Abstract
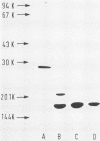
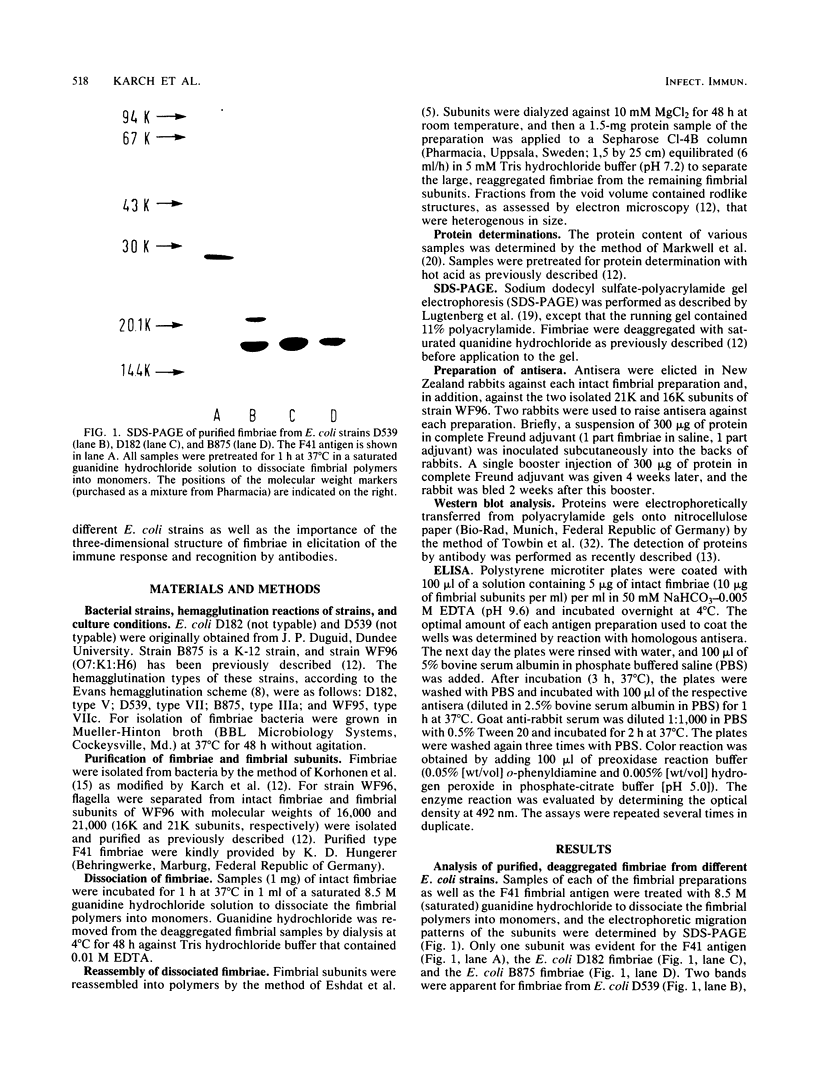
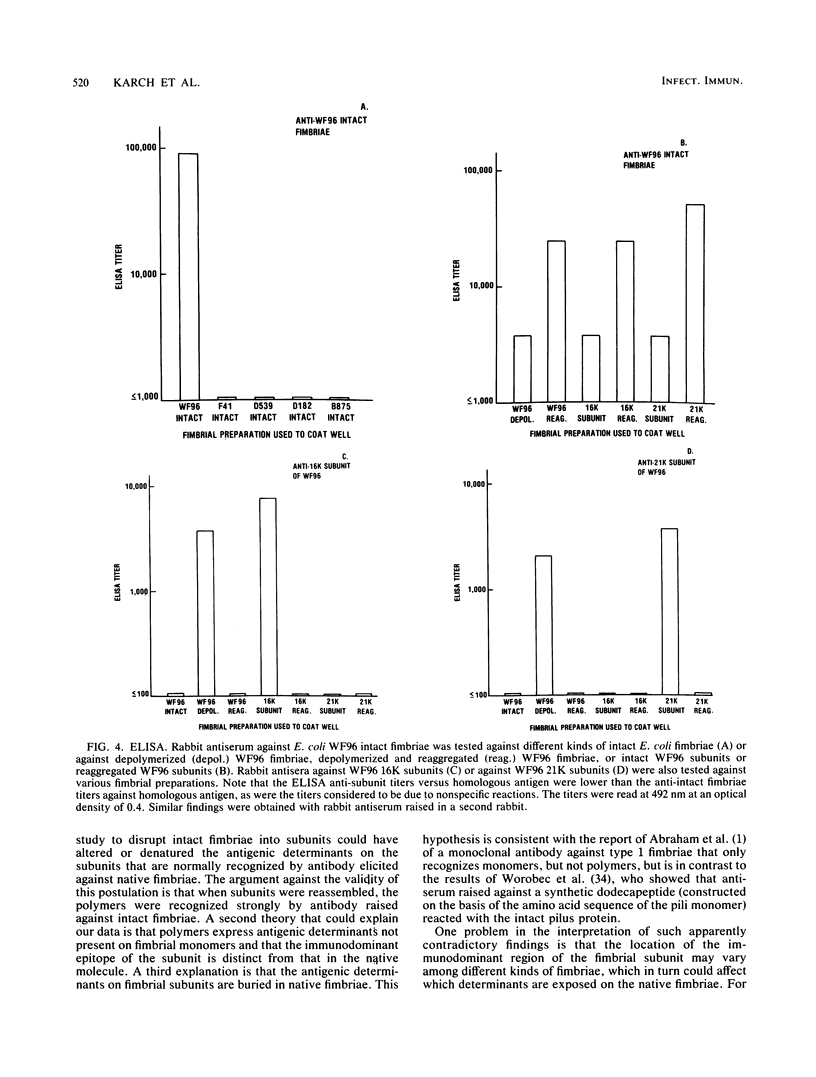
We recently described how a fraction of isolated fimbriae from a multifimbriated strain of Escherichia coli O7:K1:H6 (WF96) could be subdivided by sequential disaggregation in disrupting agents into individual subunits with different molecular weights. In this study, antibodies were raised in rabbits against these isolated fimbrial subunits and against purified intact WF96 fimbriae. These sera were tested by Western blot analysis or by enzyme-linked immunosorbent assays for reactivity against the following antigens: intact WF96 fimbriae, dissociated WF96 fimbriae, dissociated and reaggregated WF96 fimbriae, the WF96 21K fimbrial subunit, reaggregated WF96 21K subunits, the WF96 16K subunits, reaggregated WF96 16K subunits, intact fimbriae from four other E. coli strains, and deaggregated fimbriae from these strains. We found that antibody against intact WF96 fimbriae only reacted strongly with intact WF96 fimbriae, depolymerized and reaggregated WF96 fimbriae, or reaggregated fimbrial subunits; no reactions were evident with intact fimbriae from four other E. coli strains. Conversely, antisera prepared against the WF96 16K subunit and against the WF96 21K subunit did not react with intact WF96 fimbriae or with depolymerized and reaggregated WF96 fimbriae, but did react with homologous isolated subunits. One cross-reaction between fimbrial subunits was apparent: anti-WF96 16K subunit bound to a 21K subunit of deaggregated fimbriae, from another E. coli strain. Taken together, the findings indicate that the three-dimensional structure of the fimbrial preparation used to immunize animals determines the specificity of the immune response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Hasty D. L., Simpson W. A., Beachey E. H. Antiadhesive properties of a quaternary structure-specific hybridoma antibody against type 1 fimbriae of Escherichia coli. J Exp Med. 1983 Oct 1;158(4):1114–1128. doi: 10.1084/jem.158.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Cravioto A., Scotland S. M., Rowe B. Hemagglutination activity and colonization factor antigens I and II in enterotoxigenic and non-enterotoxigenic strains of Escherichia coli isolated from humans. Infect Immun. 1982 Apr;36(1):189–197. doi: 10.1128/iai.36.1.189-197.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr New surface-associated heat-labile colonization factor antigen (CFA/II) produced by enterotoxigenic Escherichia coli of serogroups O6 and O8. Infect Immun. 1978 Aug;21(2):638–647. doi: 10.1128/iai.21.2.638-647.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Young L. S., Pitt J. Hemagglutination typing of Escherichia coli: definition of seven hemagglutination types. J Clin Microbiol. 1980 Aug;12(2):235–242. doi: 10.1128/jcm.12.2.235-242.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Nagy B., Moon H. W. Colonization of porcine small intestine by Escherichia coli: colonization and adhesion factors of pig enteropathogens that lack K88. J Infect Dis. 1977 Apr;135(4):531–539. doi: 10.1093/infdis/135.4.531. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Leying H., Büscher K. H., Kroll H. P., Opferkuch W. Isolation and separation of physicochemically distinct fimbrial types expressed on a single culture of Escherichia coli O7:K1:H6. Infect Immun. 1985 Feb;47(2):549–554. doi: 10.1128/iai.47.2.549-554.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. The complete amino-acid sequence of the K88 antigen, a fimbrial protein from Escherichia coli. Eur J Biochem. 1981 Jul;117(3):617–627. doi: 10.1111/j.1432-1033.1981.tb06382.x. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. New Method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980 Feb;27(2):569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen V., Saxén H., Hultberg H., Svenson S. B. P-antigen-recognizing fimbriae from human uropathogenic Escherichia coli strains. Infect Immun. 1982 Jul;37(1):286–291. doi: 10.1128/iai.37.1.286-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Ristaino P., Marley G., Smyth C., Knutton S., Boedeker E., Black R., Young C., Clements M. L., Cheney C. Coli surface antigens 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: morphology, purification, and immune responses in humans. Infect Immun. 1984 May;44(2):409–420. doi: 10.1128/iai.44.2.409-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Nagy B., Isaacson R. E., Orskov I. Occurrence of K99 antigen on Escherichia coli isolated from pigs and colonization of pig ileum by K99+ enterotoxigenic E. coli from calves and pigs. Infect Immun. 1977 Feb;15(2):614–620. doi: 10.1128/iai.15.2.614-620.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Birch-Andersen A. Comparison of Escherichia coli fimbrial antigen F7 with type 1 fimbriae. Infect Immun. 1980 Feb;27(2):657–666. doi: 10.1128/iai.27.2.657-666.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Serology of Escherichia coli fimbriae. Prog Allergy. 1983;33:80–105. [PubMed] [Google Scholar]
- Orskov I., Orskov F., Smith H. W., Sojka W. J. The establishment of K99, a thermolabile, transmissible escherichia coli K antigen, previously called "Kco", possessed by calf and lamb enteropathogenic strains. Acta Pathol Microbiol Scand B. 1975 Feb;83(1):31–36. doi: 10.1111/j.1699-0463.1975.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Further observations on Escherichia coli enterotoxins with particular regard to those produced by atypical piglet strains and by calf and lamb strains: the transmissible nature of these enterotoxins and of a K antigen possessed by calf and lamb strains. J Med Microbiol. 1972 May;5(2):243–250. doi: 10.1099/00222615-5-2-243. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Observations on the pathogenic properties of the K88, Hly and Ent plasmids of Escherichia coli with particular reference to porcine diarrhoea. J Med Microbiol. 1971 Nov;4(4):467–485. doi: 10.1099/00222615-4-4-467. [DOI] [PubMed] [Google Scholar]
- Smyth C. J. Two mannose-resistant haemagglutinins on enterotoxigenic Escherichia coli of serotype O6:K15:H16 or H-isolated from travellers' and infantile diarrhoea. J Gen Microbiol. 1982 Sep;128(9):2081–2096. doi: 10.1099/00221287-128-9-2081. [DOI] [PubMed] [Google Scholar]
- Stirm S., Orskov F., Orskov I., Birch-Andersen A. Episome-carried surface antigen K88 of Escherichia coli. 3. Morphology. J Bacteriol. 1967 Feb;93(2):740–748. doi: 10.1128/jb.93.2.740-748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen-Rhen V., Elo J., Väisänen E., Siitonen A., Orskov I., Orskov F., Svenson S. B., Mäkelä P. H., Korhonen T. K. P-fimbriated clones among uropathogenic Escherichia coli strains. Infect Immun. 1984 Jan;43(1):149–155. doi: 10.1128/iai.43.1.149-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worobec E. A., Taneja A. K., Hodges R. S., Paranchych W. Localization of the major antigenic determinant of EDP208 pili at the N-terminus of the pilus protein. J Bacteriol. 1983 Feb;153(2):955–961. doi: 10.1128/jb.153.2.955-961.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]