Abstract
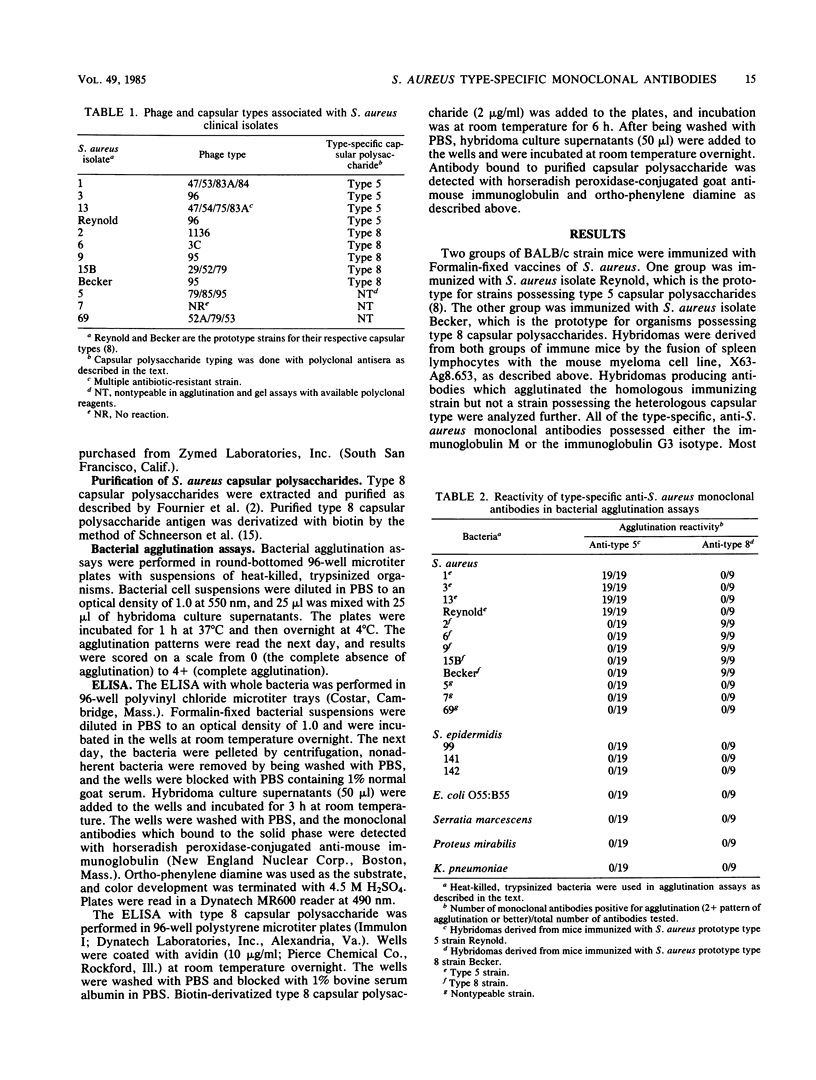
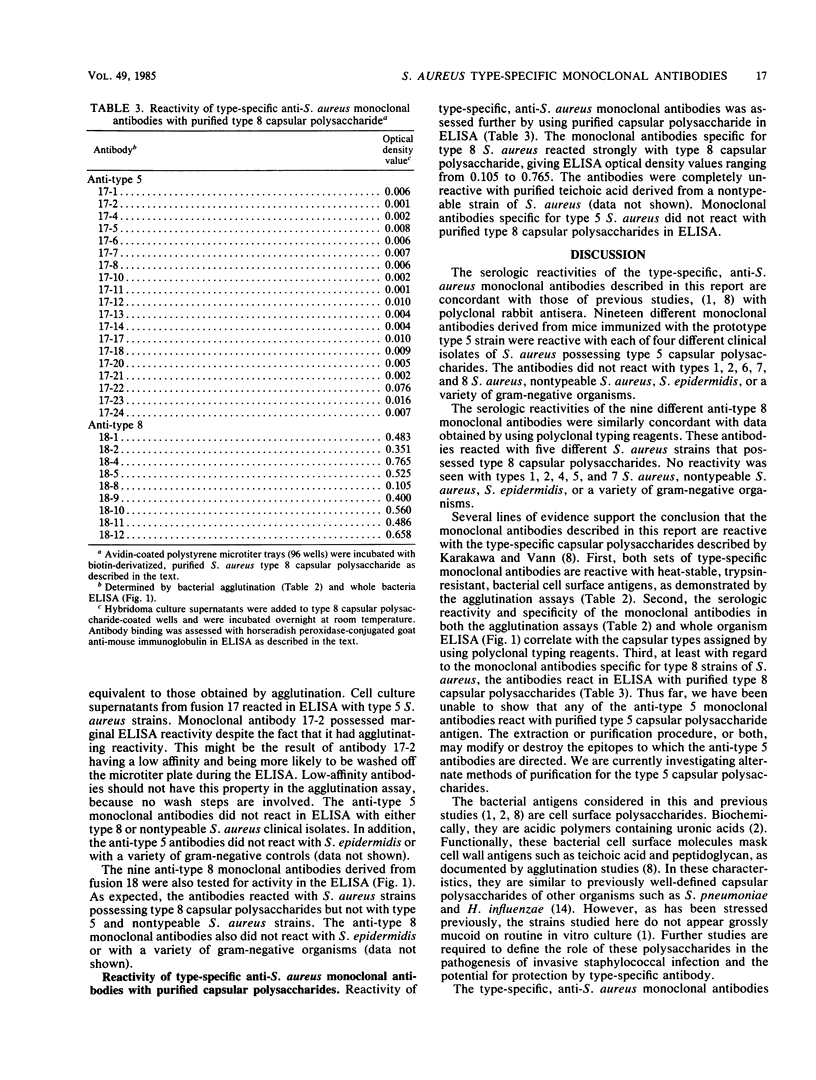
Staphylococcus aureus has been classified into at least eight different capsular types by using polyclonal rabbit antisera specific for their associated capsular polysaccharides. We produced and characterized monoclonal antibodies reactive with two serologically distinct capsular types, types 5 and 8, which account for more than 70% of all S. aureus bacteremias. These type-specific, monoclonal antibodies reacted with S. aureus clinical isolates possessing the homologous capsular type and exhibited no cross-reactivity against S. aureus clinical isolates possessing the heterologous capsular type, nontypeable S. aureus clinical isolates, Staphylococcus epidermidis clinical isolates, or a variety of gram-negative organisms. The anti-type 8 monoclonal antibodies also reacted with purified capsular polysaccharide derived from the prototype type 8 S. aureus strain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeit R. D., Karakawa W. W., Vann W. F., Robbins J. B. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis. 1984 Apr;2(2):85–91. doi: 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- Fournier J. M., Vann W. F., Karakawa W. W. Purification and characterization of Staphylococcus aureus type 8 capsular polysaccharide. Infect Immun. 1984 Jul;45(1):87–93. doi: 10.1128/iai.45.1.87-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Kaplan M. H., Tenenbaum M. J. Staphylococcus aureus: cellular biology and clinical application. Am J Med. 1982 Feb;72(2):248–258. doi: 10.1016/0002-9343(82)90817-8. [DOI] [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A. Characterization of the surface antigens of Staphylococcus aureus, strain K-93M. J Immunol. 1972 May;108(5):1199–1208. [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A. Immunochemical analysis of a Smith-like antigen isolated from two human strains of Staphylococcus aureus. J Immunol. 1975 Aug;115(2):564–568. [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A., Smith M. R. Isolation of an acidic surface antigen from a conventional strain of Staphylococcus aureus. Infect Immun. 1974 Mar;9(3):511–518. doi: 10.1128/iai.9.3.511-518.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Young D. A. Immunochemical study of diverse surface antigens of a Staphylococcus aureus isolate from an osteomyelitis patient and their role in in vitro phagocytosis. J Clin Microbiol. 1979 Mar;9(3):399–408. doi: 10.1128/jcm.9.3.399-408.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Young D. A., Kane J. A. Structural analysis of the cellular constituents of a fresh clinical isolate of Staphylococcus aureus, and their role in the interaction between the organisms and polymorphonuclear leukocytes in the presence of serum factors. Infect Immun. 1978 Aug;21(2):496–505. doi: 10.1128/iai.21.2.496-505.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Hansburg D., Briles D. E., Nicolotti R. A., Davie J. M. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978 Aug;121(2):566–572. [PubMed] [Google Scholar]
- SMITH J. M., DUBOS R. J. The behavior of virulent and avirulent staphylococci in the tissues of normal mice. J Exp Med. 1956 Jan 1;103(1):87–108. doi: 10.1084/jem.103.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. C. A capsulate Staphylococcus aureus. J Med Microbiol. 1969 Aug;2(3):253–260. doi: 10.1099/00222615-2-3-253. [DOI] [PubMed] [Google Scholar]
- Sheagren J. N. Staphylococcus aureus. The persistent pathogen (first of two parts). N Engl J Med. 1984 May 24;310(21):1368–1373. doi: 10.1056/NEJM198405243102107. [DOI] [PubMed] [Google Scholar]
- Wu T. C., Park J. T. Chemical characterization of a new surface antigenic polysaccharide from a mutant of Staphylococcus aureus. J Bacteriol. 1971 Nov;108(2):874–884. doi: 10.1128/jb.108.2.874-884.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


