitor was, however, very potent in blocking the phospho-Abl signal in K562 cells, which reflects the kinase activity of BCR–Abl in this cell line (Figure 6A, panel II). Similar to the basal activity of the Erk kinases (Figure 6A, panel IV), phosphorylation of tyrosine 245 in Abl was consistently reduced in unstimulated J.CaM2 cells, resulting in a fluorescence signal not significantly above isotype control (Figure 6A, panels V and VI). The phospho-Abl signal observed in Jurkat T cells probably reflects both phosphorylated c-Abl and phosphorylated Arg since the two kinases are highly conserved in the tyrosine 245 region. Importantly, phospho-Abl levels were restored in the J.CaM2-LAT line, pointing to an essential role for LAT in Abl phosphorylation (Figure 6A, panel V; Figure 6B). The reduction of c-Abl-specific phosphorylation in J.CaM2 cells was confirmed by c-Abl immunoprecipitations followed by total phosphotyrosine immunoblotting and was restored in J.CaM2 cells complemented with LAT cDNA (Figure 6B). Induced expression of the phosphatase CD148 for 7 d also ablated this constitutive phospho-c-Abl signal (right panel in Figure 6B).
Figure 6. LAT Is Required for Two Largely Separate Pathways Marked by Constitutive Phosphorylation of Erk and Abl Kinases.
(A) Intracellular FACS staining for phospho-Erk (I and IV), phospho-Abl (II and V), and isotype control (III and VI) in the indicated cell lines. Histograms are an example of a representative experiment. The accompanying bar graphs are depicted in the same color-coding and represent the mean levels of fluorescence and standard deviation of three independent assays for all experiments. Mean levels of phosphoproteins measured by fluorescence in a resting Jurkat population were arbitrarily set at 100, and the mean fluorescence of the isotype control samples is indicated by the dotted line as a point of reference. Specifics are mentioned in the text.
(B) Analysis of tyrosine phosphorylation levels of c-Abl in the indicated cell samples. c-Abl or control immunopercipitations were immunoblotted for total tyrosine phosphorylation by 4G10. The same blot was stripped and reprobed for c-Abl. Doxycyclin was administered for 7 d to induce CD148 expression.
(C) Resting Jurkat T cells were treated with the indicated inhibitors for 24 h, and intracellular FACS staining for phospho-Abl and isotype control was compared to that detected in vehicle-treated Jurkat cells and the J.CaM2 line. Bar graphs (representing three experiments) display the four conditions depicted in the histograms as well as three additional samples.
The signals that elicit Abl phosphorylation were limited to Src and Abl kinases since blockade of PLCγ, MEK-1 and MEK-2, or calcineurin activity had no effect on the phosphorylation status of tyrosine 245 (Figure 6C). PP2 and STI-571 reduced the phospho-Abl level to that observed in J.CaM2, virtually equal to isotype control staining. It should be noted that PP2 has recently been reported to inhibit to some extent c-Kit and BCR–Abl (Tatton et al. 2003). Therefore, the PP2 effect may be the combined result of blocking Src family kinases and direct inhibition of Abl kinases. Together, these findings suggest that constitutive activity of Abl represses RAG gene expression, requires the presence of LAT, and is blocked by Src and Abl kinase inhibitors, but is unaffected by Erk activity.
Both Erk and Abl Kinases Repress RAG-1 Expression and Require Phosphorylation of Tyrosine 132 in LAT
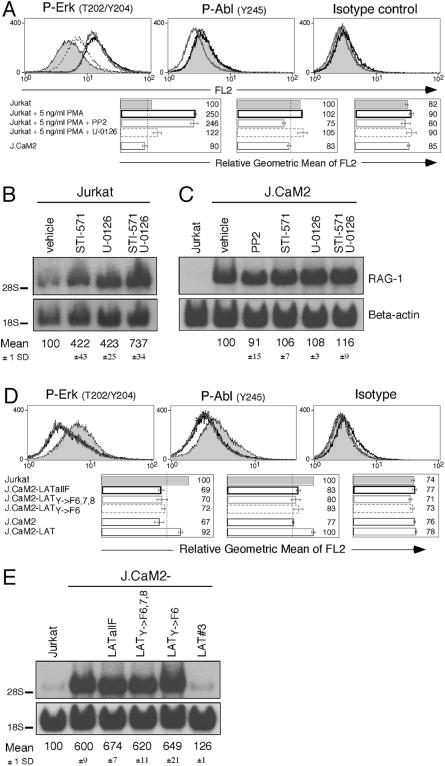
The unaltered phosphorylation status of Abl in U-0126-treated cells suggested that the MEK–Erk pathway does not contribute to basal Abl activity in our model. To further test this, we elevated the basal MAPK signal in Jurkat T cells by addition of 5 ng/ml PMA for 24 h. This treatment induced a higher level of phospho-Erk that is largely abrogated by U-0126, but not by PP2 (left panel of Figure 7A). By contrast, low amounts of PMA did not induce phosphorylation of Abl kinases (middle panel of Figure 7A). Therefore, whereas inhibition of Abl has modest effects on Erk phosphorylation, Erk activity does not enhance phosphorylation of tyrosine 245 in Abl. These observations further support the notion that Abl and Erk operate independently to regulate the expression of RAG-1. This notion is illustrated by the additive effects of STI-571 and U-0126 in inducing RAG-1 expression (Figure 7B). Moreover, it appears that the regulatory roles of both kinases in this pathway depend on LAT. Addition of STI-571, U-0126, or a combination of these inhibitors, or even targeting Src family kinases upstream of Abl and Erk, did not result in a hyperinduction of RAG-1 in the J.CaM2 line (Figure 7C).
Figure 7. Erk and Abl Synergize to Repress RAG-1 Expression and Require Tyrosine 132 in LAT.
(A) Jurkat T cells were stimulated with 5 ng/ml PMA for 24 h to mimic a low, constitutive MAPK signal. Intracellular FACS staining for phospho-Erk, phospho-Abl, and isotype control was performed on the indicated samples.
(B) The combined effect of blocking both Abl and MEK-1 and MEK-2 on RAG-1 expression was determined by Northern blot analysis. Cells were treated for 24 h.
(C) RAG-1 expression levels in LAT-deficient J.CaM2 cells treated with the designated inhibitors compared to vehicle-treated and Jurkat T cells as control.
(D) Intracellular FACS analysis using phospho-Erk, phospho-Abl, and isotype control antibodies on Jurkat, J.CaM2, and J.CaM2 cells stably reconstituted with cDNA expression vectors carrying the indicated mutations in LAT.
(E) Consequences of a signaling defective LAT molecule on RAG-1 mRNA levels in the specified resting cell lines. LATY→F6,7,8 reflects mutations in tyrosine residues 132, 171, and 191, whereas LATY→F6 harbors only a single mutation at tyrosine position 132.
Finally, the results above demonstrated that the roles of Erk and Abl kinases in controlling RAG-1 gene expression are largely parallel rather than sequential. MAPK activation by TCR stimulation has been known to rely on LAT (Finco et al. 1998; Zhang et al. 1999). How does LAT transduce a unique signal to Abl? To study a possible LAT–Abl connection, we made use of a panel of J.CaM2 cell lines reconstituted with LAT molecules that carry point mutations of tyrosine residues (see Figure 3A). We and others have previously demonstrated that induction of phospho-Erk upon TCR stimulation is impaired in J.CaM2 lines reconstituted with LATY→F6,7,8 or LATY→F6 (Zhang et al. 2000; Lin and Weiss 2001). These studies demonstrated the importance of PLCγ recruitment to tyrosine 132 (#6) in LAT for TCR-induced calcium flux and Erk activation. As demonstrated in Figure 7D, constitutive phosphorylation of both Erk and Abl kinases in these two lines was decreased compared to wild-type Jurkat and comparable to the levels seen in J.CaM2-LATallF and J.CaM2 cells. Consistent with the reduced phosphorylation of these two kinases, RAG-1 expression was increased in J.CaM2 cells reconstituted with LAT carrying only a single substitution of tyrosine residue 132 by phenylalanine (Figure 7E). Thus, Abl, like Erk, depends upon phosphorylation of tyrosine 132 in LAT.
Discussion
Here we have demonstrated a ligand-independent constitutive signaling pathway that is functional in Jurkat T cells, two murine thymomas, and in thymocytes. Our studies reveal that even without TCR engagement, the signaling pathways normally responsive to TCR stimulation are not inert. Instead the components of these pathways deliver unique constitutive instructions to the nucleus that maintains proper gene expression programs. RAG gene expression is tightly regulated during thymopoiesis and is expressed in at least two waves. The tonic basal signal we characterized in this study maintains repression of RAG gene expression and involves a TCR-like signal transduction pathway. One arm of the pathway requires Src family kinase activity, presence of LAT–SLP-76, and the enzyme activities of PLCγ1, PKC, PI3K, MEK-1, and calcineurin, culminating in basal kinase activity of Erk (Figure 8). We also identified a novel pathway that is responsible for constitutive phosphorylation of Abl. Proper signal transduction relies on Src family kinase activity and the presence of tyrosine 132 in LAT and can be blocked by STI-571 (Figure 8). We postulate that these constitutive signals may function to repress RAG gene expression during thymopoiesis and, as such, allow the locus to remain accessible for later usage without inappropriate expression.
Previous reports have demonstrated the importance of constitutive signals triggered by the antigen receptor or coreceptors in mature lymphocytes. In order to survive, B cells require surface expression of the BCR that generates a basal signal independently of endogenous antigen (Lam et al. 1997). Naive peripheral T cells appear to be less dependent on their receptors for survival (Polic et al. 2001), although they do depend on either Lck or Fyn as well as CD4 and MHC molecules for survival (Zamoyska et al. 2003). They also maintain a high degree of sensitivity for foreign antigen by having constitutive phosphorylation of TCRζ (Stefanova et al. 2002). Similar signals were suggested to occur in thymocytes (Nakayama et al. 1989; van Oers et al. 1994).
In this study, we have identified a TCR-independent and MHC-independent signaling pathway that maintains repression of RAG gene expression in a constitutive fashion. It is perhaps not surprising that in Jurkat cells and in thymocytes this pathway shares components with conventional TCR signaling. Key effector molecules for this repressive signal are Erk and Abl kinases. One branch of this constitutive signaling pathway involves Src family kinase activity, presumably phosphorylation of TCRζ and ZAP-70, phosphorylation of LAT, and recruitment and activation of PLCγ (Figure 8). This results, most likely, through low-level generation of the second messengers IP3 and DAG in constitutive activation of Erk kinases. We also identified a novel second pathway that results in constitutive phosphorylation and activity of Abl, which requires phosphorylation of tyrosine residue 132 in LAT, but not PLCγ, activation. The specific requirement of tyrosine residue 132 suggests that this pathway involves the formation of a LAT–PLCγ–Abl or LAT–Abl complex. Recently a complex of c-Abl and PLCγ1 has been reported in growth factor receptor signaling (Plattner et al. 2003). In agreement with these results, we observed a decrease in basal Abl phosphorylation in PLCγ1-deficient Jurkat cells (J.gamma1) and normal Abl phosphorylation in PLCγ1-reconstituted cells (J.gamma1.WT1; data not shown). However, basal activity of Erk kinases in this mutant cell line was hardly affected, possibly because of compensation by PLCγ2 in this cell line. As a result RAG-1 expression was only moderately increased, yet it was still repressed in J.gamma1.WT1 (data not shown). Together these results imply an unappreciated role for Abl kinases in thymopoiesis and TCR signaling. c-Abl-deficient mice are born runted, are less viable, and show signs of lymphopenia, but no stage-specific block in lymphocyte development (Schwartzberg et al. 1990; Tybulewicz et al. 1991). c-Abl−/−/Arg−/− mice have an embryonic lethal phenotype (Koleske et al. 1998). It will therefore be interesting to assess lymphocyte development in doubly deficient mice using a conditional deletion. Various mechanisms initiating c-Abl activation have been suggested, such as displacement of the myristate group or the SH2 or SH3 domain, all disrupting the inactive conformation and allowing tyrosine phosphorylation of residue 245 in the linker region or of 412 in the activation loop (Hantschel et al. 2003). We hypothesize that in this signaling pathway, binding of Abl to PLCγ opens up the inactive conformation. In addition, formation of a LAT–PLCγ–Abl complex could bring Abl in close proximity to Src family kinases, facilitating phosphorylation of Abl by Src.
Two elegant studies have addressed the specific role of tyrosine residue 136 of LAT, the murine equivalent of human tyrosine 132, in vivo. Thymocytes of 2-wk-old LATY136F knock-in mice demonstrate an incomplete block at the pre-TCR signaling stage (Aguado et al. 2002; Sommers et al. 2002). Paradoxically, a few weeks later the animals develop lymphadenopathy and splenomegaly. Lymph node CD4+ cells exhibit an activated phenotype, are of the T helper 2 type, and are autoreactive (Aguado et al. 2002; Sommers et al. 2002). Interestingly, these peripheral cells and DP and SP thymocytes display dramatically reduced surface TCR levels in comparison to their wild-type counterparts. This could reflect selection of a different repertoire due to altered strength of signal, preferentially selecting autoreactive cells. Alternatively, our data suggest that these thymocytes may aberrantly express Rag genes, resulting in undesirable further rearrangement leading to self-reacting TCRs.
Rag loci seem to remain accessible at least up to the DP stage when surface αβTCR is expressed. When ex vivo DP thymocytes are stimulated overnight with anti-TCR antibody, half of the cells have inactivated the Rag locus by repositioning to centromeric regions (Brown et al. 1999). Incubation of these DP cells with stroma results in the development into SP thymocytes combined with complete centromeric repositioning of the Rag locus. Permanent, heritable silencing subsequently prevents further Rag expression when thymocytes exit the thymus and become peripheral T cells. In agreement with this model, we failed to detect induced expression of Rag-1 in purified T cells from peripheral lymphoid organs using our panel of inhibitors. Similarly, Yu et al. (1999) could not observe any fluorescent activity in T cells of the RAG reporter mice. In lymph node T cells, this cascade may very well regulate expression patterns of other genes, like those depicted in Figure 3D, and sensitize the T cell to respond to foreign antigens (Stefanova et al. 2002).
Repositioning of the Rag locus over time requires transient repression, consistent with our findings here. In addition, repression may ensure physiologic Rag levels, allowing the DP thymocyte to test the newly assembled αβTCR before additional TCRα rearrangements are started. At the moment we can only speculate on the exact timing of the constitutive signal during thymopoiesis. Total thymocyte populations, as analyzed in Figure 4C, typically contain 80% DP cells. Given the effects we observe with the various inhibitors on total thymocytes with DP thymocytes being by far the largest population, this is the most likely subset where basal repression of Rag expression takes place.
Nevertheless, many components that transduce this repressive signal are expressed in thymocytes well before TCR or pre-TCR signaling occurs. It is therefore possible that Rag genes are repressed even before the first wave of Rag expression occurrs in CD25-positive DN thymocytes. If so, a cytokine signal may consequently be required to release the constitutive repression of Rag genes to allow Rag expression and rearrangement of the TCRβ chain. We are currently adapting our intracellular FACS staining to determine phospho-Erk and phospho-Abl in thymocyte subsets. In addition, we are planning to study this pathway in Rag-GFP indicator mice (Yu et al. 1999).
Our results support the notion that receptor-independent signaling occurs in T cells and thymocytes. This tonic basal signaling pathway utilizes many of the same signaling components as a TCR transduction pathway triggered by receptor engagement does. However, the activity of the pathway is far less than is seen when the TCR is stimulated. The basal flux of phosphorylation, second messengers, or both in the pathway is well compensated by negative regulators, thereby establishing a basal, steady-state tone. Our results indicate that this basal level of activity, nonetheless, has significant biological consequences and functions in T cells, as revealed here by the regulation of the RAG genes. The existence of a constitutive, ligand-independent regulatory role of this pathway has implications for interpreting the effects of mutations and chemical agents that interfere with its activity. It is likely that such basal activity in other signaling systems and in other cell types likewise has significant biological functions.
Materials and Methods
Cell lines, inhibition assays, and stimulations
References to all cell lines are cited in the text. To minimize variation in this and other studies, we isolated a single clone by limiting dilution of Jurkat E6-1 T cells, designated Jurkat E6-1-clone1. Clone numbers of derived cell lines are mentioned in the text or here: J.CaM2-LAT#3 (similar results were obtained with J.CaM2-LAT#70), J.CaM2-LATallF#11, J.CaM2-CtoS#2, Jurkat-CD148 (L12), J.CaM2-LATY→F6,7,8#55, and J.CaM2-LATY→F6#107. Jurkat T cells and derived cell lines were grown in RPMI (Cellgrow, Heydon, Virginia, United States) supplemented with 5% FCS (Hyclone, South Logan, Utah, United States) and pen/strep–glutamine (Irvine Scientific, Santa Ana, California, United States) at densities ranging between 0.4 × 106 and 1.0 × 106 cells per milliliter. SCB-29 cells were cultured in RPMI and DPK cells in CLICK's medium (Irvine Scientific), both with 10% FCS, 50 μM β-mercaptoethanol, and antibiotics. Thymi of 1- to 3-mo-old wild-type C57BL/6 mice or β2M−/−/MHCII−/− mice on C57BL/6 background (Taconic, Germantown, New York, United States) were removed, and single-cell suspensions were filtered, pelleted, and resuspended at approximately 25 × 106 to 50 × 106 cells per milliliter in RPMI with 20% FCS, 50 μM β-mercaptoethanol, antibiotics, 20 μM Caspase-3 inhibitor II (Calbiochem, San Diego, California, United States), and inhibitors. Inhibitor assays were set up in the appropriate medium. Final inhibitors concentrations were 20 μM PP2, 10 nM rapamycin (Calbiochem), 20 μM D609, 10 μM H89 (Sigma, St. Louis, Missouri, United States), 1 μM Ro 31-8220 (Alexis Biochemicals, Montreal, Quebec, Canada), 5 μM LY294002, 30 μM PD98059, 10 μM U0126 (Cell Signaling Technology, Beverly, Massachusetts, United States), 100 ng/ml FK506, and 10 μM STI-571 (University of California San Francisco School of Pharmacy, San Francisco, California, United States). All were dissolved in DMSO as a 1,000-fold stock, except STI-571, which was dissolved in water (pH 4). Cycloheximide (Sigma) was added at 10 μg/ml and actinomycin D (ICN Biochemicals, Costa Mesa, California, United States) at 40 μg/ml. PMA and PDBu (Sigma) were added at 25 ng/ml unless mentioned otherwise: ionomycin (Calbiochem) at 1 μM, carbachol (ICN Biochemicals) at 100 μM. Efficacy of inhibitors and stimulators was tested in independent assays, like phosphorylation of Erk and NFAT luciferase assays. CD148 expression was induced by 1 μg/ml doxycyclin (Sigma), as was 48-h induction of PTEN expression.
DNA arrays
The cDNA microarrays contained 37,632 elements, representing approximately 18,000 different genes, and were manufactured and hybridized as previously described (Eisen and Brown 1999; Alizadeh et al. 2000; see also http://brownlab.stanford.edu) and were scanned using a 4000B GenePix scanner at 10 mm resolution (Axon Instruments Inc., Union City, California, United States). In each analysis, mRNA from a cell line was used as a template for Cy-5-labeled cDNA synthesis. As a reference pool, RNA derived from wild-type Jurkat cells was used to prepare Cy-3-labeled cDNA. The Cy-5-labeled cell line cDNA and the Cy-3-labeled reference cDNA were mixed and hybridized to microarrays. Comparison of all experimental samples to the same reference allowed the relative expression level of each gene to be compared across all of the experiments. The resulting images were processed using GenePix Pro 3.0. The data were then normalized and indexed in the Stanford Microarray Database (SMD). Expression data for some of the genes in Figure 3 did not make the selection in Figure 1A and 1B due to high stringency used in the experiments presented in Figure 1A and 1B. All of the raw microarray data are available through SMD, linked via http://microarray-pubs.stanford.edu/tonicsignal/.
RNA isolation and Northern blot analysis
RNA was isolated as described (Chomczynski and Sacchi 1987). Total RNA (25 μg) was separated by electrophoresis, transferred to Hybond N+ nylon (Amersham Biosciences, Piscataway, New Jersey, United States), and UV cross-linked, all according to standard protocols. Hybridization was carried out in ExpressHyb (Clontech, Palo Alto, California, United States) with randomly labeled fragments using the Rediprime II Random Prime Labeling System and [32P]α-dCTP (Amersham Biosciences). For human RAG-1, mixed 400 bp and 800 bp EcoRI cDNA fragments were used; for mouse Rag-1, a 500 bp EcoRI fragment; for TCRα, a 350 bp HindIII–PvuII fragment encompassing the constant region; for β-actin, a 540 bp PCR fragment (primers: 5′-gtgggccgctctaggcaccaa-3′ and 5′-ctctttgatgtcacgcacgatttc-3′). Exposed films were quantified avoiding image saturation using a Kodak Image Station 440CF and Kodak ID Image Analysis Software (Eastman Kodak Corporation, Rochester, New York, United States). Relative expression of RAG-1 was calculated by subtracting background intensity from all the hybridizing bands. RAG-1 intensity was subsequently divided by β-actin intensity, and the result was adjusted to match 100% in vehicle-treated samples.
Western blot analysis and immunopercipitations
Western blot analysis of RIPA lysates was performed according to standard procedures (see Lin and Weiss 2001) using antibodies following the manufacturers' suggestions: phospho-p44/42 MAP kinase (Thr204/Tyr204) antibody (Cell Signaling Technology), antibodies to Erk-1 (C16) and Erk-2 (C14), and c-Abl (24-11) for immunopercipitations and c-Abl (K12) for blotting (all Santa Cruz Biotechnology, Santa Cruz, California, United States). c-Abl or control (using purified mouse IgG2b; ICN Biochemicals) immunopercipitations were performed on 500 μl RIPA lysates of 50 × 106 cells with 5 μg of 24-11 antibody bound to protein G–sepharose (Pharmacia, Peapack, New Jersey, United States) in 500 μl of NP40 buffer (Lin and Weiss 2001). Total phosphotyrosine levels were detected by 4G10 (Upstate Biotechnology, Lake Placid, New York, United States) and visualized using SuperSignal ECL reagent (Pierce Biotechnology, Rockford, Illinois, United States) and a Kodak Imaging Station.
Surface and intracellular FACS analysis
To determine the composition of thymocyte populations, cells were stained in FACS buffer (PBS, 1% BSA) with antibodies against CD4 (conjugated to FITC), CD8 (conjugated to tricolor) (CalTag Laboratories, Burlingame, California, United States), CD3ɛ (conjugated to PE) (PharMingen, San Diego, California, United States) and B220, DX-5, Gr-1, Mac-1 (eBioscience, San Diego, California, United States), and CD11b (Pharmigen), all conjugated to APC or Cy-5 using FL-4 as a negative gate. In addition, thymocytes negative for CD8, CD3, B220, DX-5, CD11b, and Mac-1 were analyzed for CD25 ( (FITC) (eBioscience) and CD44 (PE) (CalTag). Jurkat cell lines were analyzed for CD3ɛ and αβTCR staining using anti-CD3ɛ (clone SK7; BD Biosciences, San Jose, California, Unied States) and anti-αβTCR (clone IP26; eBioscience).
Intracellular FACS staining was performed using Fix & Perm (CalTag) according to the manufacturer's protocol. In short, cells were seeded in plates at 0.4 × 106 cells per milliliter, 5 ml per well. The next day, 4 × 106 cells were fixed in 100 μl of buffer A for 15 min and twice washed in FACS buffer, and one-sixth of each sample per staining was transferred to a concave 96-well plate. Samples were preblocked for 10 min in buffer B containing 5% normal goat serum (NGS) (Jackson Immunoresearch Laboratories, West Grove, Pennsylvania, United States). Subsequently, cells were stained in buffer B with 5% NGS for 1 h at 4°C in the dark with 1:50 phospho-p44/42 MAP kinase (Thr204/Tyr204) antibody, 1:10 phospho-c-Abl (Tyr245) antibody (Cell Signaling Technology), or control rabbit IgG. After two washes in FACS buffer, cells were stained for 1 h in buffer B with 5% NGS and 1:50 PE-conjugated AffiniPure F(ab′)2 fragment donkey anti-rabbit IgG (Jackson Immunoresearch Laboratories), washed two times, and analyzed.
Supporting Information
Figure 1A and 1B can interactively explored at http://microarray-pubs.stanford.edu/tonicsignal/.
All of the raw microarray data in this paper are available through the Stanford Microarray Database, linked via http://microarray-pubs.stanford.edu/tonicsignal/.
Accession Numbers
Locus Link ID numbers (http://www.ncbi.nlm.nih.gov/LocusLink/) for the loci discussed in this paper are ABCB10 (23456), c-Abl (25), Arg (27), BIN2 (51411), Cbl (867), CD2 (914), CD3δ (915), CD3ɛ (916), CD3γ (917), CD4 (920), CD5 (921), CD6 (923), CD7 (924), CD8 (925), CD18 (281877), CD25 (3559), CD28 (940), CD44 (960), CD62L (6402), CD148 (5795), DTR (1839), Erk-2 (5594), Fyn (2534), Fyn-binding protein (2533), Gads (9402), Grap (71520), Grb2 (2885), c-Kit (3815), Lck (3932), MAGEA-8 (4107), MAGEA-11 (4110), MEK-1 (5604), MEK-2 (5605), MHC class II (85498), mTOR (2475), muscarinic receptor (1128), PDGF (5159), PI3K (5295), PKA (5566), PLCγ1 (5335), PTEN (5728), RAG-1 (human) (5896), Rag-1 (mouse) (19373), RAG-2 (human) (5897), Rag-2 (mouse) (19374), SCN2A2 (6326), SHIP (3635), SLP-76 (3937), Syk (6850), TCF7 (6932), TCRα (6955), TCRβ (6957), TCRζ (919), Vav (7409), and ZAP-70 (7535).
Acknowledgments
The authors express their gratitude to David Schatz for RAG cDNAs, Jonathan Kaye for providing DPK cells, and Joseph DeRisi and Adam Carroll for assistance with DNA microarray experiments. We thank Nigel Killeen, Tony DeFranco, and Larry Kane for critically reading the manuscript; Mark Schlissel for sharing data prior to publication; Rene Bernards for continuous support; and Kristin Hogquist and the Weiss lab for suggestions and comments. JPR is grateful for grants from The Netherlands Organization for Scientific Research (NWO) and the Dutch Cancer Society (KWF). MD and AAA were supported by the Medical Scientist Training Program. POB is an investigator of the Howard Hughes Medical Institute. This work was supported in part by a grant from the National Cancer Institute (CA 72531).
Abbreviations
- Abl
Abelson
- AP-1
activating protein 1
- β2M
β2-microglobulin
- BCR
B cell receptor
- Cbl
casitas B-lineage lymphoma
- DAG
diacylglycerol
- DMSO
dimethyl sulphoxide
- DN
double negative
- DP
double positive
- Erk
extracellular signal-regulated kinase
- FACS
fluorescence-activated cell sorting
- Fyn
Fgr/Yes-related novel protein tyrosine kinase
- Gads
Grb-2-like adapter downstream of Shc
- GEM
glycolipid-enriched microdomain
- Grb-2
growth factor receptor-bound protein 2
- HM-I
human muscarinic receptor
- IP3
inositol trisphosphate
- ITAM
immunoreceptor tyrosine-based activation motif
- LAT
linker for activation in T cells
- Lck
lymphocyte-specific protein tyrosine kinase
- MAPK
mitogen-activated protein kinase
- MEK
MAP kinase/Erk kinase
- MHC
major histocompatibility complex
- mTOR
mammalian target of rapamycin
- NFAT
nuclear factor for activation of T cells
- NGS
normal goat serum
- PDBu
phorbol-12,13-dibutyrate
- PDGF
platelet-derived growth factor
- PI3K
phosphoinositide 3′-kinase
- PIP3
phosphatidylinositol 3,4,5-trisphosphate
- PKA
protein kinase A
- PKC
protein kinase C
- PLCγ1
phospholipase Cγ1
- PMA
phorbol myristate acetate
- PTEN
3′-phosphatase and tensin homolog deleted on chromosome 10
- PTK
protein tyrosine kinase
- RAG
recombinase-activating gene
- RGS
regulator of G-protein signaling
- RIPA
radioimmunoprecipitation assay
- SH2
Src homology 2
- SH3
Src homology 3
- SHIP
SH2-containing inositol polyphosphate 5′-phosphatase
- SLP-76
SH2 domain-containing leukocyte protein
- SMD
Stanford Microarray Database
- SP
single positive
- Syk
spleen tyrosine kinase
- TCR
T cell receptor
- ZAP-70
ζ-associated protein 70
Conflicts of Interest. The authors have declared that no conflicts of interest exist.
Author Contributions. JPR and AW conceived and designed the experiments. JPR, MD, MGT, JL, and AAA performed the experiments. JPR, MD, MGT, and AAA analyzed the data. MD, JL, DB, and POB contributed reagents/materials/analysis tools. JPR wrote the paper. POB assisted in preparing the manuscript. AW guided JPR's project and assisted in writing the manuscript.
Academic Editor: Philippa Marrack, National Jewish Medical and Research Center
¤1Present address: Department of Microbiology and Immunology, University of California, San Francisco, San Francisco, California, United States of America
¤2Present address: Lewis-Sigler Institute, Princeton University, Princeton, New Jersey, United States of America
References
- Aguado E, Richelme S, Nuñez-Cruz S, Miazek A, Mura AM, et al. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science. 2002;296:2036–2040. doi: 10.1126/science.1069057. [DOI] [PubMed] [Google Scholar]
- Aifantis I, Borowski C, Gounari F, Lacorazza HD, Nikolich-Zugich J, et al. A critical role for the cytoplasmic tail of pTα in T lymphocyte development. Nat Immunol. 2002;3:483–488. doi: 10.1038/ni779. [DOI] [PubMed] [Google Scholar]
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- Astoul E, Edmunds C, Cantrell DA, Ward SG. PI 3-K and T-cell activation: Limitations of T-leukemic cell lines as signaling models. Trends Immunol. 2001;22:490–496. doi: 10.1016/s1471-4906(01)01973-1. [DOI] [PubMed] [Google Scholar]
- Baker JE, Majeti R, Tangye SG, Weiss A. Protein tyrosine phosphatase CD148-mediated inhibition of T-cell receptor signal transduction is associated with reduced LAT and phospholipase Cγ1 phosphorylation. Mol Cell Biol. 2001;21:2393–2403. doi: 10.1128/MCB.21.7.2393-2403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C, Muradore I, Corizzato M, Cornacchini G, Beretta L, et al. The expression of the nonreceptor tyrosine kinases Arg and c-Abl is differently modulated in B lymphoid cells at different stages of differentiation. FEBS Lett. 2002;527:216–222. doi: 10.1016/s0014-5793(02)03233-7. [DOI] [PubMed] [Google Scholar]
- Brasher BB, Van Etten RA. c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J Biol Chem. 2000;275:35631–35637. doi: 10.1074/jbc.M005401200. [DOI] [PubMed] [Google Scholar]
- Brown KE, Baxter J, Graf D, Merkenschlager M, Fisher AG. Dynamic repositioning of genes in the nucleus of lymphocytes preparing for cell division. Mol Cell. 1999;3:207–217. doi: 10.1016/s1097-2765(00)80311-1. [DOI] [PubMed] [Google Scholar]
- Chen YY, Wang LC, Huang MS, Rosenberg N. An active v-Abl protein tyrosine kinase blocks immunoglobulin light-chain gene rearrangement. Genes Dev. 1994;8:688–697. doi: 10.1101/gad.8.6.688. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clements JL, Yang B, Ross-Barta SE, Eliason SL, Hrstka RF, et al. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- Crespo P, Xu N, Simonds WF, Gutkind JS. Ras-dependent activation of MAP kinase pathway mediated by G-protein βγ subunits. Nature. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Brown PO. DNA arrays for analysis of gene expression. Methods Enzymol. 1999;303:179–205. doi: 10.1016/s0076-6879(99)03014-1. [DOI] [PubMed] [Google Scholar]
- Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- Fisher AG, Merkenschlager M. Gene silencing, cell fate and nuclear organisation. Curr Opin Genet Dev. 2002;12:193–197. doi: 10.1016/s0959-437x(02)00286-1. [DOI] [PubMed] [Google Scholar]
- Fraser JD, Newton ME, Weiss A. CD28 and T cell antigen receptor signal transduction coordinately regulate interleukin 2 gene expression in response to superantigen stimulation. J Exp Med. 1992;175:1131–1134. doi: 10.1084/jem.175.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Panana EM, Monroe JG. Ligand-dependent and -independent processes in B-cell-receptor-mediated signaling. Springer Semin Immunopathol. 2001;23:333–350. doi: 10.1007/s281-001-8163-6. [DOI] [PubMed] [Google Scholar]
- Goldsmith MA, Desai DM, Schultz T, Weiss A. Function of a heterologous muscarinic receptor in T cell antigen receptor signal transduction mutants. J Biol Chem. 1989;264:17190–17197. [PubMed] [Google Scholar]
- Groettrup M, Baron A, Griffiths G, Palacios R, von Boehmer H. T cell receptor (TCR) beta chain homodimers on the surface of immature but not mature alpha, gamma, delta chain deficient T cell lines. EMBO J. 1992;11:2735–2745. doi: 10.1002/j.1460-2075.1992.tb05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusby MJ, Auchincloss H, Lee R, Johnson RS, Spencer JP, et al. Mice lacking major histocompatibility complex class I and class II molecules. Proc Natl Acad Sci U S A. 1993;90:3913–3917. doi: 10.1073/pnas.90.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haks MC, Belkowski SM, Ciofani M, Rhodes M, Lefebvre JM, et al. Low activation threshold as a mechanism for ligand-independent signaling in pre-T cells. J Immunol. 2003;170:2853–2861. doi: 10.4049/jimmunol.170.6.2853. [DOI] [PubMed] [Google Scholar]
- Hantschel O, Nagar B, Guettler S, Kretzschmar J, Dorey K, et al. A myristoyl/phosphotyrosine switch regulates c-Abl. Cell. 2003;112:845–857. doi: 10.1016/s0092-8674(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Huby RD, Weiss A, Ley SC. Nocodazole inhibits signal transduction by the T cell antigen receptor. J Biol Chem. 1998;273:12024–12031. doi: 10.1074/jbc.273.20.12024. [DOI] [PubMed] [Google Scholar]
- Irving BA, Alt FW, Killeen N. Thymocyte development in the absence of pre-T cell receptor extracellular immunoglobulin domains. Science. 1998;280:905–908. doi: 10.1126/science.280.5365.905. [DOI] [PubMed] [Google Scholar]
- Kane LP, Lin J, Weiss A. Signal transduction by the TCR for antigen. Curr Opin Immunol. 2000;12:242–249. doi: 10.1016/s0952-7915(00)00083-2. [DOI] [PubMed] [Google Scholar]
- Kane LP, Mollenauer MN, Xu Z, Turck CW, Weiss A. Akt-dependent phosphorylation specifically regulates Cot induction of NF-κB-dependent transcription. Mol Cell Biol. 2002;22:5962–5974. doi: 10.1128/MCB.22.16.5962-5974.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J, Ellenberger DL. Differentiation of an immature T cell line: A model of thymic positive selection. Cell. 1992;71:423–435. doi: 10.1016/0092-8674(92)90512-b. [DOI] [PubMed] [Google Scholar]
- Kharbanda S, Ren R, Pandey P, Shafman TD, Feller SM, et al. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature. 1995;376:785–788. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- Koleske AJ, Gifford AM, Scott ML, Nee M, Bronson RT, et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- Koretzky GA, Picus J, Schultz T, Weiss A. Tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc Natl Acad Sci U S A. 1991;88:2037–2041. doi: 10.1073/pnas.88.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Lin J, Weiss A. Identification of the minimal tyrosine residues required for linker for activation of T cell function. J Biol Chem. 2001;276:29588–29595. doi: 10.1074/jbc.M102221200. [DOI] [PubMed] [Google Scholar]
- Lin J, Weiss A, Finco TS. Localization of LAT in glycolipid-enriched microdomains is required for T cell activation. J Biol Chem. 1999;274:28861–28864. doi: 10.1074/jbc.274.41.28861. [DOI] [PubMed] [Google Scholar]
- Lindsten T, June CH, Thompson CB. Transcription of T cell antigen receptor genes is induced by protein kinase C activation. J Immunol. 1988;141:1769–1774. [PubMed] [Google Scholar]
- McGargill MA, Derbinski JM, Hogquist KA. Receptor editing in developing T cells. Nat Immunol. 2000;1:336–341. doi: 10.1038/79790. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Muljo SA, Schlissel MS. A small molecule Abl kinase inhibitor induces differentiation of Abelson virus-transformed pre-B cell lines. Nat Immunol. 2003;4:31–37. doi: 10.1038/ni870. [DOI] [PubMed] [Google Scholar]
- Nagaoka H, Yu W, Nussenzweig MC. Regulation of RAG expression in developing lymphocytes. Curr Opin Immunol. 2000;12:187–190. doi: 10.1016/s0952-7915(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Singer A, Hsi ED, Samelson LE. Intrathymic signalling in immature CD4+CD8+ thymocytes results in tyrosine phosphorylation of the T-cell receptor ζ chain. Nature. 1989;341:651–654. doi: 10.1038/341651a0. [DOI] [PubMed] [Google Scholar]
- Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Ohashi PS, Mak TW, van den Elsen P, Yanagi Y, Yoshikai Y, et al. Reconstitution of an active surface T3/T-cell antigen receptor by DNA transfer. Nature. 1985;316:606–609. doi: 10.1038/316606a0. [DOI] [PubMed] [Google Scholar]
- Ono S, Ohno H, Saito T. Rapid turnover of the CD3 ζ chain independent of the TCR–CD3 complex in normal T cells. Immunity. 1995;2:639–644. doi: 10.1016/1074-7613(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Pivniouk V, Tsitsikov E, Swinton P, Rathbun G, Alt FW, et al. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner R, Irvin BJ, Guo S, Blackburn K, Kazlauskas A, et al. A new link between the c-Abl tyrosine kinase and phosphoinositide signalling through PLC-γ1. Nat Cell Biol. 2003;5:309–319. doi: 10.1038/ncb949. [DOI] [PubMed] [Google Scholar]
- Pluk H, Dorey K, Superti-Furga G. Autoinhibition of c-Abl. Cell. 2002;108:247–259. doi: 10.1016/s0092-8674(02)00623-2. [DOI] [PubMed] [Google Scholar]
- Polic B, Kunkel D, Scheffold A, Rajewsky K. How αβT cells deal with induced TCRα ablation. Proc Natl Acad Sci U S A. 2001;98:8744–8749. doi: 10.1073/pnas.141218898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D, Mollenauer MN, Weiss A. Dominant-negative ζ-associated protein 70 inhibits T cell antigen receptor signaling. J Exp Med. 1996;183:611–620. doi: 10.1084/jem.183.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Ruf C, Panigada M, Azogui O, Debey P, von Boehmer H, et al. Different initiation of pre-TCR and γδTCR signalling. Nature. 2000;406:524–527. doi: 10.1038/35020093. [DOI] [PubMed] [Google Scholar]
- Schatz DG, Oettinger MA, Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989;59:1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Schatz DG, Oettinger MA, Schlissel MS. V(D)J recombination: Molecular biology and regulation. Annu Rev Immunol. 1992;10:359–383. doi: 10.1146/annurev.iy.10.040192.002043. [DOI] [PubMed] [Google Scholar]
- Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, et al. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- Schwartzberg PL, Robertson EJ, Goff SP. Targeted gene disruption of the endogenous c-abl locus by homologous recombination with DNA encoding a selectable fusion protein. Proc Natl Acad Sci U S A. 1990;87:3210–3214. doi: 10.1073/pnas.87.8.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Siekhaus DE, Drubin DG. Spontaneous receptor-independent heterotrimeric G-protein signalling in an RGS mutant. Nat Cell Biol. 2003;5:231–235. doi: 10.1038/ncb941. [DOI] [PubMed] [Google Scholar]
- Sommers CL, Park CS, Lee J, Feng C, Fuller CL, et al. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science. 2002;296:2040–2043. doi: 10.1126/science.1069066. [DOI] [PubMed] [Google Scholar]
- Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- Straus DB, Weiss A. Genetic evidence for the involvement of the Lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- Tatton L, Morley GM, Chopra R, Khwaja A. The Src-selective kinase inhibitor PP1 also inhibits Kit and Bcr–Abl tyrosine kinases. J Biol Chem. 2003;278:4847–4853. doi: 10.1074/jbc.M209321200. [DOI] [PubMed] [Google Scholar]
- Taylor ML, Dastych J, Sehgal D, Sundstrom M, Nilsson G, et al. The Kit-activating mutation D816V enhances stem cell factor-dependent chemotaxis. Blood. 2001;98:1195–1199. doi: 10.1182/blood.v98.4.1195. [DOI] [PubMed] [Google Scholar]
- Tomlinson MG, Lin J, Weiss A. Lymphocytes with a complex: Adapter proteins in antigen receptor signaling. Immunol Today. 2000;21:584–591. doi: 10.1016/s0167-5699(00)01716-3. [DOI] [PubMed] [Google Scholar]
- Turka LA, Schatz DG, Oettinger MA, Chun JJ, Gorka C, et al. Thymocyte expression of RAG-1 and RAG-2: Termination by T cell receptor cross-linking. Science. 1991;253:778–781. doi: 10.1126/science.1831564. [DOI] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Van Etten RA. Cycling, stressed-out and nervous: Cellular functions of c-Abl. Trends Cell Biol. 1999;9:179–186. doi: 10.1016/s0962-8924(99)01549-4. [DOI] [PubMed] [Google Scholar]
- van Oers NS, Killeen N, Weiss A. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCRζ in murine thymocytes and lymph node T cells. Immunity. 1994;1:675–685. doi: 10.1016/1074-7613(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Wayne J, Suh H, Misulovin Z, Sokol KA, Inaba K, et al. A regulatory role for recombinase activating genes, RAG-1 and RAG-2, in T cell development. Immunity. 1994;1:95–107. doi: 10.1016/1074-7613(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Weiss A, Stobo JD. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984;160:1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BL, Schreiber KL, Zhang W, Wange RL, Samelson LE, et al. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: Reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol Cell Biol. 1998;18:1388–1399. doi: 10.1128/mcb.18.3.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Held W, MacDonald HR. Two waves of recombinase gene expression in developing thymocytes. J Exp Med. 1994;179:1355–1360. doi: 10.1084/jem.179.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Stokoe D, Kane LP, Weiss A. The inducible expression of the tumor suppressor gene PTEN promotes apoptosis and decreases cell size by inhibiting the PI3K/Akt pathway in Jurkat T cells. Cell Growth Differ. 2002;13:285–296. [PubMed] [Google Scholar]
- Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-γ1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- Yu W, Misulovin Z, Suh H, Hardy RR, Jankovic M, et al. Coordinate regulation of RAG1 and RAG2 by cell type-specific DNA elements 5′ of RAG2. Science. 1999;285:1080–1084. doi: 10.1126/science.285.5430.1080. [DOI] [PubMed] [Google Scholar]
- Zamoyska R, Basson A, Filby A, Legname G, Lovatt M, et al. The influence of the Src-family kinases, Lck and Fyn, on T cell differentiation, survival and activation. Immunol Rev. 2003;191:107–118. doi: 10.1034/j.1600-065x.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- Zawadzka M, Kaminska B. Immunosuppressant FK506 affects multiple signaling pathways and modulates gene expression in astrocytes. Mol Cell Neurosci. 2003;22:202–209. doi: 10.1016/s1044-7431(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: The ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, et al. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, et al. Association of Grb2, Gads, and phospholipase C-γ1 with phosphorylated LAT tyrosine residues: Effect of LAT tyrosine mutations on T cell antigen receptor-mediated signaling. J Biol Chem. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]