Abstract
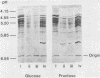
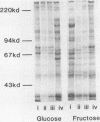
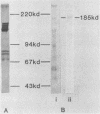
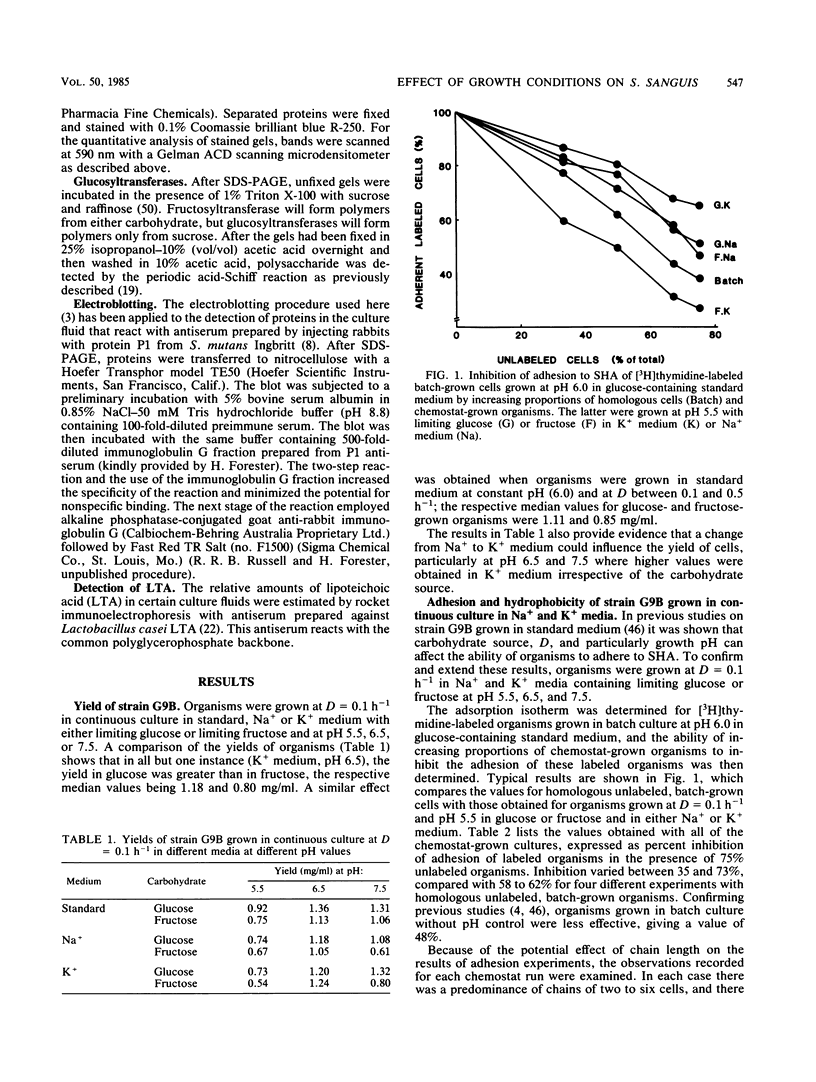
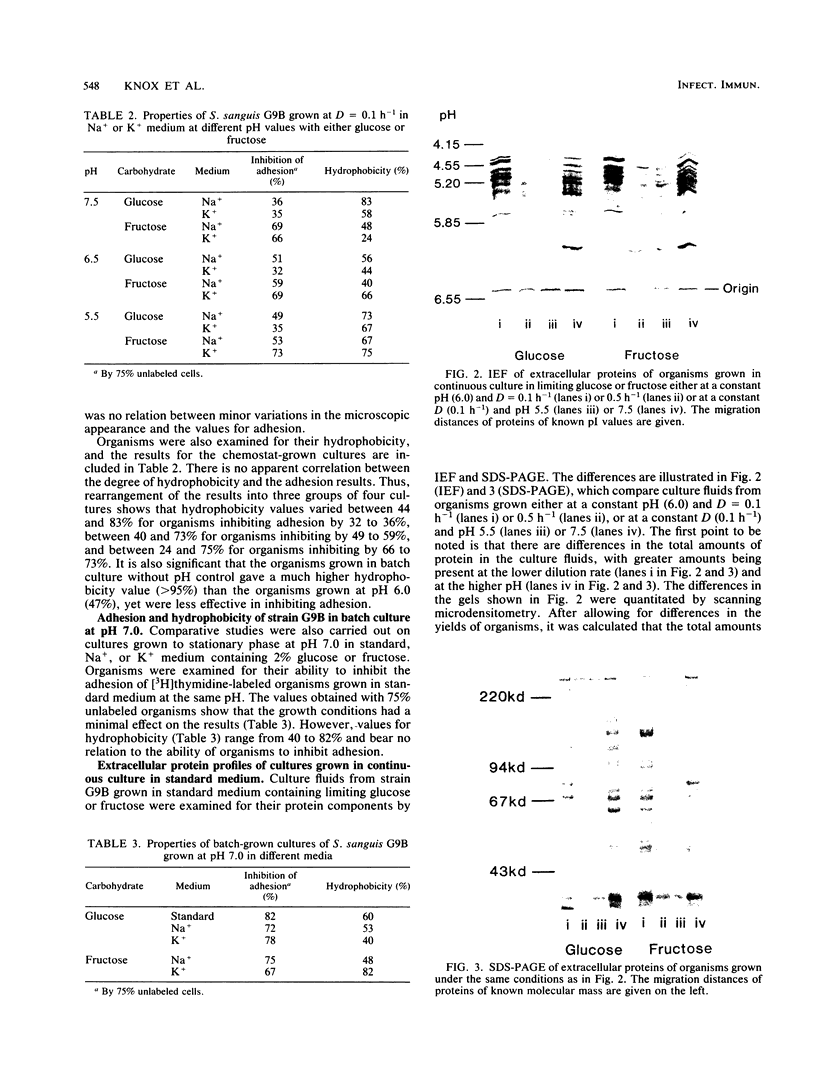
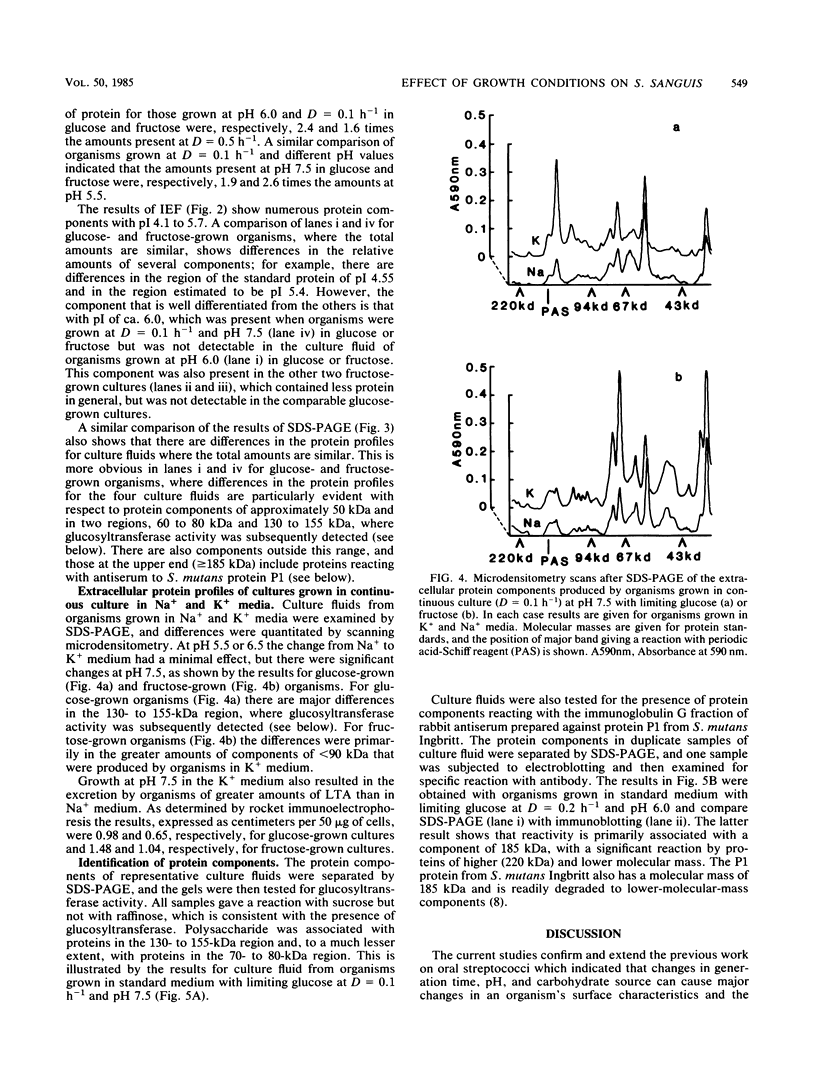
Streptococcus sanguis G9B was grown in continuous culture at different generation times and pH values in media containing either glucose or fructose and differing in the concentrations of Na+ and K+. The growth pH, carbohydrate, and cation concentration each affected the yield of organisms, their ability to adhere to saliva-coated hydroxyapatite beads, and their hydrophobicity, as measured by adhesion to hexadecane. There was no correlation between adhesion to saliva-coated hydroxyapatite beads and hydrophobicity, the values for hydrophobicity varying between 44 and 83% for organisms that adhered poorly and between 24 and 75% for those that adhered effectively. For organisms grown in batch culture at pH 6.0 or 7.0 there was similarly no correlation between adhesion and hydrophobicity. The growth conditions also had a considerable influence on the production of extracellular protein. The total amount was greater at pH 7.5 than at other pH values, and there were also differences in the individual components in response to changes in generation time, pH, carbohydrate source, and cation concentration. Two protein bands were identified, namely, glucosyltransferase and protein P1 (also called antigen B or I/II). However, there was no correlation between a particular protein component and adhesion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum B., Rosan B. Cell surface proteins of oral streptococci. Infect Immun. 1984 Oct;46(1):245–250. doi: 10.1128/iai.46.1.245-250.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. K., Knox K. W., Wicken A. J. Influence of growth conditions on adherence of Streptococcus mutans ingbritt to saliva-coated hydroxyapatite. Infect Immun. 1983 Jan;39(1):445–448. doi: 10.1128/iai.39.1.445-448.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Keevil C. W., Marsh P. D., Brown C. M., Wardell J. N. Surface-associated growth. Philos Trans R Soc Lond B Biol Sci. 1982 Jun 11;297(1088):517–532. doi: 10.1098/rstb.1982.0058. [DOI] [PubMed] [Google Scholar]
- Forester H., Hunter N., Knox K. W. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J Gen Microbiol. 1983 Sep;129(9):2779–2788. doi: 10.1099/00221287-129-9-2779. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Comparative hydrophobicities of oral bacteria and their adherence to salivary pellicles. Infect Immun. 1983 Sep;41(3):1190–1196. doi: 10.1128/iai.41.3.1190-1196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I., Moreno E. C. Association of neuraminidase-sensitive receptors and putative hydrophobic interactions with high-affinity binding sites for Streptococcus sanguis C5 in salivary pellicles. Infect Immun. 1983 Dec;42(3):1006–1012. doi: 10.1128/iai.42.3.1006-1012.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I., Moreno E. C. Contribution of stereochemical interactions in the adhesion of Streptococcus sanguis C5 to experimental pellicles. J Dent Res. 1985 Feb;64(2):96–101. doi: 10.1177/00220345850640021801. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I., Skobe Z. Association of fimbriae with the hydrophobicity of Streptococcus sanguis FC-1 and adherence to salivary pellicles. Infect Immun. 1983 Jul;41(1):414–417. doi: 10.1128/iai.41.1.414-417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Etherden I. Concentration-dependent multiple binding sites on saliva-treated hydroxyapatite for Streptococcus sanguis. Infect Immun. 1983 Jan;39(1):280–289. doi: 10.1128/iai.39.1.280-289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame D. A., Mayer R. M. The origin and composition of multiple forms of dextransucrase from Streptococcus sanguis. Biochim Biophys Acta. 1984 Apr 27;786(1-2):42–48. doi: 10.1016/0167-4838(84)90151-1. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Phipps P. J., Ellwood D. C. Effect of growth rate and glucose concentration on the biochemical properties of Streptococcus mutans Ingbritt in continuous culture. Infect Immun. 1979 Dec;26(3):861–869. doi: 10.1128/iai.26.3.861-869.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- Hardy L., Jacques N. A., Forester H., Campbell L. K., Knox K. W., Wicken A. J. Effect of fructose and other carbohydrates on the surface properties, lipoteichoic acid production, and extracellular proteins of Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1981 Jan;31(1):78–87. doi: 10.1128/iai.31.1.78-87.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg S. D., Embery G. Blood-group-reactive glycoprotein from human saliva interacts with lipoteichoic acid on the surface of Streptococcus sanguis cells. Arch Oral Biol. 1982;27(3):261–268. doi: 10.1016/0003-9969(82)90060-7. [DOI] [PubMed] [Google Scholar]
- Jacques N. A., Hardy L., Campbell L. K., Knox K. W., Evans J. D., Wicken A. J. Effect of carbohydrate source and growth conditions on the production of lipoteichoic acid by Streptococcus mutans Ingbritt. Infect Immun. 1979 Dec;26(3):1079–1087. doi: 10.1128/iai.26.3.1079-1087.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques N. A., Hardy L., Knox K. W., Wicken A. J. Effect of growth conditions on the formation of extracellular lipoteichoic acid by Streptococcus mutans BHT. Infect Immun. 1979 Jul;25(1):75–84. doi: 10.1128/iai.25.1.75-84.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keevil C. W., West A. A., Bourne N., Marsh P. D. Inhibition of the synthesis and secretion of extracellular glucosyl- and fructosyltransferase in Streptococcus sanguis by sodium ions. J Gen Microbiol. 1984 Jan;130(1):77–82. doi: 10.1099/00221287-130-1-77. [DOI] [PubMed] [Google Scholar]
- Keevil C. W., Williamson M. I., Marsh P. D., Ellwood D. C. Evidence that glucose and sucrose uptake in oral streptococcal bacteria involves independent phosphotransferase and proton-motive force-mediated mechanisms. Arch Oral Biol. 1984;29(11):871–878. doi: 10.1016/0003-9969(84)90085-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G. Isolation of a protein-containing cell surface component from Streptococcus sanguis which affects its adherence to saliva-coated hydroxyapatite. Infect Immun. 1981 Nov;34(2):428–434. doi: 10.1128/iai.34.2.428-434.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markevics L. J., Jacques N. A. Enhanced secretion of glucosyltransferase by changes in potassium ion concentrations is accompanied by an altered pattern of membrane fatty acids in Streptococcus salivarius. J Bacteriol. 1985 Mar;161(3):989–994. doi: 10.1128/jb.161.3.989-994.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh P. D., Keevil C. W., Ellwood D. C. Relationship of bioenergetic processes to the pathogenic properties of oral bacteria. J Dent Res. 1984 Mar;63(3):401–406. doi: 10.1177/00220345840630030901. [DOI] [PubMed] [Google Scholar]
- Marsh P. D., Williamson M. I., Keevil C. W., McDermid A. S., Ellwood D. C. Influence of sodium and potassium ions on acid production by washed cells of Streptococcus mutans ingbritt and Streptococcus sanguis NCTC 7865 grown in a chemostat. Infect Immun. 1982 May;36(2):476–483. doi: 10.1128/iai.36.2.476-483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Song M., Krasse B., Olsson J. Biochemical and immunological differences between hydrophobic and hydrophilic strains of Streptococcus mutans. Infect Immun. 1984 Apr;44(1):68–75. doi: 10.1128/iai.44.1.68-75.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miörner H., Johansson G., Kronvall G. Lipoteichoic acid is the major cell wall component responsible for surface hydrophobicity of group A streptococci. Infect Immun. 1983 Jan;39(1):336–343. doi: 10.1128/iai.39.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris E. J., McBride B. C. Adherence of Streptococcus sanguis to saliva-coated hydroxyapatite: evidence for two binding sites. Infect Immun. 1984 Feb;43(2):656–663. doi: 10.1128/iai.43.2.656-663.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G. Hydrophobic interactions and the adherence of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Nov;38(2):637–644. doi: 10.1128/iai.38.2.637-644.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G., Staat R. H., Arnold R. R. Positive coooperativity in the binding of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Jan;35(1):157–165. doi: 10.1128/iai.35.1.157-165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A. H., Pilowsky K., Zilm P. S. The effect of growth rate on the adhesion of the oral bacteria Streptococcus mutans and Streptococcus milleri. Arch Oral Biol. 1984;29(2):147–150. doi: 10.1016/0003-9969(84)90119-5. [DOI] [PubMed] [Google Scholar]
- Rosan B., Appelbaum B., Campbell L. K., Knox K. W., Wicken A. J. Chemostat studies of the effect of environmental control on Streptococcus sanguis adherence to hydroxyapatite. Infect Immun. 1982 Jan;35(1):64–70. doi: 10.1128/iai.35.1.64-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R. Distribution of cross-reactive antigens A and B in Streptococcus mutans and other oral streptococci. J Gen Microbiol. 1980 Jun;118(2):383–388. doi: 10.1099/00221287-118-2-383. [DOI] [PubMed] [Google Scholar]
- Schöller M., Klein J. P., Sommer P., Frank R. Common antigens of streptococcal and nonstreptococcal oral bacteria: characterization of wall-associated protein and comparison with extracellular protein antigen. Infect Immun. 1983 Jun;40(3):1186–1191. doi: 10.1128/iai.40.3.1186-1191.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatevossian A., Gould C. T. The composition of the aqueous phase in human dental plaque. Arch Oral Biol. 1976;21(5):319–323. doi: 10.1016/0003-9969(76)90055-8. [DOI] [PubMed] [Google Scholar]
- Wadström T., Schmidt K. H., Kühnemund O., Havlícek J., Köhler W. Comparative studies on surface hydrophobicity of streptococcal strains of groups A, B, C, D and G. J Gen Microbiol. 1984 Mar;130(3):657–664. doi: 10.1099/00221287-130-3-657. [DOI] [PubMed] [Google Scholar]
- Westergren G., Olsson J. Hydrophobicity and adherence of oral streptococci after repeated subculture in vitro. Infect Immun. 1983 Apr;40(1):432–435. doi: 10.1128/iai.40.1.432-435.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Broady K. W., Ayres A., Knox K. W. Production of lipoteichoic acid by lactobacilli and streptococci grown in different environments. Infect Immun. 1982 Jun;36(3):864–869. doi: 10.1128/iai.36.3.864-869.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven J. S., Franken H. C. Production of acids in rat dental plaque with or without Streptococcus mutans. Caries Res. 1982;16(5):375–383. doi: 10.1159/000260623. [DOI] [PubMed] [Google Scholar]