Abstract
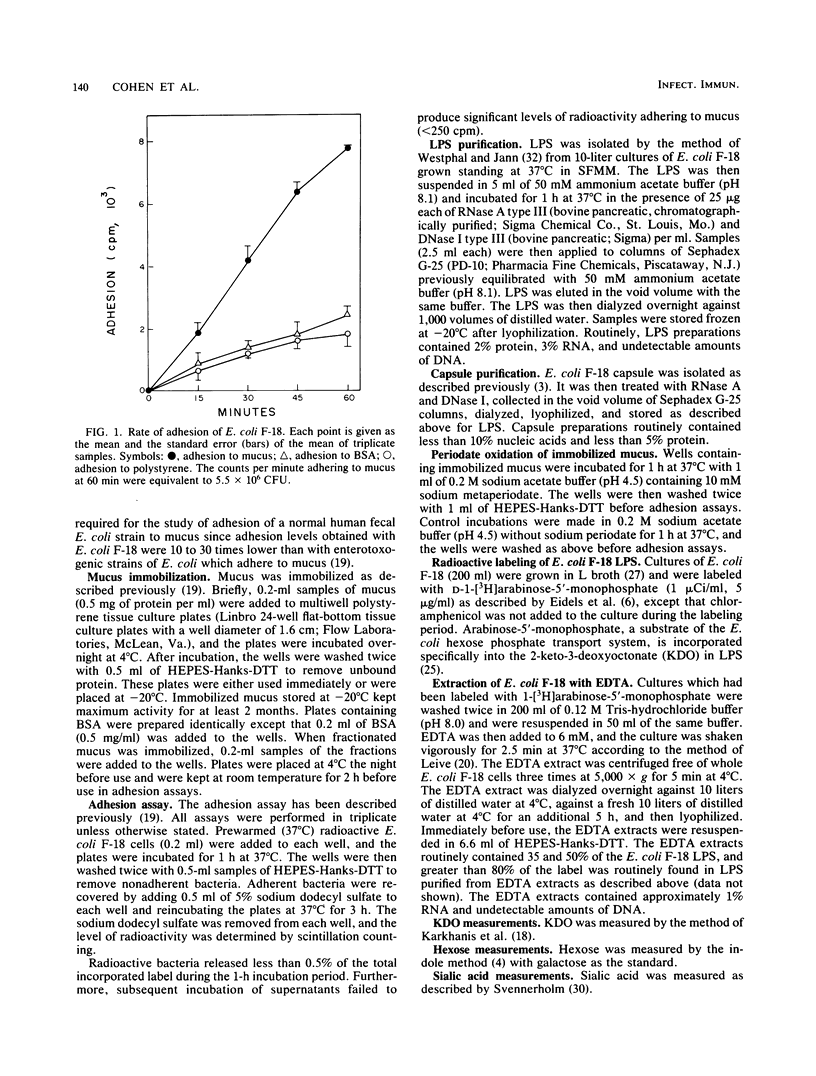
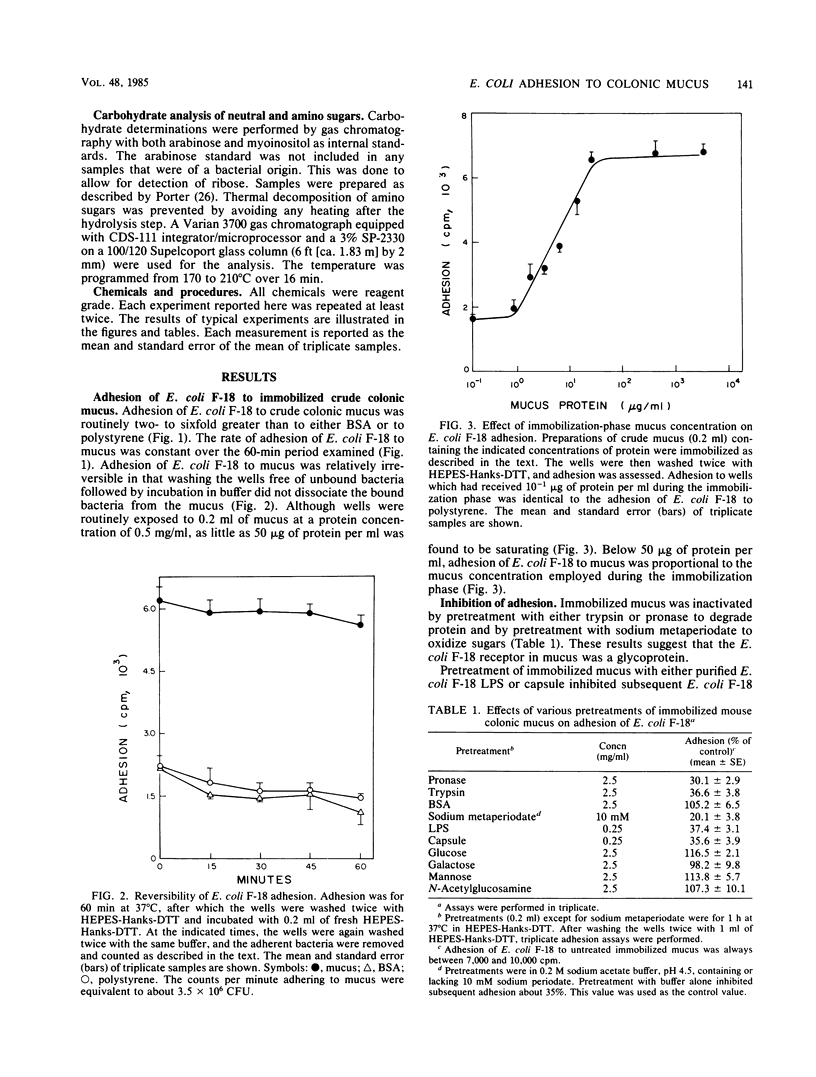
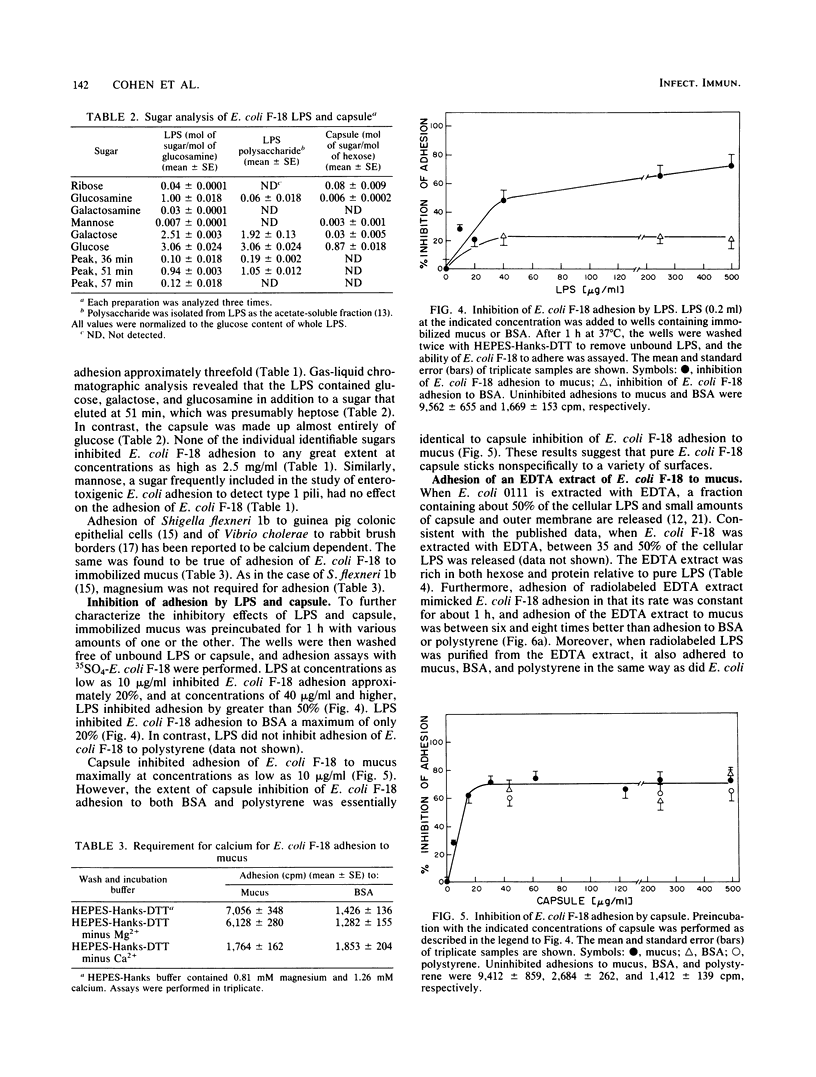
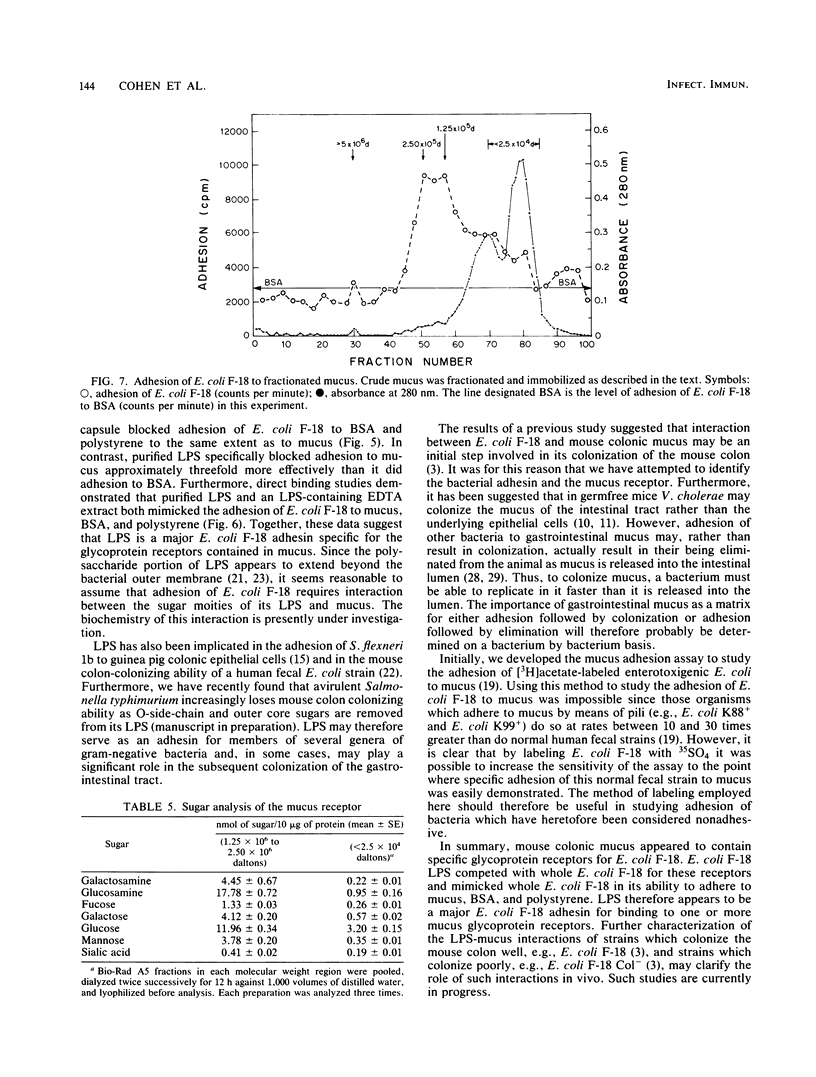
Escherichia coli F-18 isolated from the feces of a healthy human is an excellent colonizer of the CD-1 mouse colon. In the present investigation, adhesion of E. coli F-18 to CD-1 mouse colonic mucus and bovine serum albumin (BSA), immobilized on polystyrene, was studied. Adhesion of E. coli F-18 to mucus was two- to sixfold greater than to either BSA or polystyrene. E. coli F-18 lipopolysaccharide specifically blocked adhesion of E. coli F-18 to mucus and mimicked adhesion of E. coli F-18 to mucus, BSA, and polystyrene. Purified capsule also blocked adhesion of E. coli F-18 to mucus, but this inhibition was found to be entirely nonspecific. The specific E. coli F-18 receptor in mucus appeared to be a glycoprotein, containing sugars normally found in mucins and having a maximum molecular weight of between 1.25 X 10(5) and 2.5 X 10(5).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Snary D. The structure and function of gastric mucus. Gut. 1972 Aug;13(8):666–672. doi: 10.1136/gut.13.8.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick S., Harber M. J., Mackenzie R., Asscher A. W. Modified method for studying bacterial adhesion to isolated uroepithelial cells and uromucoid. Infect Immun. 1981 Oct;34(1):256–261. doi: 10.1128/iai.34.1.256-261.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Rossoll R., Cabelli V. J., Yang S. L., Laux D. C. Relationship between the mouse colonizing ability of a human fecal Escherichia coli strain and its ability to bind a specific mouse colonic mucous gel protein. Infect Immun. 1983 Apr;40(1):62–69. doi: 10.1128/iai.40.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corliss T. L., Cohen P. S., Cabelli V. J. R-Plasmid Transfer to and from Escherichia coli Strains Isolated from Human Fecal Samples. Appl Environ Microbiol. 1981 Apr;41(4):959–966. doi: 10.1128/aem.41.4.959-966.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- Eidels L., Rick P. D., Stimler N. P., Osborn M. J. Transport of D-arabinose-5-phosphate and D-sedoheptulose-7-phosphate by the hexose phosphate transport system of Salmonella typhimurium. J Bacteriol. 1974 Jul;119(1):138–143. doi: 10.1128/jb.119.1.138-143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner J. F. Intestinal mucins in health and disease. Digestion. 1978;17(3):234–263. doi: 10.1159/000198115. [DOI] [PubMed] [Google Scholar]
- Freter R., Allweiss B., O'Brien P. C., Halstead S. A., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect Immun. 1981 Oct;34(1):241–249. doi: 10.1128/iai.34.1.241-249.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R. Mechanisms of association of bacteria with mucosal surfaces. Ciba Found Symp. 1981;80:36–55. doi: 10.1002/9780470720639.ch4. [DOI] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Halstead S. A. Adhesion and chemotaxis as determinants of bacterial association with mucosal surfaces. Adv Exp Med Biol. 1978;107:429–437. doi: 10.1007/978-1-4684-3369-2_48. [DOI] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Macsai M. S. Effect of chemotaxis on the interaction of cholera vibrios with intestinal mucosa. Am J Clin Nutr. 1979 Jan;32(1):128–132. doi: 10.1093/ajcn/32.1.128. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., White D., Orskov F., Orskov I., Rick P. D., Lewis M. S., Bhattacharjee A. K., Leive L. A surface polysaccharide of Escherichia coli O111 contains O-antigen and inhibits agglutination of cells by O-antiserum. J Bacteriol. 1982 Sep;151(3):1210–1221. doi: 10.1128/jb.151.3.1210-1221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley C. L., Neumann C. S., Richmond M. H. Adhesion of commensal bacteria to the large intestine wall in humans. Infect Immun. 1979 Jan;23(1):128–132. doi: 10.1128/iai.23.1.128-132.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhar M., Nuchamowitz Y., Mirelman D. Adherence of Shigella flexneri to guinea pig intestinal cells is mediated by a mucosal adhesion. Infect Immun. 1982 Mar;35(3):1110–1118. doi: 10.1128/iai.35.3.1110-1118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann K., Schmidt G., Blumenstock E., Vosbeck K. Escherichia coli adhesion to Saccharomyces cerevisiae and mammalian cells: role of piliation and surface hydrophobicity. Infect Immun. 1981 May;32(2):484–489. doi: 10.1128/iai.32.2.484-489.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Myhal M. L., Cohen P. S., Laux D. C. Altered colonizing ability for mouse large intestine of a surface mutant of a human faecal isolate of Escherichia coli. J Gen Microbiol. 1983 May;129(5):1549–1558. doi: 10.1099/00221287-129-5-1549. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Birch-Andersen A. Comparison of Escherichia coli fimbrial antigen F7 with type 1 fimbriae. Infect Immun. 1980 Feb;27(2):657–666. doi: 10.1128/iai.27.2.657-666.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter W. H. Application of nitrous acid deamination of hexosamines to the simultaneous GLC determination of neutral and amino sugars in glycoproteins. Anal Biochem. 1975 Jan;63(1):27–43. doi: 10.1016/0003-2697(75)90186-4. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun. 1984 Jul;45(1):197–202. doi: 10.1128/iai.45.1.197-202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


