Abstract
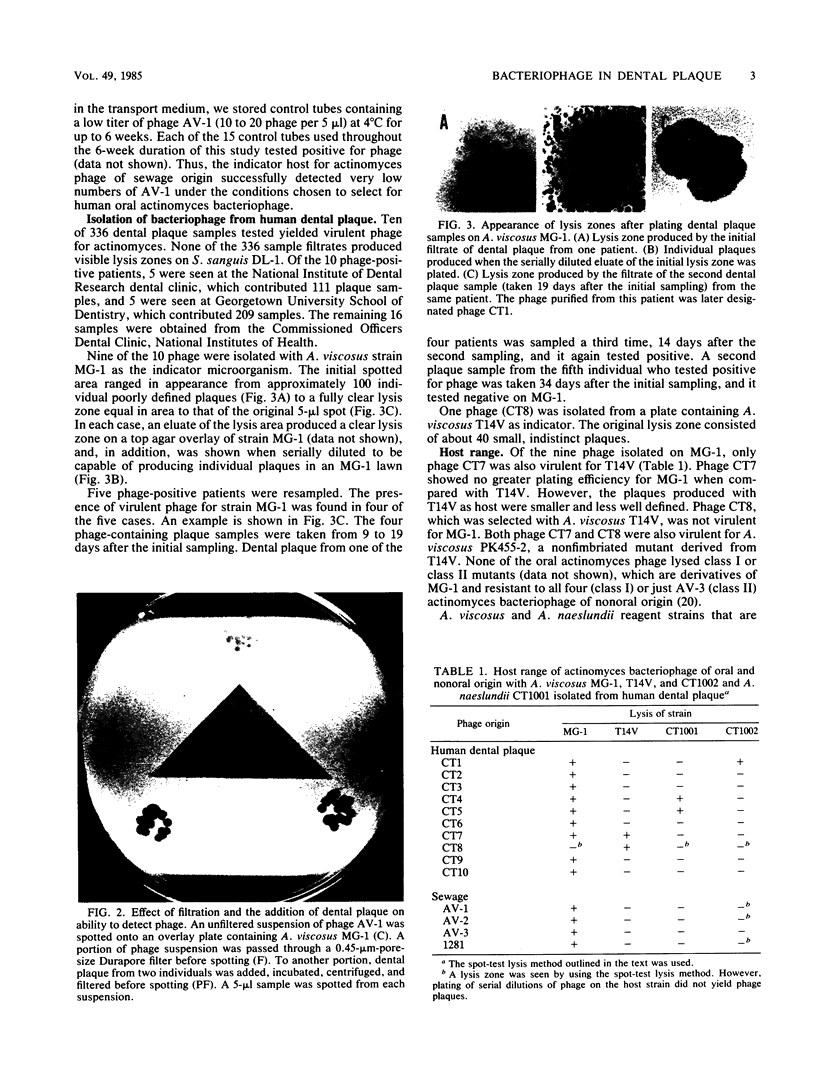
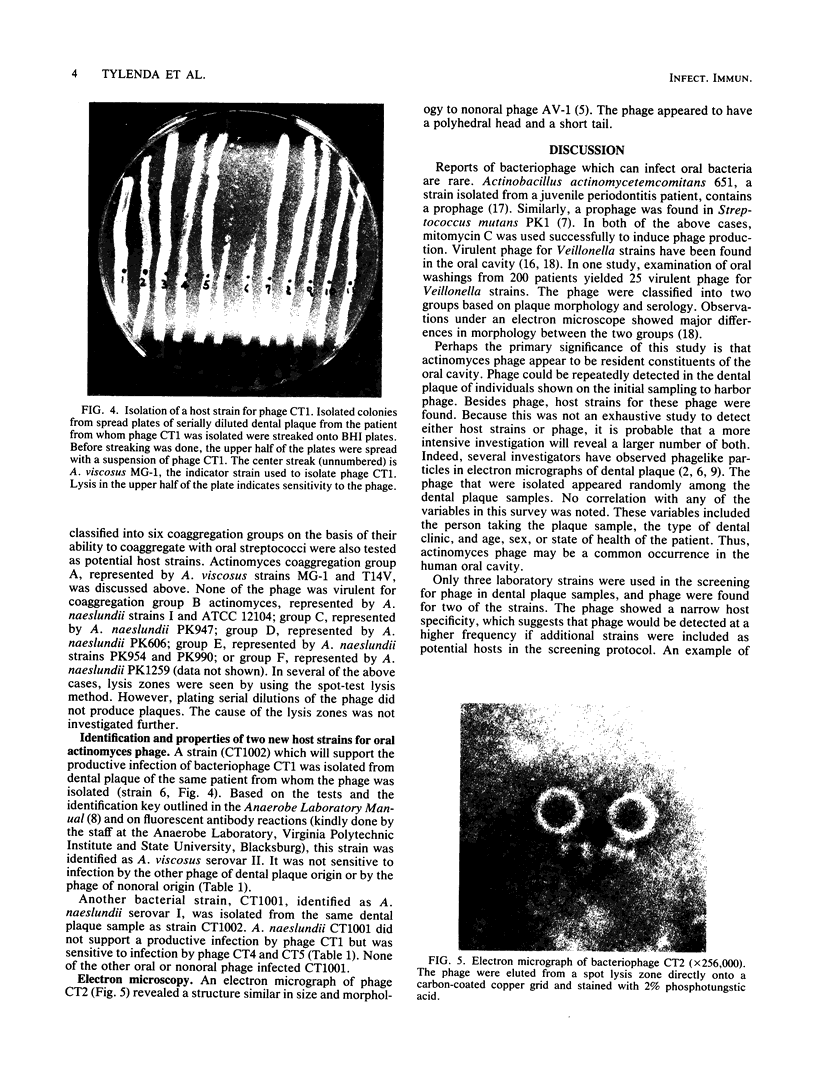
Human dental plaque samples were screened for the presence of bacteriophage for Actinomyces viscosus and Streptococcus sanguis. None of the 336 samples yielded phage for S. sanguis, but 10 contained virulent actinomyces phage. A high host cell specificity was observed in that one phage isolate infected only A. viscosus T14V, eight phage isolates infected only A. viscosus MG-1, and one infected both strains. None was capable of productively infecting various other actinomyces strains that represented the six actinomyces coaggregation groups. Because phage-containing samples occurred randomly in this survey, no correlation between the individual collecting the samples, dental clinic, or type of patient and the presence of phage in the sample was noted. Examination of one of the samples that yielded phage for the presence of a natural host strain for that particular phage resulted in the isolation of two strains which were identified as A. viscosus serotype II and Actinomyces naeslundii serotype I. This is the first report of an A. naeslundii host strain and actinomyces bacteriophage of human dental plaque origin. The finding of both phage and host strains in the same dental plaque sample along with the observation of high host cell specificity by these phage provide indicators that support an active role for actinomyces bacteriophage in oral microbial ecology. The use of these freshly isolated phage as probes to study actinomyces coaggregation properties is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady J. M., Gray W. A., Caldwell M. A. The electron microscopy of bacteriophage-like particles in dental plaque. J Dent Res. 1977 Aug;56(8):991–993. doi: 10.1177/00220345770560082901. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Barsumian E. L., Curl S. H., Vatter A. E., Sandberg A. L., Siraganian R. P. Detection and localization of a lectin on Actinomyces viscosus T14V by monoclonal antibodies. J Immunol. 1981 Oct;127(4):1318–1322. [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisle A. L., Nauman R. K., Minah G. E. Isolation of a bacteriophage for actinomyces viscosus. Infect Immun. 1978 Apr;20(1):303–306. doi: 10.1128/iai.20.1.303-306.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halhoul N., Colvin J. R. Virus-like particles in association with a microorganism from human gingival plaque. Arch Oral Biol. 1975 Dec;20(12):833–836. doi: 10.1016/0003-9969(75)90062-x. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Rhee G. H., Araya S., Higuchi M. Bacteriophage deoxyribonucleic acid-induced mutation of Streptococcus mutans. Infect Immun. 1977 Mar;15(3):938–944. doi: 10.1128/iai.15.3.938-944.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerebel B., Clergeau-Guerithault S., Forlot P. Etude ultrastructurale de plaques microbiennes dentaires chez des sujets indemnes de carie. Ann Microbiol (Paris) 1975 Feb-Mar;126(2):203–229. [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Cell to cell interactions of Capnocytophaga and Bacteroides species with other oral bacteria and their potential role in development of plaque. J Periodontal Res. 1984 Nov;19(6):564–569. doi: 10.1111/j.1600-0765.1984.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N., Holdeman L. V. Coaggregation of oral Bacteroides species with other bacteria: central role in coaggregation bridges and competitions. Infect Immun. 1985 Jun;48(3):741–746. doi: 10.1128/iai.48.3.741-746.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E. Isolation and characterization of coaggregation-defective mutants of Actinomyces viscosus, Actinomyces naeslundii, and Streptococcus sanguis. Infect Immun. 1982 Sep;37(3):1200–1208. doi: 10.1128/iai.37.3.1200-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Prevalence of viridans streptococci exhibiting lactose-inhibitable coaggregation with oral actinomycetes. Infect Immun. 1983 Aug;41(2):449–452. doi: 10.1128/iai.41.2.449-452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y. [Experimental studies on the bacterio phages of the Veillonella strains isolated from the oral cavity]. Shigaku. 1968 Mar;55(4):533–541. [PubMed] [Google Scholar]
- Stevens R. H., Hammond B. F., Lai C. H. Characterization of an inducible bacteriophage from a leukotoxic strain of Actinobacillus actinomycetemcomitans. Infect Immun. 1982 Jan;35(1):343–349. doi: 10.1128/iai.35.1.343-349.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsuka M. Studies on veillonellophages isolated from washings of human oral cavity. Bull Tokyo Med Dent Univ. 1976 Dec;23(4):261–273. [PubMed] [Google Scholar]
- Tylenda C. A., Enriquez E., Kolenbrander P. E., Delisle A. L. Simultaneous loss of bacteriophage receptor and coaggregation mediator activities in Actinomyces viscosus MG-1. Infect Immun. 1985 Apr;48(1):228–233. doi: 10.1128/iai.48.1.228-233.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylenda C. A., Kolenbrander P. E., Delisle A. L. Use of bacteriophage-resistant mutants to study Actinomyces viscosus cell surface receptors. J Dent Res. 1983 Nov;62(11):1179–1181. doi: 10.1177/00220345830620111801. [DOI] [PubMed] [Google Scholar]







