Abstract
The guanine nucleotide exchange factor (GEF) Vav1 plays an important role in T-cell activation and tumorigenesis. In the GEF superfamily, Vav1 has the ability to interact with multiple families of RhoGTPases. The structure of the Vav1-DH-PH-CRD/Rac1 complex to 2.6 Å resolution reveals a unique intramolecular network of contacts between the Vav-1 cysteine rich domain (CRD) and the C-terminal helix of the Vav1 Dbl homology (DH) domain. These unique interactions stabilize the Vav-1 DH domain for its intimate association with the Switch-II region of Rac1 that is critical for the displacement of the guanine nucleotide. Further, a mutational analysis confirms that the atypical CRD is critical for maintaining both optimal guanine nucleotide exchange activity and broader specificity of Vav family GEFs. Taken together, the data outline the detailed nature of Vav1’s ability to contact a range of RhoGTPases using a novel protein-protein interaction network.
Introduction
RhoGTPases function as bi-molecular switches that transition between the inactive GDP-bound state and the active GTP-bound state1. The Dbl family of guanine nucleotide exchange factors (GEFs) activates RhoGTPases by facilitating the exchange of GDP for GTP, mediating vital and diverse cellular processes such as cytoskeletal rearrangements, mitogenesis, transcriptional changes, lymphocyte activation, and malignant transformation1. This cycle is also tightly regulated by GAP’s (RhoGTPase activating proteins), which enhance the intrinsic catalytic activity of the RhoGTPase, and GDI’s (GDP dissociation inhibitors), which maintain the RhoGTPase in the GDP-bound form in the cytosol. Due to the critical role of RhoGTPases in numerous cellular processes, it is not surprising that this family of proteins is overexpressed or hyperactivated in human cancers, a direct result of dysregulation of the GDP-GTP cycle2; 3.
The Vav subgroup of Dbl GEFs consists of three family members (Vav1, Vav2, and Vav3) in mammals with distinctive patterns of cellular expression; Vav1 is preferentially expressed in the hematopoietic system, while Vav2 and Vav3 are described by broader expression patterns4; 5. Similar to other members of the Dbl family, Vav proteins contain a ~300 residue region of sequence homology with the Dbl oncoprotein composed of the Dbl homology domain (DH domain) and the pleckstrin homology domain (PH domain; Figure 1A). The DH domain, comprising ~200 amino acids, contains the structural information required for RhoGTPase recognition and selectivity, and stimulates the reorganization of the switch regions for GDP/GTP exchange. Interaction of the DH domain of the GEF with the β2/β3 region of the RhoGTPase has been shown to control the specificity of GEF/RhoGTPase recognition6. The PH domain, which consists of ~100 amino acids and resides C-terminal to the DH domain, has been implicated in directing membrane localization, allosteric regulation of guanine nucleotide exchange activity (GNE), and as a phospholipid- dependent regulator of GEF activity7; 8; 9; 10. The invariable association of the DH and PH domains is suggestive of a conserved functional interdependence that remains poorly defined. Crystal structures of numerous DH-PH cassettes in complex with RhoGTPases have been reported, and describe the PH domain situated against the C-terminal helix of the DH domain, although the position of the PH domain is inevitably translated differentially with respect to the DH domain11; 12; 13. This rotational discrepancy results in variable interactions of the PH domain with respect to the DH domain and the RhoGTPase that are critical for guanine nucleotide exchange. Unlike other GEF family members, however, the Vav family conserves a cysteine rich domain (CRD) C-terminal to the PH domain, which has been described to control the on-rate of the nucleotide exchange reaction14. Interestingly, Vav family members belong to a small subset of GEFs that are promiscuous towards several RhoGTPases including Rac1, RhoA, RhoG, and Cdc42, while other members of the GEF family are specific for a single RhoGTPase (eg Tiam1/Rac1), suggestive that the CRD may play a role in promoting the promiscuity inherent of the Vav family15; 16; 17.
Figure 1.


Figure 1A. Domain architecture of the Vav family of guanine nucleotide exchange factors. Vav1 consists of a calponin homology domain (CH, 1–116), an acidic region (Ac, 132–176), a Dbl homology domain (DH, 185–375), a pleckstrin homology domain (PH, 398–508), a cysteine rich domain (CRD, 516–565), a proline rich region (607–610), and an SH3-SH2-SH3 domain (612–844).
Figure 1B. Overall structure of the Vav-1 DH-PH-CRD in complex with the Rac1 RhoGTPase solved to a resolution of 2.6 Å and a free R factor of 29%. The Vav1 DH domain is depicted in blue, the PH domain is depicted in cyan, and the CRD is shown in magenta, with grey spheres representing coordinating zinc ions. Rac1 is shown in green, with the Switch I and Switch II regions highlighted in red.
Herein, we present the crystal structure of the Vav1 (DH-PH-CRD) GEF bound to the Rac1 RhoGTPase. Vav1 interacts with Rac1 through the two switch regions of the RhoGTPase, a feature which is highly conserved amongst GEF/RhoGTPase interacting partners. However, this interaction lacks several conserved structural elements observed in previously described crystal structures, most notably at the interface of the DH domain and the Rac1 β2/β3 region, which have been predicted to contain the molecular determinants for specificity between the GEF/RhoGTPase interaction6. Further, the specificity factor, Trp-56, plays a mitigated role in the context of the Vav1/Rac1 interaction, as compared to previously studied GEF/RhoGTPase interactions. We describe for the first time the structure and positioning of the atypical CRD in the context of the multidomain protein-protein interaction. The CRD resides adjacent to the α11 helix of the DH domain and stabilizes Rac1 binding through an additional interaction network at the interface of the Vav1-CRD-DH(α11)/Rac1-Switch II.
Results
Overall Structure
Crystals of the human Vav1/Rac1 complex (relative molecular mass (Mr) of ~73,000) were obtained from a Vav1-DH-PH-CRD construct (residues 176–575) in complex with Rac1 (residues 1–177)18. The crystals diffracted to 2.6 Å resolution in the P21 spacegroup, with two molecules each of Vav1 and Rac1 in the asymmetric unit (Figure 1B). Both molecules in the asymmetric unit share similar overall structural features and conserve similar interactions between Vav1 and Rac1 proteins. The majority of the Vav1/Rac1 interface is mediated through the DH domain of Vav1 and the two switch regions of Rac1 (Switch I and Switch II). The PH domain stably positions the cysteine rich domain adjacent to the long C-terminal helix of the DH domain, providing the functional interaction network between the DH domain and Rac1. The Switch I (residues 25–39) region of Rac1 interacts with both Conserved Region 1 (CR1) and Conserved Region 3 (CR3) of the Vav1 DH domain, while the Switch II (residues 60–75) region interacts with CR3 and with the α11 helix of the DH domain. These interface elements are strictly conserved amongst the GEF/RhoGTPase interacting partners, and have been widely reported to mediate the release of GDP in the binding cavity in order to allow exchange with GTP13; 19.
The DH domain (residues 189–375) of Vav1 consists of an oblong and elongated helical bundle comprised of 11 α-helices. The architecture of this domain is similar to previously described crystal structures of Dbl-family GEF’s, with root mean square deviations (r.m.s.d.) of 5.1 Å over equivalent Cα atoms with the DH domain of Tiam113, and 1.8 Å with the previously reported NMR structure of the isolated Vav1 DH domain19. The higher r.m.s. deviation between the Tiam and Vav1 DH domains is a direct result of several highly flexible loop regions, while the overall core of the proteins conserve similar structural features. The C-terminal end helix, α11, of the DH domain extends into the PH domain, providing a large complementary surface at the interface with the DH domain. The flexibility of the C-terminal helix of the DH domain allows the PH domain to reside in variable positions with respect to the DH domain, which has been described in previous crystal structures11; 13; 20.
The PH domain (residues 402–505) is composed of a 7-stranded anti-parallel β-sandwich consisting of two sheets comprising strands β1–β4 and β5–β7, and is capped by a C-terminal α-helix. Although this domain generally displays high degrees of conformational flexibility11, the extensive interaction network between the DH-PH domains and the PH-CRD, allowed us to model the entire domain, with the sole exception of residues 458–461, which are not depicted in the final model due to a lack of electron density. Noticeably different for the Vav family of GEF’s is a 19 amino acid truncation of the β3/β4 loop. This flexible loop has been previously described to increase the nucleotide exchange potential in other GEF/RhoGTPase pairs, including the Trio/Rac and Dbs/Cdc42 complexes, wherein the β3/β4 loop forms interactions with residues from the respective RhoGTPases11; 21. Unlike the Tiam1/Rac structure, where the β3/β4 loop is disordered, this short loop is ordered in the Vav1/Rac structure, and positioned towards the C-terminal end of the α11 helix of the DH domain, forming hydrogen bonds with main-chain atoms of residues lining this helix, and stabilizing the C-terminal end of the DH domain. The C-terminal α-helix of the PH domain extends into the cysteine rich domain (CRD).
The CRD (residues 506–566) resides adjacent to the α11 helix of the DH domain. The positioning of the PH domain appears to stabilize a novel conformation of the CRD adjacent to the α11 helix of the Vav1 DH domain. The interaction between the DH domain and the CRD is maintained by numerous polar and hydrophobic interactions. This interaction network is further stabilized through interactions with the Switch II region of Rac1 through the DH domain (Figure 2A). For instance, Arg-537 (CRD) interacts with Gln-368 (DH), and Gln-368 (DH) also forms a hydrogen bond with Tyr-64 of the Rac1 switch II region (Figure 6A). The CRD is comprised of five β-strands which together form 2 β-sheets, and a short α-helical region at the C-terminus. Three cysteine residues and one histidine residue are involved in coordinating Zn2+ ions, and form two zinc finger motifs that are characteristic for this atypical C1 domain. The overall structure is similar to the Raf1 CRD previously described by NMR, with an overall r.m.s. deviation of 5.2 Å (Cα atoms), with the major structural deviations occurring around the N and C termini22 (Figure 2B).
Figure 2.

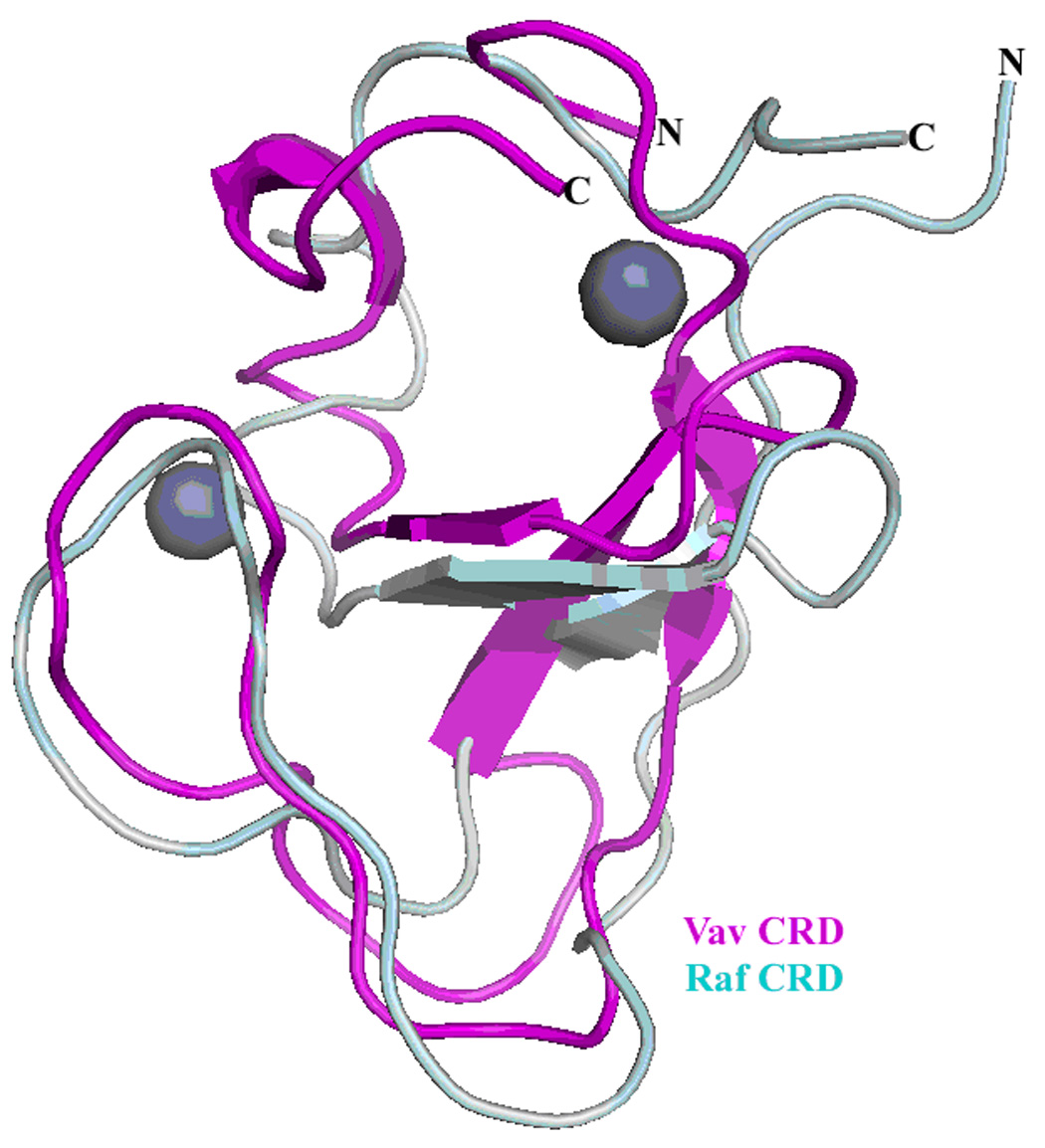
Figure 2A. Complete interlock network between the Vav1 DH domain, PH domain, and CRD. The DH domain is depicted in blue, the PH domain in cyan, and the CRD in magenta. Critical interactions between the domains are shown with dotted black lines.
Figure 2B. Superposition of the Vav1 cysteine rich domain; magenta) on the Raf1 cysteine rich domain (grey) previously described22. The overall structures are similar, with both similarly coordinating two zinc ions, depicted as spheres.
Figure 6.

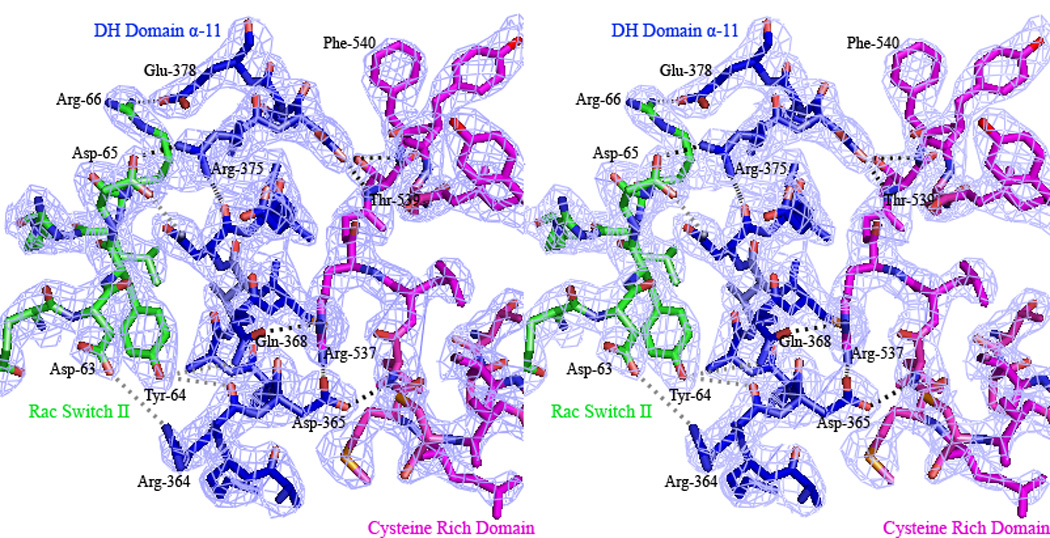
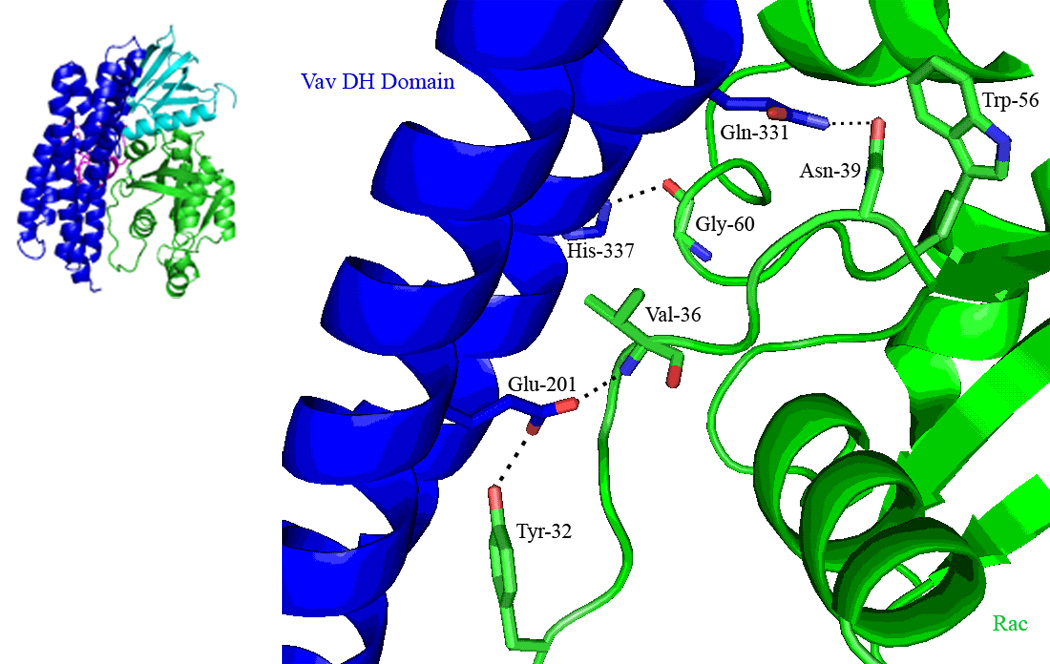
Figure 6A. Interlock system between the Vav1 CRD and Rac1 Switch II region with the α11 C-terminal helix of the Vav1 DH domain. The CRD is shown in magenta, the DH domain in blue, and the Rac1 in green. Polar interactions are depicted in grey, while hydrogen bonds in black. The series of interactions at this interface is predicted to stabilize this flexible helix for an integrated interaction with a particular RhoGTPase.
Figure 6B. σ-A weighted 2|Fobs | − |Fcalc| electron density at 2.6 Å resolution, contoured at 1.5 σ for the interlock system between the Vav1 CRD and Rac1 Switch II region with the α11 C-terminal helix of the Vav1 DH domain.
Figure 6C. The interaction network between the Rac1 Switch I region and the Vav1 DH domain. The DH domain is shown in blue, and Rac1 in green. Hydrogen bonds are depicted as dotted black lines. The spatial orientation of Trp-56 is also shown, although this residue forms no interactions with residues from Vav1.
Rac1 is composed of a central core comprised of 6 β-sheets (5 parallel and 1 antiparallel) that along with 6 α-helices and two 310 helices form the overall structure. Although residues 123–127 of Rac1 could not be properly modeled as a result of deficient electron density, Rac1 nonetheless retains many of the structural elements observed in previously described GEF-bound crystal structures, deviating only minimally in equivalent Ca positions (r.m.s. deviation of 0.84 Å) with Rac1 from the Tiam1/Rac1 complex13.
SAXS solution analysis of Vav1-DH-PH-CRD/Rac1 complex
Previous studies have provided disparate mechanistic explanations for the role of the Vav CRD in mediating nucleotide exchange. Campbell and co-workers observed in NMR experiments that a number of residues in Rac1 undergo a chemical shift upon addition of Maltose-Binding-Protein-Vav1-CRD or MBP-Vav1-PH-CRD, implicating direct interactions between the Vav1 CRD and Rac114. Yet, electron microscopy (EM) analysis of the homologous Vav3 protein indicated the presence of a fairly extended conformation of the DH-PH-CRD module. This conformation would disallow a direct interaction between the CRD and Rac1 23. These EM studies differed however from NMR experiments and also from our crystallographic analysis, by lacking a RhoGTPase component. Thus, to further clarify the Vav/Rac interaction we utilized small angle X-ray scattering (SAXS), which provides structural details of the complex in solution24. Repeated short exposures were necessary for data collection, because the Vav1-DH-PH-CRD/Rac1 protein complex showed radiation sensitivity. Also, an extrapolation of scattering profiles to zero concentration was required for analysis, due to the concentration dependence of the protein complex. This extrapolated experimental curve determined that the complex has a radius of gyration (RG) of 29 Å, and a maximum dimension (Dmax) of 91 Å. Notably, the calculated scattering curve from the crystal structure has an RG of 28 Å and a Dmax of 87 Å, in close agreement with the experimental results. However, an extended, Vav3-like model of the complex differs significantly, having an RG of 38 Å and Dmax of 119 Å. Comparison of the experimental scattering profile, with theoretical profiles generated from the crystal structure and from a Vav3-like extended configuration also revealed similar results (Fig. 3). The crystal structure fits the experimental data with a χ2 agreement of 2.12, compared with the extended model fitting with a χ2 of 10.9. Moreover, models constructed from the crystal structure, having a slight rotation and ~1 Å spreading of the gap between the PH domain and Rac1, increased the χ2 agreement to below 1.0 (data not shown). Flexibility in the C-terminal helix of the DH domain has been described in previous studies; this flexibility would adequately describe the small differences in the solution structure relative to the low energy minimum found under the crystallization conditions.
Figure 3.
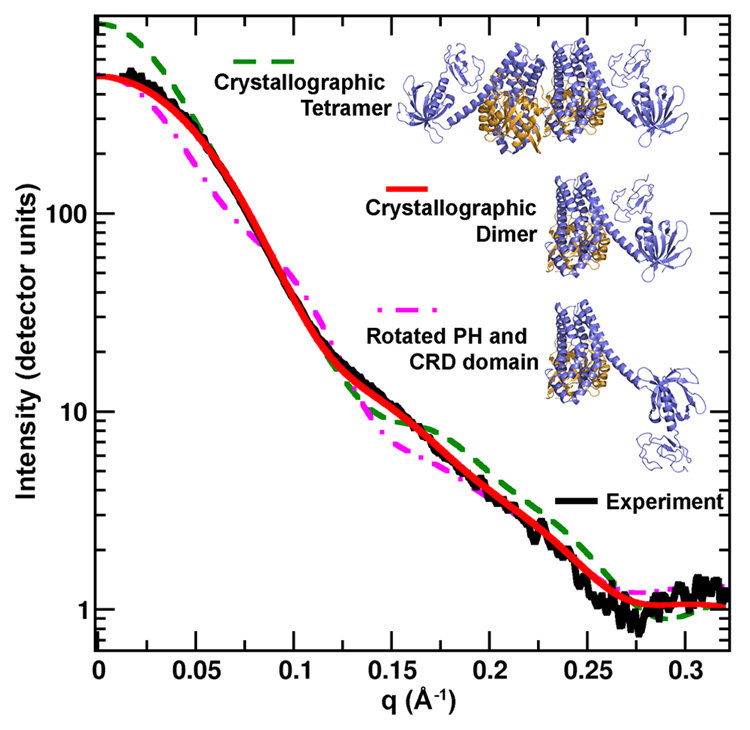
SAXS analysis of Vav1-DH-PH-CRD/Rac1 structure. The scattering profiles are depicted for the experimental data, shown in black, for the crystal structure, in red, for both molecules present in asymmetric unit, green dashed line, and for the model proposed by Llorca et al. that contains a different orientation of the CRD and PH domains23, pink dashed line. The crystal structure is in close agreement with the experimental scattering profile.
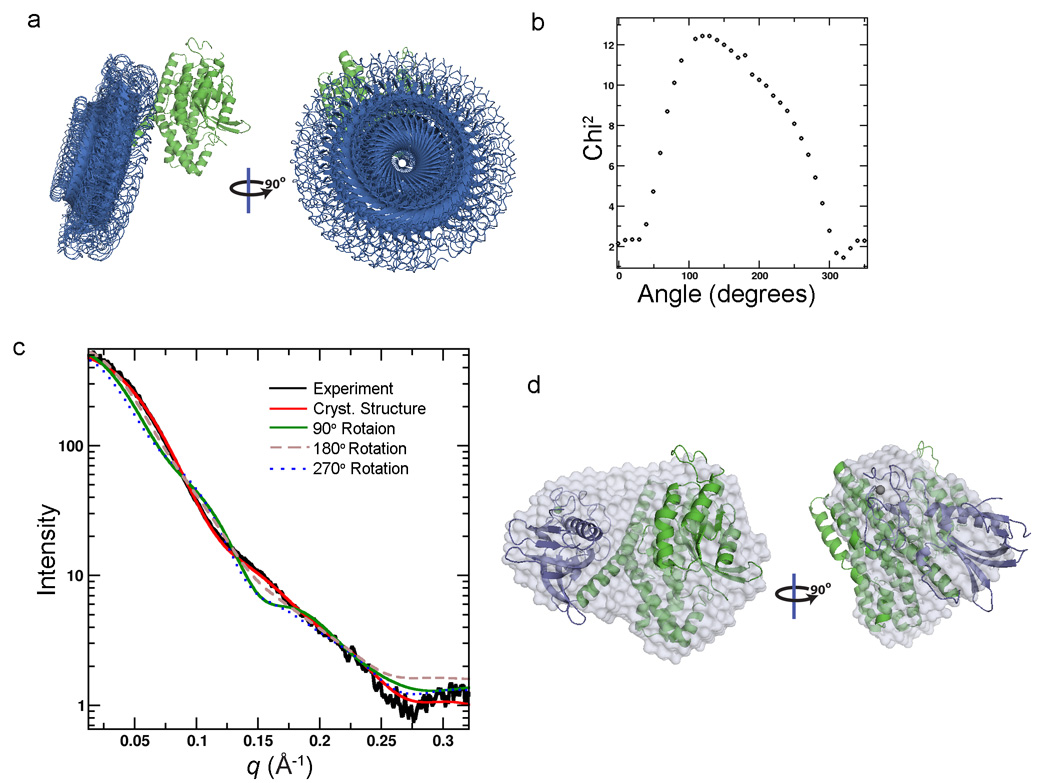
To further support the agreement of the compact configuration of the Vav/Rac crystal complex with the in solution SAXS data, an analysis of a range of extended structures was calculated. The PH/CRD region was rotated 360° at 10° increments about the base of the C-terminal DH connecting helix (Fig. 4a). Each 10° change in conformation was compared to the experimental SAXS curve (using CRYSOL25). A plot of the angle of rotation against the χ2 fit to the experimental data clearly indicates that the crystal structure, and angles of rotation close to this structure, best agree with the experimental data (Fig. 4b). We also compared four key conformations against the experimental scattering profile: the crystal structure, a PH/CRD rotation of 90°, a rotation of 180° (as in Fig. 3) and a rotation of 270°. The theoretical SAXS curve of the compact crystal structure of the Vav/Rac complex, rather than the three extended structures, clearly fits the scattering profile best (Fig. 4c). Additionally, an ab initio SAXS shape was generated from the experimental scattering curve, by averaging 10 independent ab initio GASBOR26 shape reconstructions. An overlay of the averaged ab initio SAXS shape with the crystal structure shows a good agreement in the compact shape, by these two independent methods (Fig 4d). Taken together, the analyses clearly indicate that the crystal structure is a considerably better fit to the SAXS data, when compared to the Vav3-like extended configuration in comparison23. Moreover, the agreement between the crystal structure and in solution SAXS data suggests that the compact Vav/Rac conformation is biologically relevant, rather than occurring due to experimental artifacts, such as crystal packing.
Figure 4.
a) The PH/CRD region (blue) of the Vav1-DH-PH-CRD/Rac1 complex rotated 360° at 10° increments about the base of the C-terminal DH connecting helix. b) A plot of the PH/CRD rotation angle vs χ2 fit of the calculated scattering curve to the experimental scattering curve. This plot indicates that the crystal structure, and angles of PH/CRD rotation close to this structure, best agree with the experimental data (Fig. 4b). c) A plot of the theoretical SAXS curve of four alternate conformations, the crystal structure, rotation of the PH/CRD region by 90°, by 180° (as in Fig 3) and by 270°, plotted with the experimental SAXS data. The crystal structure is the optimal fit of these four conformations. d) The ab initio SAXS shape, generated by averaging 10 independent runs of GASBOR48, overlaid on the Vav1-DH-PH-CRD/Rac1 crystal structure. The ab initio SAXS shape and crystal structure are in agreement, further supporting the closed conformation of the crystal structure rather than an extended conformation indicated by EM analysis.
Mutational analysis of Vav1/Rac1 Switch I and Switch II interfaces
A number of conserved interactions are observed in the Vav1/Rac1 structure that are similar to previously described GEF/RhoGTPase complexes, particularly those between the Switch I and Switch II regions of a RhoGTPase with their cognate GEF that are critical for efficient guanine nucleotide exchange. Therefore, we probed the role of those regions based upon the structural predictions from the Vav1/Rac1 structure using a mutational analysis of both Vav1 and Rac1 residues using an in vitro guanine nucleotide exchange (GEF) assay and an in vivo Rac1 activation assay.
To further explore the role of residues lining the switch II region of Rac1, point mutations were generated at residues lining the Switch2/DH interface (Asp-63-Ala, Asp-65-Ala, and Arg-66-Ala), and analyzed in a GEF assay. The above residues are highly conserved in Rac1, RhoA and Cdc42 RhoGTPases, all of which are recognized by Vav family GEFs. The assay results indicate that mutation of either Asp-63 or Arg-66 results in a ~3–5-fold reduction of rate of guanine nucleotide exchange when compared with wild type Rac1, consistent with the structural information (Figure 5). Although both Asp-63 and Arg-66 form polar interactions with residues lining the Vav1 DH domain, they are either not within hydrogen bonding distance (Asp-63), or not in an optimal orientation for participating in a hydrogen bond (Arg-66) (Figure 6A). On the other hand, mutation of residue Asp-65 results in the ~10-fold reduction of rate of GNE (Figure 5). Asp-65 forms a hydrogen bond with Arg-375 from the Vav1 DH domain (Figure 6A), and is predicted to impart stability to the formation of the Vav1/Rac1 complex. Asp-65 of Rac1 was also recently described to interact with Gln-1430 of the Trio PH domain, and its mutation was shown to significantly impair Trio- mediated GNE 11. Not surprisingly, residues at the center of this intricate interaction network are well ordered in the crystal structure (Figure 6B).
Figure 5.
Guanine nucleotide exchange assay analysis of representative Rac1 Switch II mutants. Exchange reactions were carried out in triplicates as described in the Materials and Methods. Exchange rates are calculated as percentages of the exchange rate of wild type Rac1.
To further explore Vav1/Rac1 binding determinants, a number of mutations were introduced in both the Vav1-DH and the Vav1-CRD to probe their role in Rac1 activation. Our initial attempts to create a new panel of mutants spanning all regions of the Vav1-DH-PH-CRD construct for biochemical analysis resulted in a low success rate of recovering soluble recombinant protein for both PH and CRD domain mutants involved in the interdomain interactions. Therefore, we have chosen an in vivo Rac1 activation assay to probe the role of those regions in Vav1 mediated nucleotide exchange.
We first probed additional residues that participate in the Vav1-DH and Rac1-Switch I interaction using a mutagenesis strategy which was generally elicited from the structural analysis. DH domain residue Glu-201 forms a hydrogen bond with the main-chain of Val-36, in addition to a side-chain—side-chain hydrogen bond with Tyr-32, which are both part of the Switch I region (Figure 6C). Mutation of Glu-201-Ala resulted in abrogated Rac1 activation (Figure 7C). This interaction network acts as the center of the polar interaction network between the switch I and the DH domain, indicative of the significant loss of guanine nucleotide exchange upon mutation at residue Glu-201. Similarly, our data indicate that another DH domain mutation Gln-331-Ala involved in Switch I region interactions also results in an inhibition of Rac1 activation (Figure 7C). Gln-331 forms a side-chain—side-chain hydrogen bond with Asn-39 of the Switch I region, and the interruption of this polar interaction would be expected to result in reduced stability in this region (Figure 6C).
Figure 7.
Endogenous Rac1 activation induced by exogenous expressed wild type Vav1 and Vav1 mutants in HEK293 cells. A) The expression levels of wild type Vav1 and Vav1 mutants in HEK293 cells were detected by Vav1 antibody and oncogenic Vav-1 was detected by HA antibody. B) The expression levels of endogenous Rac1 in HEK 293 cells expressing wild type Vav1 and Vav1 mutants were detected by Rac1 antibody. C) Active Rac1 in HEK293 cells expressing wild type Vav1 and Vav1 mutant was detected by Pull-down assay (described in methods and materials).
Our nucleotide exchange data indicated that the mutation at the Rac1 Asp-65 position nucleotide exchange due to the disruption of a critical hydrogen bond between Asp-65 of the Rac1 Switch II region and Arg-375 of the DH domain (Figure 6A). We therefore mutated Vav1 DH domain residue Arg-375 to an Ala, and a Rac1 pull down experiment revealed no detectable activated Rac1 for the reciprocal mutation (Figure 7C). This suggests a critical overall feature between Vav1/Rac1 interactions. Mutation of the adjacent residue, Asp-376, was also characterized as it participates in a side-chain—main-chain interaction with Thr-539 (CRD) as well as side-chain—side-chain interaction with Lys-404 (PH domain) (Figure 2A, Figure 6A). Not surprisingly, mutation of this residue resulted in a full abrogation of exchange activity with Rac1, a likely result of the absence of an interaction network between the DH, PH and CRD regions. The final DH domain mutation was centered at Glu-378, which interacts via hydrogen bond with Arg-66 of the Switch II region (Figure 6A). Mutation of Arg-66 to Ala exhibited a moderate loss of guanine nucleotide exchange potential. The reciprocal data with the Glu mutant indicates a moderate loss of Rac1 activation (Figure 7C). A mutation was also generated at position Asp-365, which interacts with the main-chain of Leu-535 (CRD) as well as the side-chain of Arg-537 (CRD; Figure 6A). Asp-365 resides adjacent to the Switch II region, but does not directly participate in an interaction with this region (Figure 6A). Mutation at this residue did not result in abrogated Rac1 activation, suggestive that mutations at residues which solely contact the Switch II region are predictive of altered GNE. It is a surprising result nonetheless, as it was structurally identified to impart stability at the α11 helix/CRD/Switch II interlock, but redundant in the context of an additional interaction between Gln-368 and Arg-537 (Figure 6A).
A critical role for Vav2 and Vav3 CRD residues in maintaining Vav GNE activity has been described in multiple reports27; 28. The previously reported mutational analysis was conducted based on a homology model using the NMR structure of the Raf-1 CRD. Some of those mutations were predicted to result in a loss of Zn2+ coordination, while others were predicted to be presented on the surface of the CRD, and were identified as critical residues for an interaction between the Raf-CRD and h-Ras. Our structural information provides a unique molecular insight into the positioning of the CRD in its critical role of mediating GNE. The significant interaction network observed between Vav1/Rac1 is further expanded into the DH-PH-CRD interlock, a network that appears to significantly stabilize Vav1 in the spatial orientation for interaction with the RhoGTPase. The critical role of residues at this interface for the proper folding of the Vav1-DH-PH-CRD protein construct is emphasized by the low success rate of recovering soluble recombinant protein for both PH and CRD domain mutants involved in the interlock
We have chosen to examine a subset of previously studied mutations (Asn-505, Arg-537 and Lys-555) in order to correlate the structural information with the level of Rac1 in an activation assay. Interestingly, all of the above mutations resulted in moderately decreased Rac1 activation, however not a complete loss of activation potential (Figure 7C). Asn-505 forms a hydrogen bond with the side-chain of Asp-428 (PH), as well as a polar interaction with Lys-555 (CRD) (Figure 2A). The moderate reduction in activity for the Arg-537 mutant is a likely result of the inability of an Ala residue to form a hydrogen bond with both Asp-365 and Gln-368 of the DH domain, which would be expected to result in defects in CRD positioning with respect to the DH domain (Figure 6A). Similarly, mutation at residue 555, a Lys, results in only a moderate reduction of Rac1 activation, a likely result of the abrogation of hydrogen bonds between the side-chain amine of Lys-555 (CRD) and the side chains of residues Ser-504 and Asn-505 (PH; Figure 2A). In addition to our studies, GEF analyses of Vav2 CRD mutants indicated that only a Lys-533-Ala mutant resulted in a significant but incomplete reduction in GNE activity towards Rac1, which was pronounced when measured against RhoA27. A moderate reduction in GNE was observed for Lys-538 and Lys-563 (structural homolog of Lys-555 in Vav1) mutants of Vav2 when measured against RhoA but not Rac1. Thus, multiple mutations at the DH-PH-CRD interface are likely required to result in a significant loss of GNE due to the extensive protein-protein interaction network involved in maintaining the overall positioning of the CRD. Interestingly, in vitro kinetic measurements conducted by Heo et al. indicated that the RhoGTPase specificity for the DH-PH-CRD cassette of Vav2 followed in this order: Rac1 > Cdc42 > RhoA14. Thus, the sensitivity of nucleotide exchange to CRD mutations is more pronounced in the context of the least robust interaction.
Structural and functional basis of Vav1/Rac1 regions contributing to Vav1/Rac1 interaction Specificity
The β2/β3 region of Rac1 has previously been reported to play a critical role as a structural recognition motif in GEF recognition6; 29, and therefore participates in mediating the selectivity between RhoGTPase/GEF pairs. In addition, Trp-56 has been characterized as the critical molecular determinant for specificity in GEF binding to Rho/Rac-specific RhoGTPases or Cdc42 –specific RhoGTPases6; 29. The critical nature of the Trp-56 molecular determinant has been supported by the elucidation of the Tiam1/Rac1 crystal structure, wherein a hydrogen bond was observed between the amide of Trp-56 and the side chain of His-1178 of Tiam1 (Figure 8). However, the corresponding position in the Vav1/Rac1 structure corresponds to an Ala (1319, Tiam numbering), and precludes the formation such an interaction with Trp-56 (Figure 8). Further, an Ile residue is conserved at position -1187 (Tiam-1) in all Rho-specific GEF’s, and has been reported to participate in a van der Waals interaction with the backbone side-chain of the Trp-56 residue. It has been described that the side chain of Ile is prime to accommodate the bulky Trp-56, unlike Cdc42 -specific GEF’s, which maintain a Leu at the corresponding position and would inevitably result in a steric clash with a Trp residue. Interestingly, members of the Vav family maintain a Met at the corresponding Ile/Leu position (Figure 8). The flexibility of the Met should allow a rotational conformation to discriminate between both Rho and Cdc42 families, and may help to potentiate the recognition of those RhoGTPase families by the Vav family of GEF’s. We have probed the role of Trp-56 in Vav1 mediated GNE using both the Vav1-DH-PH-CRD and Vav1-DH constructs. As a control, a Tiam-1 DH-PH construct was concurrently examined as it was described previously to be dependent on Trp-56 for optimal displacement of the guanine nucleotide29; 30. Our experiments also indicate that this GEF displayed the rate of guanine nucleotide exchange similar to the control reaction in a GEF assay with the Rac1 Trp-56-Phe mutant (Figure 9D). The same assay analysis indicates that the rate of nucleotide exchange for Vav1-DH-PH-CRD is only reduced 6-fold towards the Rac1 Trp-56-Phe mutant compare to Rac1 WT (Figure 9 A, D), while the isolated Vav1-DH lacking the structural elements participating in Switch II interactions exhibits a rate of nucleotide exchange similar to the GEF control. (Figure 9 A, D). This data is consistent with the inherent lack of specificity of Vav1 for RhoGTPases, and suggests that Trp-56 plays a mitigated role for recognition of Vav1 proteins in context of their interaction with Rac1. However, the measurable reduction in rate of GNE towards Vav-DH-PH-CRD for the Trp-56 mutant does suggest that the residue indeed still maintains a role in GEF recognition.
Figure 8.
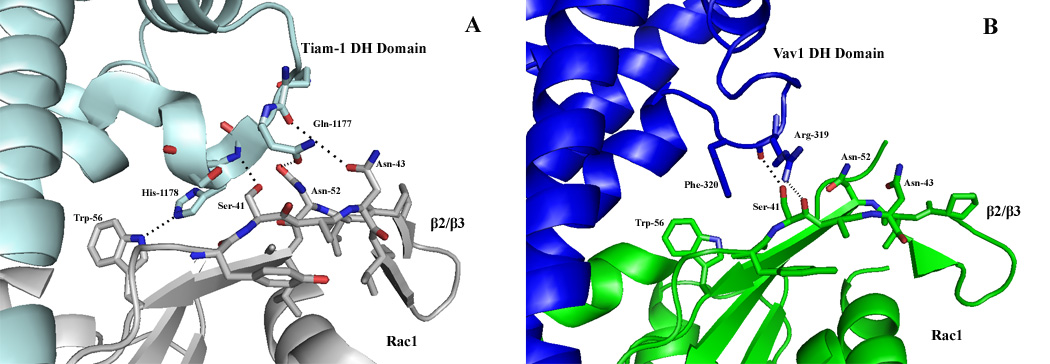
Disruption of the β2/β3 region interface in Vav1/Rac1 structure. Comparison of the Tiam1/Rac β2/β3 region (left) and the Vav/Rac β2/β3 region (right). Tiam-1 (cyan) forms an integrated network with the B2/B3 region of Rac1 (grey), in addition to a hydrogen bond between Trp-56 of Rac1 and His-1178 of Tiam1. Vav1 (blue) forms only one hydrogen bond in this region, between Ser-41 of the B2/B3 of Rac1 (green) and Arg-319 of the DH domain of Vav1. In addition, Trp-56 of Rac1 does not form any interaction with the DH domain of Vav1, polar or hydrophobic.
Figure 9.
Guanine nucleotide exchange assay analysis of Rac1 Trp 56 and β2/β3 region mutants. Exchange reactions were carried out in triplicates as described in the Materials and Methods. A) Wild type Rac1; B) Rac1 S41A; C) Rac1 N43A; D) Rac1 W56F. Molar ratio of GEF/RhoGTPase were adjusted to generate similar dynamic range (Vav-DH-PH-CRD 1:8; Tiam DH-PH 2:1; Vav-DH 8:1).
Additionally, the elucidation of the Tiam1/Rac1 structure suggested that the β2/β3 region acted as a selectivity monitor for GEF/RhoGTPase interactions. Interactions are formed by side-chain--main-chain hydrogen bonds between the β2/β3 loop (residues Ser-41, Asn-43, and Asn-52) of Rac1 and the DH domain of Tiam1 (residues 1,177–1,179)6; 30. Interestingly, the corresponding interaction network surrounding the β2/β3 region of Rac1 and Vav1 is noticeably absent in the Vav1/Rac1 structure due to the displacement of the DH domain from the β2/β3 region (Figure 8). In fact, only a single interaction is observed between the β2/β3 region of Rac1 and the DH domain of Vav1 that corresponds to the Tiam/Rac structure; the Ser-41 side-chain of Rac1 interacts with the main-chain nitrogen of Arg-319 of Vav1. The compromised interaction network within this region indicates that the β2/β3 region might play a diminished overall structural role in defining the selectivity determinants for the Vav1/Rac1 interaction, and that the Vav family has evolved a different means of substrate recognition, likely the evolution of the CRD. To further probe the role of the β2/β3 region in Vav1 mediated GNE, two mutations were generated surrounding the β2/β3 region of Rac1 and similarly tested in a GEF assay as above (Figure 9 B, C). A Tiam-1 construct was again utilized as a control. Our results indicate that while the Tiam-1 Ser-41-Ala construct exhibits 26-fold reduction of rate of GNE, the Vav1-DH-PH-CRD construct maintains rate of GNE similar to wild-type Rac1 (Figure A, B), suggestive that the β2/β3 region is less functionally significant in Vav1/Rac1 interaction (Figure 9B). This is further supported by the observation that Vav-DH domain retains similar rate of GNE to both Rac1 wild type and Rac1 Ser-41-Ala mutant. Similar to Tiam1, mutation at residue Asn-43 reveals that the Vav1 DH-PH-CRD construct also remains fully active (Figure 9C). Thus, it appears that the enhanced RhoGTPase recognition profile exhibited by Vav family members is contingent on the dissipated functional requirement of the β2/β3 region in addition to the strict requirement for a Trp at position 56 of Rac1.
Discussion
The Vav family belongs to a small subset of GEF superfamily members capable of recognizing various classes of RhoGTPases31. This recognition is critically dependent on the atypical CRD domain, which is unique to Vav GEFs. Additionally, the Vav family (Vav1-3) have also evolved an elaborate intramolecular activation mechanism involving a sequential phosphorylation cascade to relieve autoinhibition mediated by the N-terminal α-helix of the DH domain, and dissociation of the intramolecular protein-protein interaction between the N-terminal CH (calponin homology) domain of Vav and the CRD23; 32. Phosphorylation of tyrosine residues within the Vav Ac domain (acidic domain) by the upstream kinase Lck initiates the relief of autoinhibition and promotes the formation of a Vav conformation capable of activating downstream RhoGTPases 32. Electron microscopy studies confirmed the global conformational changes between the autoinhibited and phosphorylated states of Vav that are manifested by the ability of the Dbl homology domain to participate in a protein-protein interaction with a cognate RhoGTPase 23. Further, the hallmark of activated Vav proteins is the requirement for the atypical CRD to achieve an optimal on-rate of interaction with the RhoGTPase14. In fact, the direct interaction between the CRD and the RhoGTPase was implicated as a mechanistic explanation of the role of the CRD in the protein-protein interaction between Vav1 and Rac114. The structure of the fully activated Vav1-DH-PH-CRD module in complex with Rac1 sheds light on the role of the CRD in guanine nucleotide exchange, and provides a possible explanation for Vav1 promiscuity. The formation of the DH-PH-CRD intramolecular interaction network generates a stable conformation of the Vav1 DH domain which is primed for an efficient interaction with the highly conserved Switch II region of the RhoGTPase, and contributes to the recognition of multiple RhoGTPases. Thus upon activation of Vav1, the CRD would be predicted to dissociate from the CH domain, and engage in a novel protein-protein interaction, which is critical for the stabilization of the Vav DH α11 interaction with the RhoGTPase. The existence of this interaction network in solution was confirmed by SAXS analysis of the Vav1-DH-PH-CRD/Rac1 complex. The role of the Switch II region in the recognition of promiscuous exchange factors was recently described for the Trio/Rac1 interaction. Trio catalyzes guanine nucleotide exchange on both RhoG and Rac1, and utilizes the PH domain to generate additional interactions with Asp-65 from the Switch II region of Rac1, increasing its functional activity 4-fold. In the case of the Vav1/Rac1 interaction, further expansion of the Asp-65-α11 interaction network with the CRD is critical for maintaining the functional activity in vitro, since the DH domain alone requires a 50-fold higher molar ratio to Rac1, as compared to the DH-PH-CRD module, to induce similar GNE 18; 19. Interestingly, the catalytic activities of Lbc (a Rho A specific GEF) and Cdc24 (a Cdc42-specfic GEF) are insensitive to mutations in the switch II region of the aforementioned RhoGTPases, while the more promiscuous GEFs Vav and Trio maintain critical functional interactions with the Switch II region of the RhoGTPase33.
Using mutational analyses of both Vav1 and Rac1 residues involved in the Vav1/Rac1 protein-protein interaction, we also show that the Vav1 complements the essential nature of interactions with the RhoGTPase Switch II region while showing less dependence on the predicted interactions reported to impart specificity for various GEF/RhoGTPase interactions, including those at the β2/β3 region, and at position 5613. Those observations suggest that the Vav family of GEF’s evolved the CRD in order to promote a diversified platform for RhoGTPase binding. Further structure based mutational analysis of Vav1 mediated GNE with both RhoA and Cdc42 is required to fully elucidate mechanism of Vav1 promiscuity. The structural and functional analysis of the Vav1/Rac1 interface also sheds some light on previous work directed towards the identification of small molecule inhibitors of the Tiam/Rac1 protein-protein interaction. In those studies, the drugability of the Trp-56 pocket was explored using a virtual library screening approach, and resulted in compounds inhibiting Rac1 activation by both Trio and Tiam1, specifically the NCS23766 compound. The inherent flexibility of Vav1 residues involved in the recognition of Trp-56, together with the critical role of Switch II interactions, provides a plausible explanation for the lack of Vav1 mediated signaling sensitivity to the NCS23766 compound, which specifically inhibits a Rac1 interaction with both Trio and Tiam134, but not Vav1. Further, the therapeutic potential of pharmacological interference of Vav1/Rac1 is validated by the mechanism of action of the immunosuppressive drug azathioprine. It was shown that pre-incubation of Rac1 with 6-thio-GTP, a metabolite of azathioprine, leads to the reduced incorporation of radiolabeled GTP into Rac1 in the presence of Vav1, and hence the accumulation of a 6-thio-GDP/Rac1 complex which results in the suppression of Vav’s GNE activity. Additional studies demonstrated that 6-thio-GTP can bind to a number of RhoGTPases from different families including Ran, Rab, RhoA, and Cdc42 but is incapable of reducing their activation level unlike for Rac1 and Rac2 upon CD3/CD28 stimulation35. One could envision that the disruption of the inter- and intramolecular network involved in the Vav-DH/Rac1-Switch II interaction is responsible for the 6-thio-GDP mechanism of action. At present, the level of thiopurine methyl transferase (TMPT) activity is considered to be responsible for the variation of therapeutic efficacy and toxicity of thiopurine drugs36. This dependence leaves much room for improvement and development of novel immunosuppressive agents using the structural information described here.
The identification of drugable protein–protein interaction interfaces represents an important avenue in the drug discovery paradigm, as the number of protein targets that can be directly modulated by small molecule compounds or antibodies is rapidly declining37. Often, detailed structural information is required prior to the initiation of a search for small molecule inhibitors, as even therapeutically validated protein pairs may lack attributes required for the development of protein-protein interaction inhibitors. The structure of Vav1-DH-PH-CRD/Rac1 complex describes for the first time the involvement of the atypical CRD in an elaborate protein-protein interaction network that provides the basis for the promiscuity of Vav family GEFs. This work also emphasizes the challenges associated with the development of protein-protein interaction inhibitors, as the identification of chemical matter targeting such non-traditional interfaces as observed in the Vav1/Rac1 structure remains a difficult task.
Experimental procedures
Expression and Purification of human Vav and Rac1 variants
Vav1 (DH-PH-CRD), Se-Met substituted Vav1 (DH-PH-CRD; residues 189–64) and nucleotide –free Rac1 (residues 1–177) were expressed in E. coli as described elsewhere18. Material generated by V8 limited proteolysis post Vav1/Rac1 complex formation was used to crystallize Vav1/Rac1 complex 18. Vav1 mutants for functional analysis were generated based on a Vav (190–575) M351T construct using the QuikChange Mutagenesis Kit (Stratagene, CA), and expressed as described for wild type protein.
Crystallization of Vav1-DH-PH-CRD/Rac1 complex
Crystallization of the Vav1-DH-PH-CRD/Rac1 complex was described elsewhere18. The Vav1 (Se-Met)-Rac1 complex was concentrated to 3 mg/mL in a buffer containing 25 mM Hepes, pH 7.2, 100 mM NaCl, and 1 mM TCEP), and crystallized in a sitting drop by vapor diffusion at 293 K against a precipitant of 100 mM Hepes, pH 7.5, 16% PEG-3350, and 200 mM ammonium sulfate. Crystals appeared in hours, and grew as thin plates with variable geometries. Crystals (Se-Met and native) were cryoprotected in 20% propylene glycol and flash frozen in liquid nitrogen. Data were collected at Beamlines 11-1 of the Stanford Synchrotron Radiation Lab (SSRL; Menlo Park, CA), GM-CA-CAT at the Advanced Photon Source (APS; Argonne, IL), and Beamline ID-29 at the European Synchrotron Radiation Facility (ESRF; Grenoble, France), and the diffraction data were processed using XDS38; 39. Crystals belong to the P21 spacegroup. The initial phases were obtained by the single wavelength anomalous dispersion (SAD) phasing method with data collected to 2.9 Å at the selenium peak wavelength by using the programs Shelx and SHARP. Shelx40 and SHARP41 located 30 of 34 selenium atoms, and the sites were refined through MLPHARE. The resultant phases from SAD were merged, improved, and extended for a native data set to 2.6 Å using the DM program as implemented in the CCP4 package42. Both the DH domain of Vav1 and the entire cassette of Rac1 could be readily placed into the model using Coot 43, while the PH domain and the CRD were built in manually.
Model building and refinement
The model was refined with iterative rounds of model building and refinement using Refmac544 from the CCP4 package. The final model contains 8349 atoms, with a final R and Rfree of 22.5 and 29.2 %, respectively. The structure exhibits good morphology with no Ramachandran outliers (Molprobity)45. A summary of data collection and refinement statistics is shown in Table 1. The ribbon figures were generated with Pymol46.
Table 1.
Crystallographic Statistics for the Vav/Rac Complex
| Native | Peak | |
|---|---|---|
| Data Collection | ||
| Source | SSRL | APS |
| Resolution (Å)1 | 45.45-2.55 (2.62-2.55) | 25-2.9 (2.99-2.9) |
| Space Group | P21 | P21 |
| Unit Cell Dimensions (Å, °) | a=78.95, b=75.08, c=114.86 α=γ=90, β=103.87 | a=78.66, b=74.88, c=114.58 α=γ=90, β=103.79 |
| Wavelength (Ǻ) | 0.981 | 0.979 |
| Completeness (%) | 100 (100) | 99.5 (97.9) |
| Rsym (%)2 | 10.4 (38.9) | 8.7 (32.5) |
| I/σ | 7.2 (2.5) | 4.5 (2.1) |
| Mean Redundancy | 3.1 | 7.4 |
| Refinement | ||
| Resolution | 30-2.55 (2.62-2.55) | |
| No. Reflections | 36,180 | |
| Rcryst (%)3 | 22.48 | |
| Rfree (%)4 | 29.25 | |
| R.m.s. deviations | 0.13 | |
| Bond length (Å) | 0.01 | |
| Bond angle (°) | 2.28 | |
| Average B Value, Main | 44.59 | |
| Chain (Ǻ2) | ||
| Average B Value, Side | 43.85 | |
| Chains and Water (Ǻ2) | ||
| Number of atoms | ||
| Protein | 8269 | |
| Solvent | 76 | |
| Zinc | 4 |
Number in parentheses is for the highest shell.
Rsym = ∑|I −〈I〉|/∑I, where I is the observed intensity and 〈I〉 is the average intensity of multiple symmetry-related observations of that reflection.
Rcryst = ∑||Fobs| −|Fcalc ||/∑|Fobs|, where Fobs and Fcalc are the observed and calculated structure factors. Rsym = ∑|I −〈I〉|/∑I, where I is the observed intensity and 〈I〉 is the average intensity of multiple symmetry-related observations of that reflection.
Rfree = ∑||Fobs| −|Fcalc ||/ ∑|Fobs| for 10% of the data not used at any stage of structural refinement.
GEF Assay
GEF analysis of Vav1 and Rac1 mutants was conducted as described elsewhere18. Briefly, Rac1 (1 µM) was preloaded with 60 nM Bodipy-GDP (Invitrogen, Carlsbad, CA) and mixed with 200 µM GTP and various concentrations of GEFs (0.125–8 µM; specific molar ratios noted elsewhere). Fluorescence measurements were taken at 2-min intervals for a total of 120 min on a Magellan Tecan (Tecan Instruments, Zurich, Switzerland) microplate reader at 535 nm (excitation wavelength of 488 nm). Data from three-independent replicates were subsequently averaged and fitted using Graph Pad Prism 4.0. All mutants were generated based on the Vav1 M351T mutant described previously and the molar ratio between the GEF and Rac1 are indicated in the figure legends.
Rac activation assay
HEK293 cells were maintained in Dulbecco modified Eagle medium (DMEM) with high glucose supplemented with 10% FBS, 4 mM glutamine and 10 mM HEPES. HEK293 cells were grown at 37 °C and 5% CO2. HEK 293 Cells at 60–80% confluence were transiently transfected with different Vav-1 constructs and PCDNA3-GFP as mock transfection using lipofectamine according to the manufacturer’s instruction. After 24 hours of the transfection, HEK 393 cell lysate was prepared in 50 mM Tris (pH 7.5), 200 mM, 30 mM MgCl2, 1% NP40 and 5% glycerol supplemented with protein inhibitors, including 1 mM PMSF, 1 mM Aprotinin, 1 µM Leupeptin and 1µg/ml Pepstatin A. For Vav-1 expression detection, 20 µl of cell lysate was examined on 8% SDS PAGE with blotting against Vav-1 antibody (Cell signaling) for different full-length Vav-1 mutants and HA antibody (from Upstate) for oncogenic Vav-1 (A4). For Rac1 expression levels, 20 µl of each cell lysate was examined on 12% SDS PAGE with blotting against Rac1 antibody (Upstate). For active Rac-1 detection, 400 µl of cell lysate was added into 200 µl PBD binding buffer (50 mM Tris, 200 mM NaCl, 30 mM MgCl2, 1 mM DTT and 1% NP-40 supplemented with 1 mM PMSF, 1 mM Aprotinin, 1 µM Leupeptin and 1µg/ml Pepstatin A) containing 10 µg of PBD (GST-fusion protein containing the Rac/Cdc42 Binding Domain of PAK, PBD). 40 µl of control cell lysate was pre-loaded with either GTPγS or GDP as positive or negative controls and mixed with 10 µg of PBD. Incubate the mixture of cell lysate and PBD for 1 hr 15 min at 4 °C, while gently tumbling. Wash the PBD three times, add 15 µl of 4x SDS sample buffer to the PBD and boil for 10 min. Centrifuge at 6000 rpm for 2 minutes. Samples are examined on 12% SDS PAGE and blotted against Rac1 antibody (Upstate).
SAXS Data Collection
Small angle X-ray scattering (SAXS) data were collected at the SIBYLS beamline (12.3.1) at the Advanced Light Source in Lawrence Berkeley National Laboratory. The incident wavelength used in the experiment was 1.03Å with a q range of 0.01 – 0.32 Å−1 ( q= 4π sin (θ/2)/ λ where θ is the scattering angle, λ is the wavelength). The detector was a MAR 165 CCD area detector. Sample volumes of 15 µL were required, and placed in a 1mm thick cuvette. Data sets were collected from concentrations of 5, 2.5 and 1.25 mg/ml. Data were initially processed using the freeware program PRIMUS47 which enables the extraction of the radius of gyration (RG) using a Guinier plot and the maximum dimension (Dmax). Calculation of the scattering profile from PDB files was completed with CRYSOL25 using an option which best fits the data by adjusting properties of the hydration shell.
Acknowledgements
The authors would like acknowledge Raymond C. Stevens, Michael Hanson, Maneesh Yadav, Joe Ng, and Michelle Kraus for insightful discussions and guidance. We also acknowledge the helpful support of the staff scientists at the Advanced Photon Source (BL GM-CA CAT), SSRL (BL 11-1), and ESRF (BL 23-ID) for help in data collection and remote crystal screening (SSRL). The General Medicine and Cancer Institutes Collaborative Access Team (GM/CA CAT) is supported by NCI, National Institutes of Health (NIH) Grant Y1-CO-1020 and NIGMS, NIH Grant Y1-GM-1104. SSRL BL11-1 is supported by the National Institutes of Health (NIH) National Center for Research Resources, NIH National Institutes of General Medical Sciences, Department of Energy, Office of Biological and Environmental Research, Stanford University, and The Scripps Research Institute (TSRI). The Tiam-1 cDNA was a generous gift from John Sondek’s lab at the University of North Carolina at Chapel Hill. GH contribution is supported by DOE Contract # DE-AC02-05CH11231. This work was supported by grant SFP-1543 and NIH Protein Structure Initiative Specialized Centers Grant GM074961 to PK and HL48008 to GB. TSRI manuscript number: 19252.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Numbers:
The atomic coordinates and structure factors (code 3BJI) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
References
- 1.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 2.Prieto-Sanchez RM, Hernandez JA, Garcia JL, Gutierrez NC, San Miguel J, Bustelo XR, Hernandez JM. Overexpression of the VAV proto-oncogene product is associated with B-cell chronic lymphocytic leukaemia displaying loss on 13q. Br J Haematol. 2006;133:642–645. doi: 10.1111/j.1365-2141.2006.06094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schapira V, Lazer G, Katzav S. Osteopontin is an oncogenic Vav1-but not wild-type Vav1-responsive gene: implications for fibroblast transformation. Cancer Res. 2006;66:6183–6191. doi: 10.1158/0008-5472.CAN-05-3735. [DOI] [PubMed] [Google Scholar]
- 4.Katzav S, Martin-Zanca D, Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. Embo J. 1989;8:2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Movilla N, Bustelo XR. Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol Cell Biol. 1999;19:7870–7885. doi: 10.1128/mcb.19.11.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karnoub AE, Worthylake DK, Rossman KL, Pruitt WM, Campbell SL, Sondek J, Der CJ. Molecular basis for Rac1 recognition by guanine nucleotide exchange factors. Nat Struct Biol. 2001;8:1037–1041. doi: 10.1038/nsb719. [DOI] [PubMed] [Google Scholar]
- 7.Baumeister MA, Martinu L, Rossman KL, Sondek J, Lemmon MA, Chou MM. Loss of phosphatidylinositol 3-phosphate binding by the C-terminal Tiam-1 pleckstrin homology domain prevents in vivo Rac1 activation without affecting membrane targeting. J Biol Chem. 2003;278:11457–11464. doi: 10.1074/jbc.M211901200. [DOI] [PubMed] [Google Scholar]
- 8.Baumeister MA, Rossman KL, Sondek J, Lemmon MA. The Dbs PH domain contributes independently to membrane targeting and regulation of guanine nucleotide-exchange activity. Biochem J. 2006;400:563–572. doi: 10.1042/BJ20061020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossman KL, Cheng L, Mahon GM, Rojas RJ, Snyder JT, Whitehead IP, Sondek J. Multifunctional roles for the PH domain of Dbs in regulating Rho GTPase activation. J Biol Chem. 2003;278:18393–18400. doi: 10.1074/jbc.M300127200. [DOI] [PubMed] [Google Scholar]
- 10.Das B, Shu X, Day GJ, Han J, Krishna UM, Falck JR, Broek D. Control of intramolecular interactions between the pleckstrin homology and Dbl homology domains of Vav and Sos1 regulates Rac binding. J Biol Chem. 2000;275:15074–15081. doi: 10.1074/jbc.M907269199. [DOI] [PubMed] [Google Scholar]
- 11.Chhatriwala MK, Betts L, Worthylake DK, Sondek J. The DH and PH domains of Trio coordinately engage Rho GTPases for their efficient activation. J Mol Biol. 2007;368:1307–1320. doi: 10.1016/j.jmb.2007.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derewenda U, Oleksy A, Stevenson AS, Korczynska J, Dauter Z, Somlyo AP, Otlewski J, Somlyo AV, Derewenda ZS. The crystal structure of RhoA in complex with the DH/PH fragment of PDZRhoGEF, an activator of the Ca(2+) sensitization pathway in smooth muscle. Structure. 2004;12:1955–1965. doi: 10.1016/j.str.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Worthylake DK, Rossman KL, Sondek J. Crystal structure of Rac1 in complex with the guanine nucleotide exchange region of Tiam1. Nature. 2000;408:682–688. doi: 10.1038/35047014. [DOI] [PubMed] [Google Scholar]
- 14.Heo J, Thapar R, Campbell SL. Recognition and activation of Rho GTPases by Vav1 and Vav2 guanine nucleotide exchange factors. Biochemistry. 2005;44:6573–6585. doi: 10.1021/bi047443q. [DOI] [PubMed] [Google Scholar]
- 15.Han J, Das B, Wei W, Van Aelst L, Mosteller RD, Khosravi-Far R, Westwick JK, Der CJ, Broek D. Lck regulates Vav activation of members of the Rho family of GTPases. Mol Cell Biol. 1997;17:1346–1353. doi: 10.1128/mcb.17.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe K, Rossman KL, Liu B, Ritola KD, Chiang D, Campbell SL, Burridge K, Der CJ. Vav2 is an activator of Cdc42, Rac1, and RhoA. J Biol Chem. 2000;275:10141–10149. doi: 10.1074/jbc.275.14.10141. [DOI] [PubMed] [Google Scholar]
- 17.Mosteller R, Han J, Das B, Broek D. Biochemical analysis of regulation of Vav, a guanine-nucleotide exchange factor for Rho family of GTPases. Methods Enzymol. 2000;325:38–51. doi: 10.1016/s0076-6879(00)25429-3. [DOI] [PubMed] [Google Scholar]
- 18.Brooun A, Foster SA, Chrencik JE, Chien EY, Kolatkar AR, Streiff M, Ramage P, Widmer H, Weckbecker G, Kuhn P. Remedial strategies in structural proteomics: expression, purification, and crystallization of the Vav1/Rac1 complex. Protein Expr Purif. 2007;53:51–62. doi: 10.1016/j.pep.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aghazadeh B, Lowry WE, Huang XY, Rosen MK. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–633. doi: 10.1016/s0092-8674(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 20.Worthylake DK, Rossman KL, Sondek J. Crystal structure of the DH/PH fragment of Dbs without bound GTPase. Structure. 2004;12:1078–1086. doi: 10.1016/j.str.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Rossman KL, Worthylake DK, Snyder JT, Siderovski DP, Campbell SL, Sondek J. A crystallographic view of interactions between Dbs and Cdc42: PH domain-assisted guanine nucleotide exchange. Embo J. 2002;21:1315–1326. doi: 10.1093/emboj/21.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mott HR, Carpenter JW, Zhong S, Ghosh S, Bell RM, Campbell SL. The solution structure of the Raf-1 cysteine-rich domain: a novel ras and phospholipid binding site. Proc Natl Acad Sci U S A. 1996;93:8312–8317. doi: 10.1073/pnas.93.16.8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llorca O, Arias-Palomo E, Zugaza JL, Bustelo XR. Global conformational rearrangements during the activation of the GDP/GTP exchange factor Vav3. Embo J. 2005;24:1330–1340. doi: 10.1038/sj.emboj.7600617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Svergun D, Baraberato C, Koch MH. CRYSOL - a Program to evaluate X-ray Solution Scattering of Biological Macromolecules from Atomic Coordinates. J Appl. Cryst. 1995;28:768–773. [Google Scholar]
- 25.Svergun D, Baraberato C, Koch MH. CRYSOL - a Program to evaluate X-ray Solution Scattering of Biological Macromolecules from Atomic Coordinates. J. Appl. Cryst. 1995;28:768–773. [Google Scholar]
- 26.Svergun DI, Petoukhov MV, Koch MH. Determination of domain structure of proteins from X-ray solution scattering. Biophys. J. 2001;76:2879–2886. doi: 10.1016/S0006-3495(01)76260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booden MA, Campbell SL, Der CJ. Critical but distinct roles for the pleckstrin homology and cysteine-rich domains as positive modulators of Vav2 signaling and transformation. Mol Cell Biol. 2002;22:2487–2497. doi: 10.1128/MCB.22.8.2487-2497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zugaza JL, Lopez-Lago MA, Caloca MJ, Dosil M, Movilla N, Bustelo XR. Structural determinants for the biological activity of Vav proteins. J Biol Chem. 2002;277:45377–45392. doi: 10.1074/jbc.M208039200. [DOI] [PubMed] [Google Scholar]
- 29.Gao Y, Xing J, Streuli M, Leto TL, Zheng Y. Trp(56) of rac1 specifies interaction with a subset of guanine nucleotide exchange factors. J Biol Chem. 2001;276:47530–47541. doi: 10.1074/jbc.M108865200. [DOI] [PubMed] [Google Scholar]
- 30.Snyder JT, Worthylake DK, Rossman KL, Betts L, Pruitt WM, Siderovski DP, Der CJ, Sondek J. Structural basis for the selective activation of Rho GTPases by Dbl exchange factors. Nat Struct Biol. 2002;9:468–475. doi: 10.1038/nsb796. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 32.Amarasinghe GK, Rosen MK. Acidic region tyrosines provide access points for allosteric activation of the autoinhibited Vav1 Dbl homology domain. Biochemistry. 2005;44:15257–15268. doi: 10.1021/bi051126h. [DOI] [PubMed] [Google Scholar]
- 33.Li R, Zheng Y. Residues of the Rho family GTPases Rho and Cdc42 that specify sensitivity to Dbl-like guanine nucleotide exchange factors. J Biol Chem. 1997;272:4671–4679. doi: 10.1074/jbc.272.8.4671. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poppe D, Tiede I, Fritz G, Becker C, Bartsch B, Wirtz S, Strand D, Tanaka S, Galle PR, Bustelo XR, Neurath MF. Azathioprine suppresses ezrin-radixin-moesin-dependent T cell-APC conjugation through inhibition of Vav guanosine exchange activity on Rac proteins. J Immunol. 2006;176:640–651. doi: 10.4049/jimmunol.176.1.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atreya R, Atreya I, Neurath MF. Novel signal transduction pathways: analysis of STAT-3 and Rac-1 signaling in inflammatory bowel disease. Ann N Y Acad Sci. 2006;1072:98–113. doi: 10.1196/annals.1326.001. [DOI] [PubMed] [Google Scholar]
- 37.Arkin M. Protein-protein interactions and cancer: small molecules going in for the kill. Curr Opin Chem Biol. 2005;9:317–324. doi: 10.1016/j.cbpa.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Kabsch W. Evaluation of single-crystal X-ray diffraction data from a position-sensitive detector. J. Appl. Cryst. 1988;21:916–924. [Google Scholar]
- 39.Kabsch W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Cryst. 1993;26:795–800. [Google Scholar]
- 40.Sheldrick GM, Schneider TR, Charles WC, Jr, Robert MS. Methods in Enzymology. Vol. Volume 277. Academic Press; 1997. [16] SHELXL: High-resolution refinement; pp. 319–343. [PubMed] [Google Scholar]
- 41.de La Fortelle E, Bricogne G, Charles W, Carter , Jr . Methods in Enzymology. Vol. Volume 276. Academic Press; 1997. [27] Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods; pp. 472–494. [DOI] [PubMed] [Google Scholar]
- 42.Potterton E, Briggs P, Turkenburg M, Dodson E. A graphical user interface to the CCP4 program suite. Acta Crystallogr D Biol Crystallogr. 2003;59:1131–1137. doi: 10.1107/s0907444903008126. [DOI] [PubMed] [Google Scholar]
- 43.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 44.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 45.Lovell SC, Davis IW, Arendall WB, 3rd, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC. Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 46.DeLano WL. The PyMOL Molecular Graphics System. 2002 on World Wide Web http://www.pymol.org.
- 47.Konarev PV, Volkov VV, Sokolova AV, M.H.J K, Svergun DI. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J. Appl. Cryst. 2003;36:1277–1282. [Google Scholar]
- 48.Svergun DI, Petoukhov MV, Koch MH. Determination of domain structure of proteins from X-ray solution scattering. Biophys J. 2001;80:2946–2953. doi: 10.1016/S0006-3495(01)76260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]