Abstract
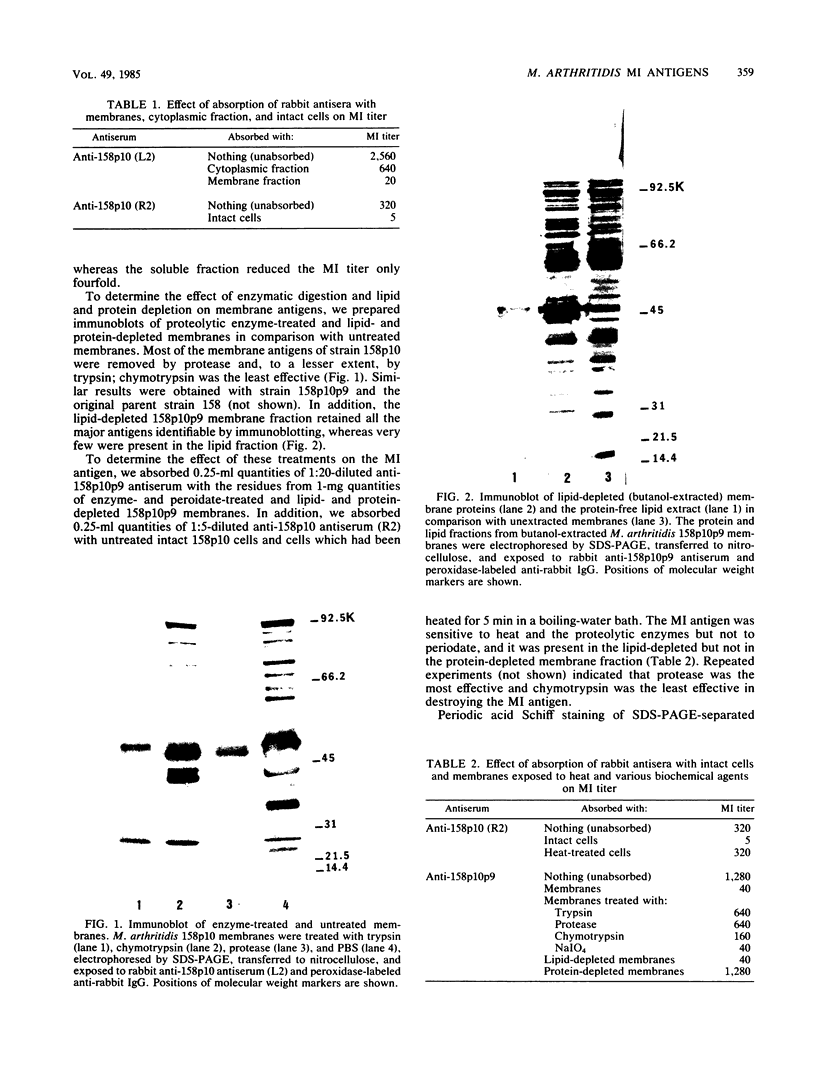
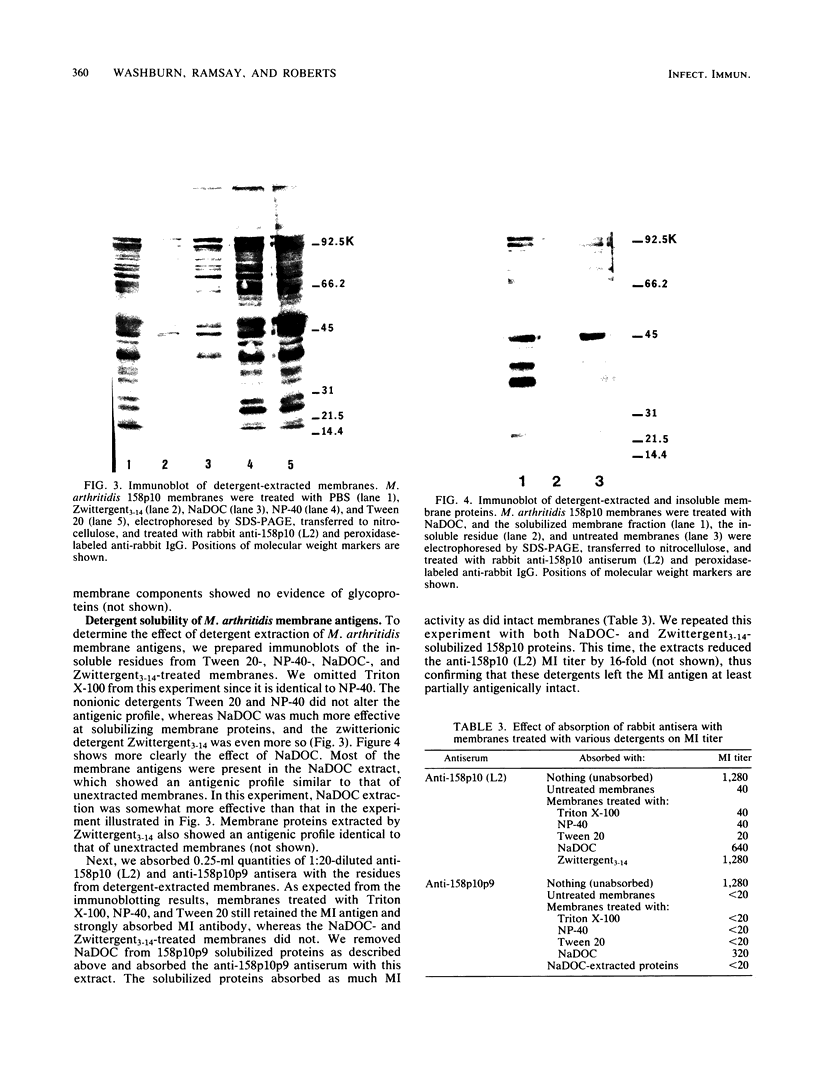
The Mycoplasma arthritidis antigen(s) responsible for eliciting metabolism-inhibiting antibodies in rabbits has been partially characterized. Metabolism-inhibiting activity was absorbed from rabbit antisera by intact M. arthritidis cells and membranes but much less so by the soluble cytoplasmic fraction, indicating that the antigen is located on the outer membrane surface. It was stable to periodate and lipid extraction but labile to heat and proteolytic enzymes, indicating that it is protein in nature. Finally, it is most likely a tightly bound integral rather than a peripheral membrane protein, since it was not extracted by low-ionic-strength solutions or by the nonionic detergents Triton X-100, Nonidet P-40, and Tween 20. It was solubilized by both the anionic agent sodium deoxycholate and the zwitterionic detergent Zwittergent. Two two monoclonal antibodies with metabolism-inhibiting activity were produced. One recognized a 45,000-dalton surface protein; however, the other recognized an antigen which is probably of cytoplasmic origin, indicating that more than one cell component may be involved in the metabolism-inhibiting antibody response.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer D. B., Rodwell A. W. Membrane proteins of Mycoplasma gallisepticum. J Bacteriol. 1982 Sep;151(3):1598–1601. doi: 10.1128/jb.151.3.1598-1601.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaman M., Razin S. Antigenic properties of mycoplasma organisms and membranes. J Gen Microbiol. 1969 Jan;55(1):45–58. doi: 10.1099/00221287-55-1-45. [DOI] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Bradbury J. M., Jordan F. T. Studies on the adsorption of certain medium proteins to Mycoplasma gallisepticum and their influence on agglutination and haemagglutination reactions. J Hyg (Lond) 1972 Jun;70(2):267–278. doi: 10.1017/s0022172400022324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery S. H. Hapten inhibition of the reaction between Mycoplasma mycoides polysaccharide and bovine antisera. Immunochemistry. 1970 Mar;7(3):305–310. doi: 10.1016/0019-2791(70)90168-0. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill J. F., Cole B. C., Wiley B. B., Ward J. R. Role of Biological Mimicry in the Pathogenesis of Rat Arthritis Induced by Mycoplasma arthritidis. Infect Immun. 1971 Jan;3(1):24–35. doi: 10.1128/iai.3.1.24-35.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Cahill J. F., Wiley B. B., Ward J. R. Immunological responses of the rat to Mycoplasma arthritidis. J Bacteriol. 1969 Jun;98(3):930–937. doi: 10.1128/jb.98.3.930-937.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Golightly-Rowland L., Ward J. R. Arthritis of mice induced by Mycoplasma pulmonis: humoral antibody and lymphocyte responses of CBA mice. Infect Immun. 1975 Nov;12(5):1083–1092. doi: 10.1128/iai.12.5.1083-1092.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Golightly-Rowland L., Ward J. R., Wiley B. B. Immunological response of rodents to murine mycoplasmas. Infect Immun. 1970 Oct;2(4):419–425. doi: 10.1128/iai.2.4.419-425.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Jones R. S., Cahill J. F. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. I. Induction of disease and histopathological characteristics. Infect Immun. 1971 Oct;4(4):344–355. doi: 10.1128/iai.4.4.344-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. J Immunol. 1976 Jul;117(1):136–142. [PubMed] [Google Scholar]
- Goel M. C., Lemcke R. M. Dissociation of Mycoplasma gallisepticum membranes with lithium diiodosalicylate and isolation of glycoprotein. Ann Microbiol (Paris) 1975 Oct-Nov;126(3):299–312. [PubMed] [Google Scholar]
- Goel M. C. New method for the isolation of membranes from Mycoplasma gallisepticum. J Bacteriol. 1973 Nov;116(2):994–1000. doi: 10.1128/jb.116.2.994-1000.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golightly-Rowland L., Cole B. C., Ward J. R., Wiley B. B. Effect of Animal Passage on Arthritogenic and Biological Properties of Mycoplasma arthritidis. Infect Immun. 1970 Jun;1(6):538–545. doi: 10.1128/iai.1.6.538-545.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hollingdale M. R., Lemcke R. M. Membrane antigens of Mycoplasma hominis. J Hyg (Lond) 1972 Mar;70(1):85–98. doi: 10.1017/s0022172400022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. S., Tittensor J. R., Walker R. T. The chemical composition of the nucleic acids and other macromolecular constituents of Mycoplasma mycoides var. capri. J Gen Microbiol. 1965 Sep;40(3):405–411. doi: 10.1099/00221287-40-3-405. [DOI] [PubMed] [Google Scholar]
- Kahane I., Brunner H. Isolation of a glycoprotein from Mycoplasma pneumoniae membranes. Infect Immun. 1977 Nov;18(2):273–277. doi: 10.1128/iai.18.2.273-277.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett R. H. Cell fusion. Methods Enzymol. 1979;58:345–359. doi: 10.1016/s0076-6879(79)58149-x. [DOI] [PubMed] [Google Scholar]
- Kenny G. E. Heat-lability and organic solvent-solubility of mycoplasma antigens. Ann N Y Acad Sci. 1967 Jul 28;143(1):676–681. doi: 10.1111/j.1749-6632.1967.tb27713.x. [DOI] [PubMed] [Google Scholar]
- Krause D. C., Baseman J. B. Inhibition of mycoplasma pneumoniae hemadsorption and adherence to respiratory epithelium by antibodies to a membrane protein. Infect Immun. 1983 Mar;39(3):1180–1186. doi: 10.1128/iai.39.3.1180-1186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LEMCKE R. M. THE SEROLOGICAL DIFFERENTIATION OF MYCOPLASMA STRAINS (PLEURO-PNEUMONIA-LIKE ORGANISMS) FROM VARIOUS SOURCES. J Hyg (Lond) 1964 Jun;62:199–219. doi: 10.1017/s0022172400039930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemcke R. M., Marmion B. P., Plackett P. Immunochemical analysis of Mycoplasma pneumoniae. Ann N Y Acad Sci. 1967 Jul 28;143(1):691–702. doi: 10.1111/j.1749-6632.1967.tb27715.x. [DOI] [PubMed] [Google Scholar]
- Lin J. S. Complement-mediated killing of Ureaplasma urealyticum by antibody directed against components of the growth medium. J Med Microbiol. 1982 Feb;15(1):135–140. doi: 10.1099/00222615-15-1-135. [DOI] [PubMed] [Google Scholar]
- Nicolet J., Paroz P., Kristensen B. Growth medium constituents contaminating mycoplasma preparations and their role in the study of membrane glycoproteins in porcine mycoplasmas. J Gen Microbiol. 1980 Jul;119(1):17–26. doi: 10.1099/00221287-119-1-17. [DOI] [PubMed] [Google Scholar]
- Purcell R. H., Taylor-Robinson D., Wong D. C., Chanock R. M. A color test for the measurement of antibody to the non-acid-forming human Mycoplasma species. Am J Epidemiol. 1966 Jul;84(1):51–66. doi: 10.1093/oxfordjournals.aje.a120627. [DOI] [PubMed] [Google Scholar]
- Ryan M. D., Noker P., Matz L. L. Immunological properties of glycolipids from membranes of Acholeplasma laidlawii. Infect Immun. 1975 Oct;12(4):799–807. doi: 10.1128/iai.12.4.799-807.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeslavský O., Prescott B., James W. D., Chanock R. M. Serological and immunogenic activities of different fractions of Mycoplasma pneumoniae. Ann N Y Acad Sci. 1967 Jul 28;143(1):682–690. doi: 10.1111/j.1749-6632.1967.tb27714.x. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Smith P. F., Langworthy T. A., Mayberry W. R. Immunological analysis of glycolipids and lipopolysaccharides derived from various mycoplasmas. Infect Immun. 1974 Dec;10(6):1273–1279. doi: 10.1128/iai.10.6.1273-1279.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Berry D. M. The evaluation of the metabolic-inhibition technique for the study of Mycoplasma gallisepticum. J Gen Microbiol. 1969 Jan;55(1):127–137. doi: 10.1099/00221287-55-1-127. [DOI] [PubMed] [Google Scholar]
- Thorns C. J., Boughton E. Studies on the effect of growth medium composition on the antigenicity of Mycoplasma bovis. J Hyg (Lond) 1980 Feb;84(1):29–36. doi: 10.1017/s0022172400026486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn L. R. Spectrophotometric method for determining titers of antimycoplasma metabolism inhibition antibody. Appl Environ Microbiol. 1983 Jan;45(1):343–346. doi: 10.1128/aem.45.1.343-346.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann B. J., Ross R. F. Modified metabolic-inhibition test for detection of antibodies to Mycoplasma hyosynoviae in swine serum. Am J Vet Res. 1977 Dec;38(12):2075–2076. [PubMed] [Google Scholar]