Abstract
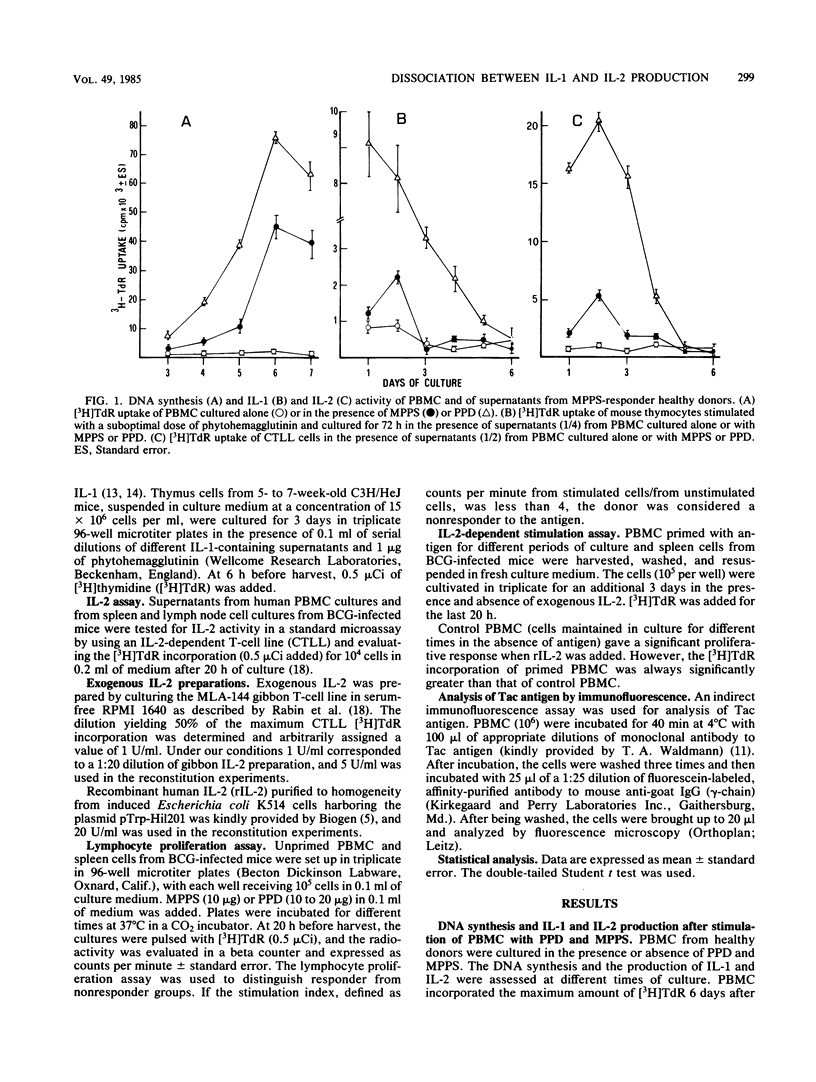
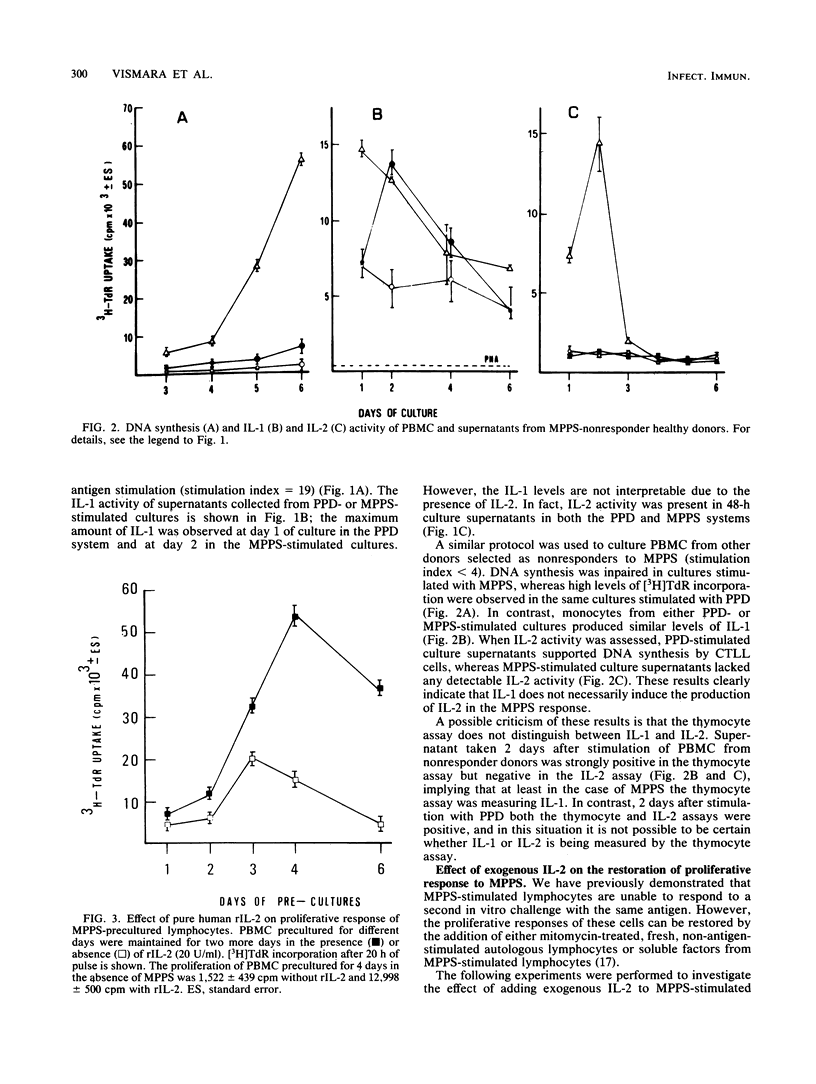
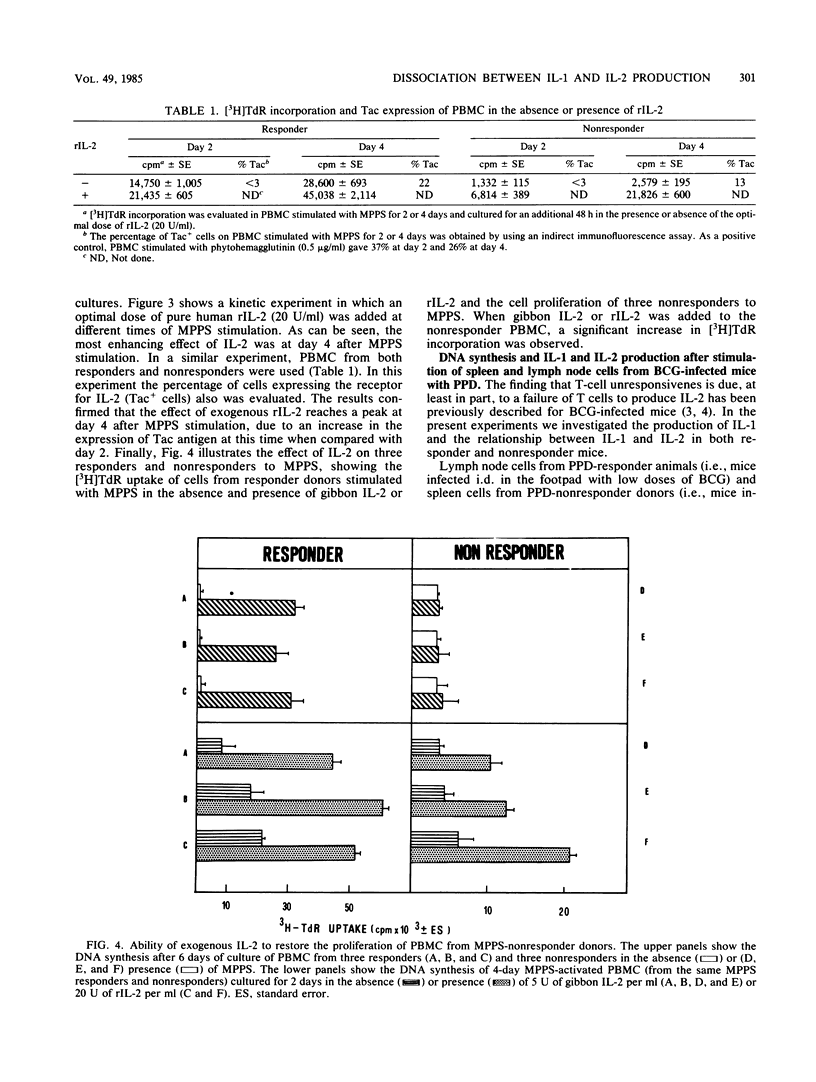
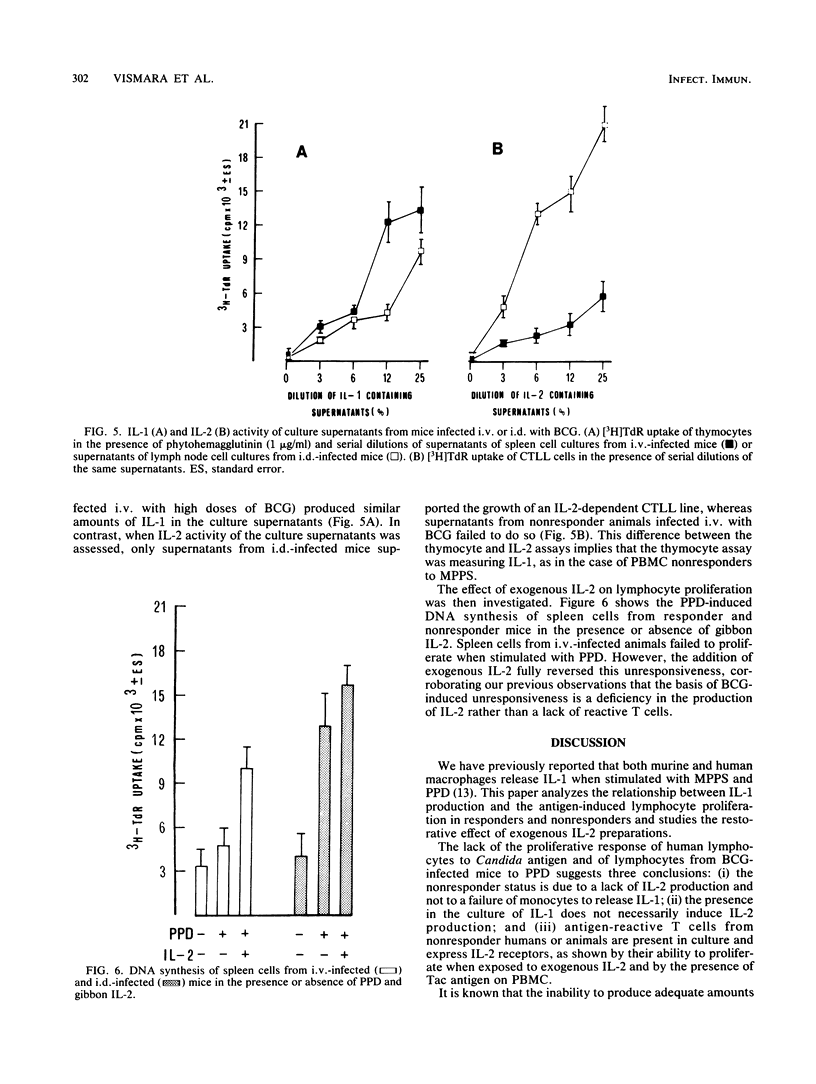
The relationship between the production of interleukin-1 (IL-1) and interleukin-2 (IL-2) after stimulation of human mononuclear cells within an antigenic extract from Candida albicans was analyzed in both responder and nonresponder donors. Culture supernatants from responders contained both IL-1 and IL-2 activity, whereas the supernatants from nonresponders contained only IL-1 and no appreciable IL-2. However, the addition of exogenous IL-2 to nonresponder cultures restored the normal proliferative response. Similar observations were made when cells from mice infected intravenously with high doses of Mycobacterium bovis BCG were cultured; these cells showed a marked impairment of the proliferative response to purified protein derivative. Spleen cells from BCG-induced unresponsive mice failed to produce IL-2 despite the fact that normal IL-1 activity was present in the culture. Again, the addition of exogenous IL-2 fully reversed the proliferative unresponsiveness. Thus, the presence of IL-1 does not necessarily induce production of IL-2, and the proliferative unresponsiveness is therefore due to a primary lack of IL-2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettens F., Kristensen F., Walker C., Schwuléra U., Bonnard G. D., de Weck A. L. Lymphokine regulation of activated (G1) lymphocytes. II. Glucocorticoid and anti-Tac-induced inhibition of human T lymphocyte proliferation. J Immunol. 1984 Jan;132(1):261–265. [PubMed] [Google Scholar]
- Bettens F., Kristensen F., Walker C., de Weck A. L. Human lymphocyte proliferation. II. Formation of activated (G1) cells. Eur J Immunol. 1982 Nov;12(11):948–952. doi: 10.1002/eji.1830121110. [DOI] [PubMed] [Google Scholar]
- Colizzi V., Ferluga J., Garreau F., Malkovsky M., Asherson G. L. Suppressor cells induced by BCG release non-specific factors in vitro which inhibit DNA synthesis and interleukin-2 production. Immunology. 1984 Jan;51(1):65–71. [PMC free article] [PubMed] [Google Scholar]
- Colizzi V. In vivo and in vitro administration of interleukin 2-containing preparation reverses T-cell unresponsiveness in Mycobacterium bovis BCG-infected mice. Infect Immun. 1984 Jul;45(1):25–28. doi: 10.1128/iai.45.1.25-28.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos R., Plaetinck G., Fiers W. Induction of cytolytic cells by pure recombinant human interleukin 2. Eur J Immunol. 1984 Nov;14(11):1057–1060. doi: 10.1002/eji.1830141118. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Godal T., Mustafa A. S., Belehu A., Yemaneberhan T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature. 1983 May 26;303(5915):342–344. doi: 10.1038/303342a0. [DOI] [PubMed] [Google Scholar]
- Harel-Bellan A., Joskowicz M., Fradelizi D., Eisen H. Modification of T-cell proliferation and interleukin 2 production in mice infected with Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3466–3469. doi: 10.1073/pnas.80.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffenbach A., Lagrange P. H., Bach M. A. Deficit of interleukin 2 production associated with impaired T-cell proliferative responses in Mycobacterium lepraemurium infection. Infect Immun. 1983 Jan;39(1):109–116. doi: 10.1128/iai.39.1.109-116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffenbach A., Lagrange P. H., Bach M. A. Influence of dose and route of Mycobacterium lepraemurium inoculation on the production of interleukin 1 and interleukin 2 in C57BL/6 mice. Infect Immun. 1984 Jun;44(3):665–671. doi: 10.1128/iai.44.3.665-671.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman L. B. Human interleukin 1: purification and properties. Fed Proc. 1983 Jun;42(9):2639–2645. [PubMed] [Google Scholar]
- Linker-Israeli M., Bakke A. C., Kitridou R. C., Gendler S., Gillis S., Horwitz D. A. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE). J Immunol. 1983 Jun;130(6):2651–2655. [PubMed] [Google Scholar]
- Lombardi G., Piccolella E., Vismara D., Colizzi V., Asherson G. L. Candida albicans polysaccharide extract (MPPS) and PPD stimulate the production of interleukin-1 and lymphocyte proliferation. Clin Exp Immunol. 1984 Dec;58(3):581–586. [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Piccolella E., Lombardi G., Morelli R. Generation of suppressor cells in the response of human lymphocytes to a polysaccharide from Candida albicans. J Immunol. 1981 Jun;126(6):2151–2155. [PubMed] [Google Scholar]
- Piccolella E., Lombardi G., Morelli R. Human lymphocyte-activating properties of a purified polysaccharide from Candida albicans: B and T cell cooperation in the mitogenic response. J Immunol. 1980 Nov;125(5):2082–2088. [PubMed] [Google Scholar]
- Piccolella E., Lombardi G., Morelli R. Mitogenic response of human peripheral blood lymphocytes to a purified C. albicans polysaccharide fraction: lack of helper activities is responsible for the in vitro unresponsiveness to a second antigenic challenge. J Immunol. 1981 Jun;126(6):2156–2160. [PubMed] [Google Scholar]
- Thoman M. L., Weigle W. O. Cell-mediated immunity in aged mice: an underlying lesion in IL 2 synthesis. J Immunol. 1982 May;128(5):2358–2361. [PubMed] [Google Scholar]
- Wagner H., Hardt C., Heeg K., Röllinghoff M., Pfizenmaier K. T-cell-derived helper factor allows in vivo induction of cytotoxic T cells in nu/nu mice. Nature. 1980 Mar 20;284(5753):278–278. doi: 10.1038/284278a0. [DOI] [PubMed] [Google Scholar]
- Watson S., Bullock W., Nelson K., Schauf V., Gelber R., Jacobson R. Interleukin 1 production by peripheral blood mononuclear cells from leprosy patients. Infect Immun. 1984 Sep;45(3):787–789. doi: 10.1128/iai.45.3.787-789.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


