Abstract
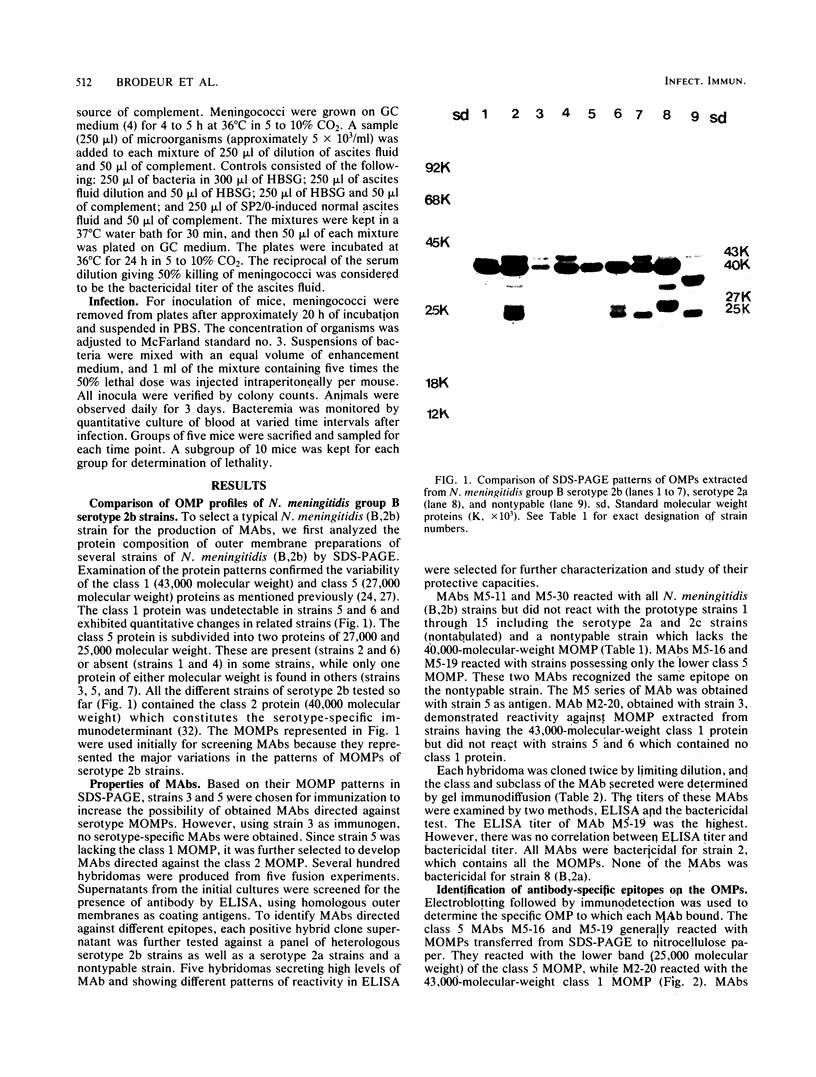
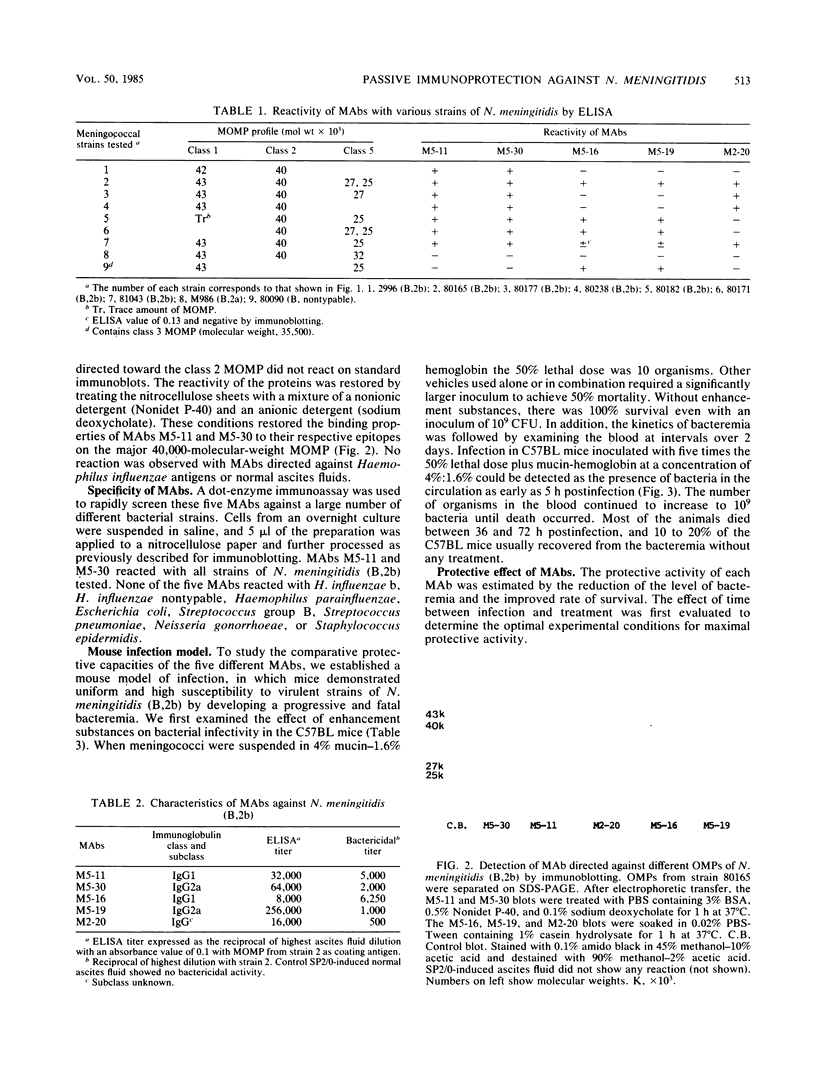
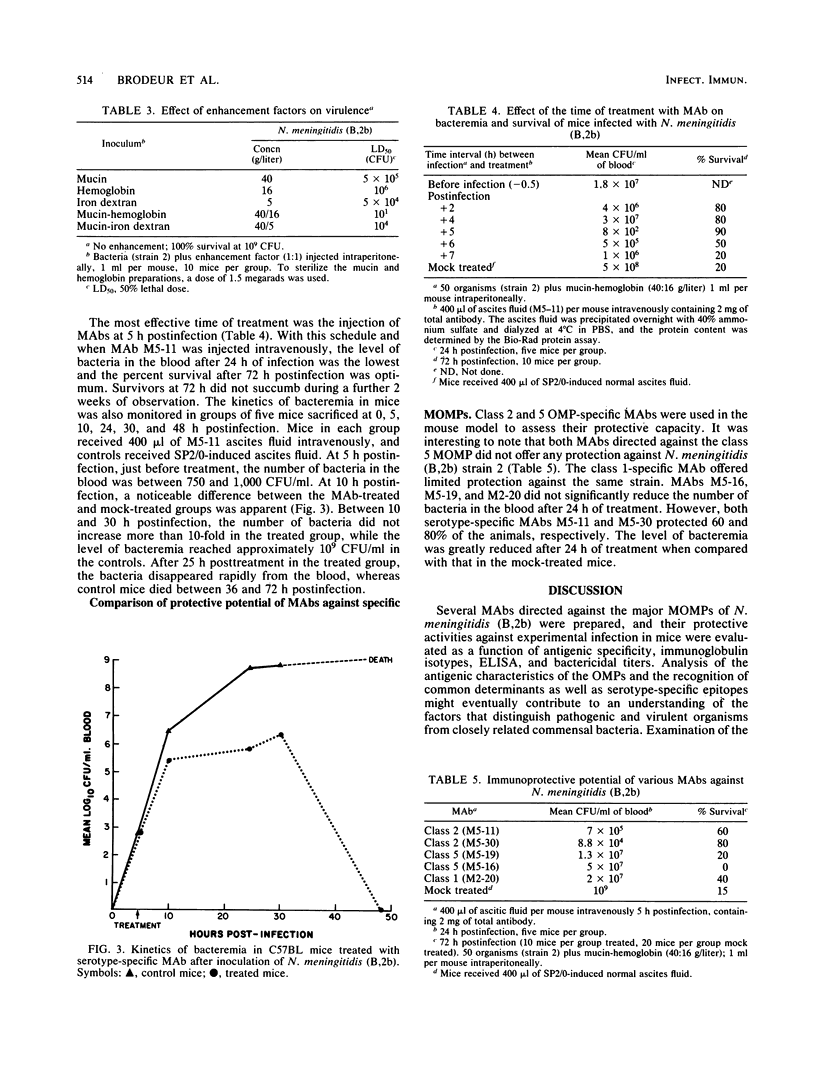
Hybridomas derived from mice immunized with Neisseria meningitidis serogroup B serotype 2b (B,2b) outer membrane preparations produced monoclonal antibodies (MAbs) specific for major outer membrane proteins of classes 1, 2, and 5. The MAbs were examined by enzyme-linked immunosorbent assay against a selected panel of seven strains of N. meningitidis (B,2b) of different sodium dodecyl sulfate-polyacrylamide gel electrophoresis patterns, a serotype 2a, and a nontypable strain. The five MAbs selected were all bactericidal and of different immunoglobulin subclasses. None of the MAbs reacted with other bacterial strains in a dot-enzyme immunoassay. The corresponding antigenic determinant for each MAb was localized on a specific outer membrane protein by immunoblotting of sodium dodecyl sulfate-polyacrylamide gel electrophoresis patterns of major outer membrane proteins. MAbs M5-11 and M5-30 bound to the class 2 protein and were serotype 2b specific. MAb M2-20 bound to the class 1 protein, and MAbs M5-16 and M5-19 bound to the class 5 protein. A mouse model of infection was established whereby a local infection progressed to lethal bacteremia over 3 days, and 50% of the animals were killed with an intraperitoneal injection of 10 meningococci plus 4% mucin and 1.6% hemoglobin. The ability of the MAbs to provide passive protection against experimental infection with N. meningitidis (B,2b) was examined. Both serotype-specific MAbs M5-11 and M5-30 were highly protective even though they were of different immunoglobulin subclasses. The class 5-specific MAb offered no protection, while the class 1-specific MAb gave limited protection. It may therefore be possible to provide protection against serotype 2b infection by using as vaccine the class 2 serotype-specific surface-exposed outer membrane protein epitopes defined by MAb M5-11 or M5-30.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton F. E., Ryan A., Diena B. B. Improved antiserum agar method for the serogroup differentiation of Neisseria meningitidis Y and W135. Can J Microbiol. 1980 May;26(5):630–632. doi: 10.1139/m80-109. [DOI] [PubMed] [Google Scholar]
- Ashton F. E., Ryan A., Diena B. B., MacKenzie A. M., Chan F. Serotypes among Neisseria meningitidis serogroups B and C strains isolated in Canada. Can J Microbiol. 1980 Dec;26(12):1480–1488. doi: 10.1139/m80-245. [DOI] [PubMed] [Google Scholar]
- Ashton F. E., Ryan J. A., Jones C., Brodeur B. R., Diena B. B. Serotypes of Neisseria meningitidis associated with an increased incidence of meningitis cases in the Hamilton area, Ontario, during 1978 and 1979. Can J Microbiol. 1983 Jan;29(1):129–136. doi: 10.1139/m83-020. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Ashton F. E., Diena B. B. Enzyme-linked immunosorbent assay with polyvalent gonococcal antigen. J Med Microbiol. 1982 Feb;15(1):1–9. doi: 10.1099/00222615-15-1-1. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Ashton F. E., Diena B. B. Enzyme-linked immunosorbent assays for the detection of Neisseria gonorrhoeae specific antibodies. Can J Microbiol. 1978 Nov;24(11):1300–1305. doi: 10.1139/m78-211. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R. Characterization of the mitogenic activity elicited by Neisseria gonorrhoeae ribosomal fractions. Can J Microbiol. 1978 May;24(5):579–585. doi: 10.1139/m78-094. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Tsang P., Larose Y. Parameters affecting ascites tumour formation in mice and monoclonal antibody production. J Immunol Methods. 1984 Jul 6;71(2):265–272. doi: 10.1016/0022-1759(84)90073-5. [DOI] [PubMed] [Google Scholar]
- Calver G. A., Kenny C. P., Lavergne G. Iron as a replacement for mucin in the establishment of meningococcal infection in mice. Can J Microbiol. 1976 Jun;22(6):832–838. doi: 10.1139/m76-120. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Frasch C. E. Protection against group B meningococcal disease: evaluation of serotype 2 protein vaccines in a mouse bacteremia model. Infect Immun. 1979 Oct;26(1):110–117. doi: 10.1128/iai.26.1.110-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein B. E., Jericho K. W., Likes G. C. Neisseria meningitidis infection in mice: influence of iron, variations in virulence among strains, and pathology. Infect Immun. 1979 May;24(2):545–551. doi: 10.1128/iai.24.2.545-551.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Kinoshita T., Kitajima H., Inoue K. Inhibitory effect of K-76 monocarboxylic acid, an anticomplementary agent, on the C3b inactivator system. J Immunol. 1981 Jul;127(1):104–108. [PubMed] [Google Scholar]
- Huet M., Suire A. Mise en évidence de la valeur protectrice des vaccins poly-saccharidiques méningococciques par la réduction de la bactéríemie chez la Souris. C R Acad Sci Hebd Seances Acad Sci D. 1976 Sep 13;283(4):421–422. [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Use of a zwitterionic detergent for the restoration of the antibody-binding capacity of electroblotted meningococcal outer membrane proteins. J Immunol Methods. 1984 Feb 24;67(1):1–11. doi: 10.1016/0022-1759(84)90080-2. [DOI] [PubMed] [Google Scholar]
- Miller C. P. EXPERIMENTAL MENINGOCOCCAL INFECTION IN MICE. Science. 1933 Oct 13;78(2024):340–341. doi: 10.1126/science.78.2024.340. [DOI] [PubMed] [Google Scholar]
- Moreno C., Lifely M. R., Esdaile J. Immunity and protection of mice against Neisseria meningitidis group B by vaccination, using polysaccharide complexed with outer membrane proteins: a comparison with purified B polysaccharide. Infect Immun. 1985 Feb;47(2):527–533. doi: 10.1128/iai.47.2.527-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P. J., Knobler R. L., Buchmeier M. J. Western and dot immunoblotting analysis of viral antigens and antibodies: application to murine hepatitis virus. J Immunol Methods. 1984 Oct 12;73(1):177–188. doi: 10.1016/0022-1759(84)90043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. Chemical analysis of major outer membrane proteins of Neisseria meningitidis: comparison of serotypes 2 and 11. J Bacteriol. 1980 Jan;141(1):169–176. doi: 10.1128/jb.141.1.169-176.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Y., Frasch C. E. Development of a Neisseria meningitidis group B serotype 2b protein vaccine and evaluation in a mouse model. Infect Immun. 1984 Nov;46(2):408–414. doi: 10.1128/iai.46.2.408-414.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wyle F. A., Artenstein M. S., Brandt B. L., Tramont E. C., Kasper D. L., Altieri P. L., Berman S. L., Lowenthal J. P. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972 Nov;126(5):514–521. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Altieri P., Berman S., Lowenthal J., Artenstein M. S. Safety and immunogenicity of a Neisseria meningitidis type 2 protein vaccine in animals and humans. J Infect Dis. 1978 Jun;137(6):728–739. doi: 10.1093/infdis/137.6.728. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Moran E. E., Connelly H., Mandrell R. E., Brandt B. Monoclonal antibodies to serotype 2 and serotype 15 outer membrane proteins of Neisseria meningitidis and their use in serotyping. Infect Immun. 1984 Oct;46(1):260–266. doi: 10.1128/iai.46.1.260-266.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]




