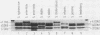Abstract
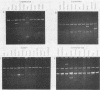
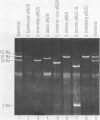
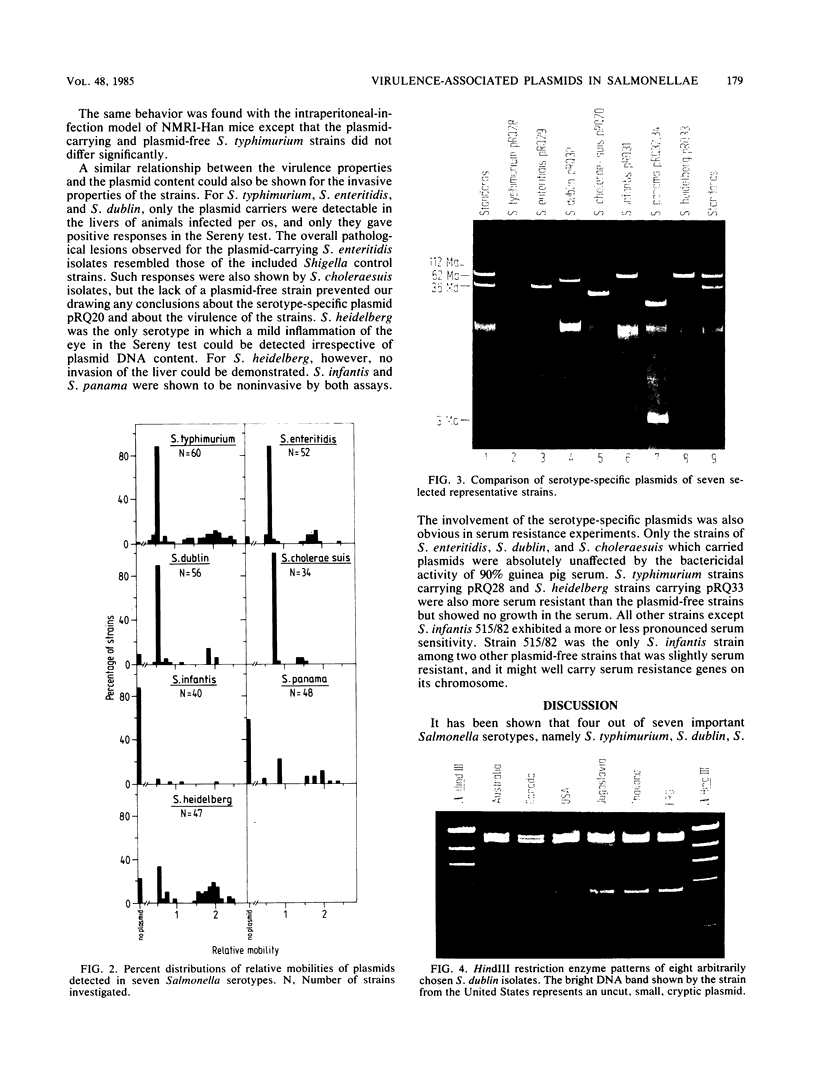
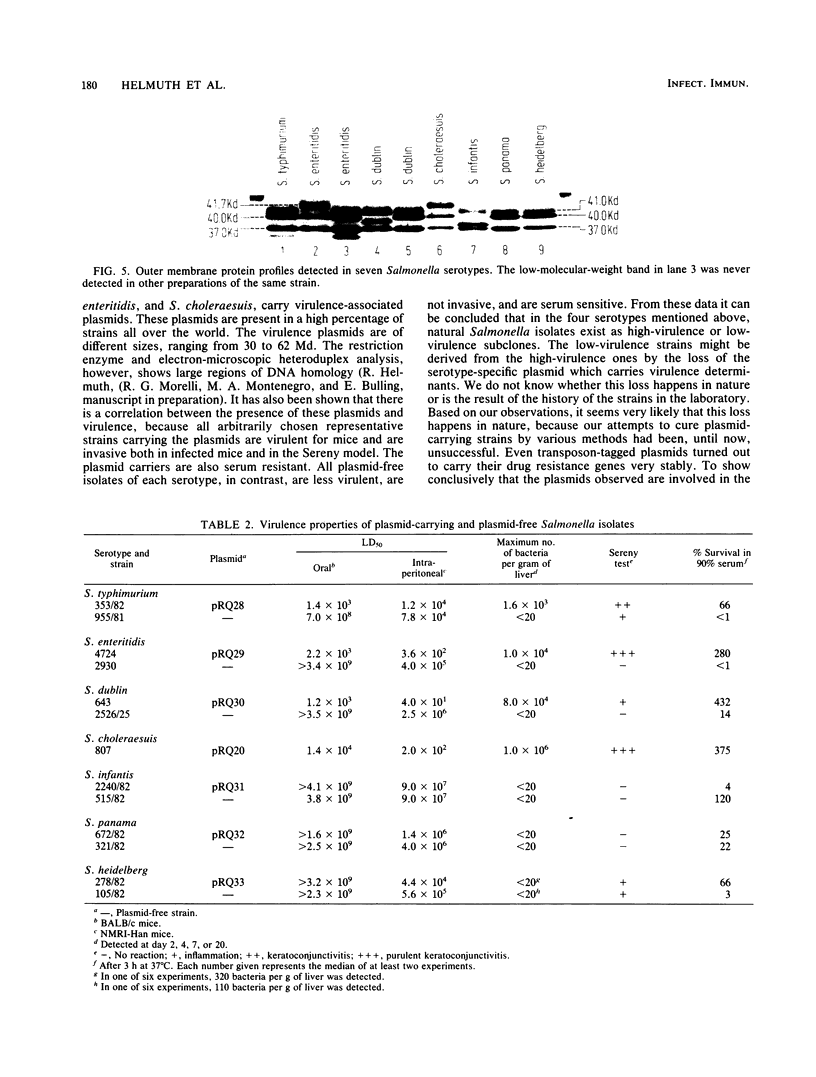
Antibiotic-sensitive Salmonella isolates belonging to seven common serotypes and originating from 29 different countries from all continents were investigated for their plasmid DNA content (337 isolates) and their outer membrane protein profiles (216 isolates). Of the S. typhimurium, S. enteritidis, S. dublin, and S. choleraesuis isolates, 90% or more carried a serotype-specific plasmid. The molecular sizes of the plasmids were 60 megadaltons (Md) for S. typhimurium, 37 Md for S. enteritidis, 56 Md for S. dublin, and 30 Md for S. choleraesuis. The outer membrane protein profiles were homogeneous within each of the seven serotypes, except that a minority of S. enteritidis and S. dublin strains were lacking one major outer membrane protein. Virulence studies were performed with 39 representative strains by measuring the 50% lethal doses (LD50S) after oral infection of mice. The LD50 values obtained for plasmid-positive strains of S. typhimurium, S. enteritidis, and S. dublin were up to 10(6)-fold lower than the values obtained for the plasmid-free strains of the same serotype. Only the plasmid-positive strains could invade the livers of orally infected mice, and only they were resistant to the bactericidal activity of 90% guinea pig serum. Strains of S. infantis were generally plasmid free, whereas S. panama and S. heidelberg isolates carried heterogeneous plasmid populations. The virulence properties of the latter three serotypes could not be correlated with the predominant plasmids found in these strains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Hildebrandt J. F. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect Immun. 1981 Jun;32(3):985–994. doi: 10.1128/iai.32.3.985-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G., Kinns H., Rood J. I. A computer program for determining the size of DNA restriction fragments. Anal Biochem. 1981 Jan 1;110(1):49–55. doi: 10.1016/0003-2697(81)90110-x. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Sansonetti P. J., Schad P. A., Austin S., Formal S. B. Characterization of virulence plasmids and plasmid-associated outer membrane proteins in Shigella flexneri, Shigella sonnei, and Escherichia coli. Infect Immun. 1983 Apr;40(1):340–350. doi: 10.1128/iai.40.1.340-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmuth R., Stephan R., Bulling E., van Leeuwen W. J., van Embden J. D., Guinée P. A., Portnoy D., Falkow S. R-factor cointegrate formation in Salmonella typhimurium bacteriophage type 201 strains. J Bacteriol. 1981 May;146(2):444–452. doi: 10.1128/jb.146.2.444-452.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rabert D. K., Svinarich D. M., Whitfield H. J. Association of adhesive, invasive, and virulent phenotypes of Salmonella typhimurium with autonomous 60-megadalton plasmids. Infect Immun. 1982 Nov;38(2):476–486. doi: 10.1128/iai.38.2.476-486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKEL D. C., LANGLEY L. F., VENICE L. A. The use of the guinea-pig conjunctivae as an experimental model for the study of virulence of Shigella organisms. Am J Hyg. 1961 Mar;73:219–223. doi: 10.1093/oxfordjournals.aje.a120179. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Patton C. M., Gorman G. W. Epidemiologic studies of serotype antigens common to groups B and C Neisseria meningitidis. J Infect Dis. 1975 Mar;131(3):286–290. doi: 10.1093/infdis/131.3.286. [DOI] [PubMed] [Google Scholar]
- Mäki M., Vesikari T., Rantala I., Sundqvist C., Grönroos P. Pathogenicity of 42-44 Mdal plasmid positive and negative Yersinia pseudotuberculosis I and Yersinia enterocolitica 0:8 and 0:9 studied in the guinea pig eye model (Serény test). Acta Pathol Microbiol Immunol Scand B. 1983 Aug;91(4):241–244. doi: 10.1111/j.1699-0463.1983.tb00040.x. [DOI] [PubMed] [Google Scholar]
- O'Brien T. F., Hopkins J. D., Gilleece E. S., Medeiros A. A., Kent R. L., Blackburn B. O., Holmes M. B., Reardon J. P., Vergeront J. M., Schell W. L. Molecular epidemiology of antibiotic resistance in salmonella from animals and human beings in the United States. N Engl J Med. 1982 Jul 1;307(1):1–6. doi: 10.1056/NEJM198207013070101. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Blank H. F., Kingsbury D. T., Falkow S. Genetic analysis of essential plasmid determinants of pathogenicity in Yersinia pestis. J Infect Dis. 1983 Aug;148(2):297–304. doi: 10.1093/infdis/148.2.297. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERENY B. Experimental shigella keratoconjunctivitis; a preliminary report. Acta Microbiol Acad Sci Hung. 1955;2(3):293–296. [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun. 1981 Oct;34(1):75–83. doi: 10.1128/iai.34.1.75-83.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terakado N., Sekizaki T., Hashimoto K., Naitoh S. Correlation between the presence of a fifty-megadalton plasmid in Salmonella dublin and virulence for mice. Infect Immun. 1983 Jul;41(1):443–444. doi: 10.1128/iai.41.1.443-444.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Breeding S. A., Lankford C. E. Enterochelin (enterobactin): virulence factor for Salmonella typhimurium. Infect Immun. 1979 Apr;24(1):174–180. doi: 10.1128/iai.24.1.174-180.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Embden J. D., van Leeuwen W. J., Guinée P. A. Interference with propagation of typing bacteriophages by extrachromosomal elements in Salmonella typhimurium: bacteriophage type 505. J Bacteriol. 1976 Sep;127(3):1414–1426. doi: 10.1128/jb.127.3.1414-1426.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]