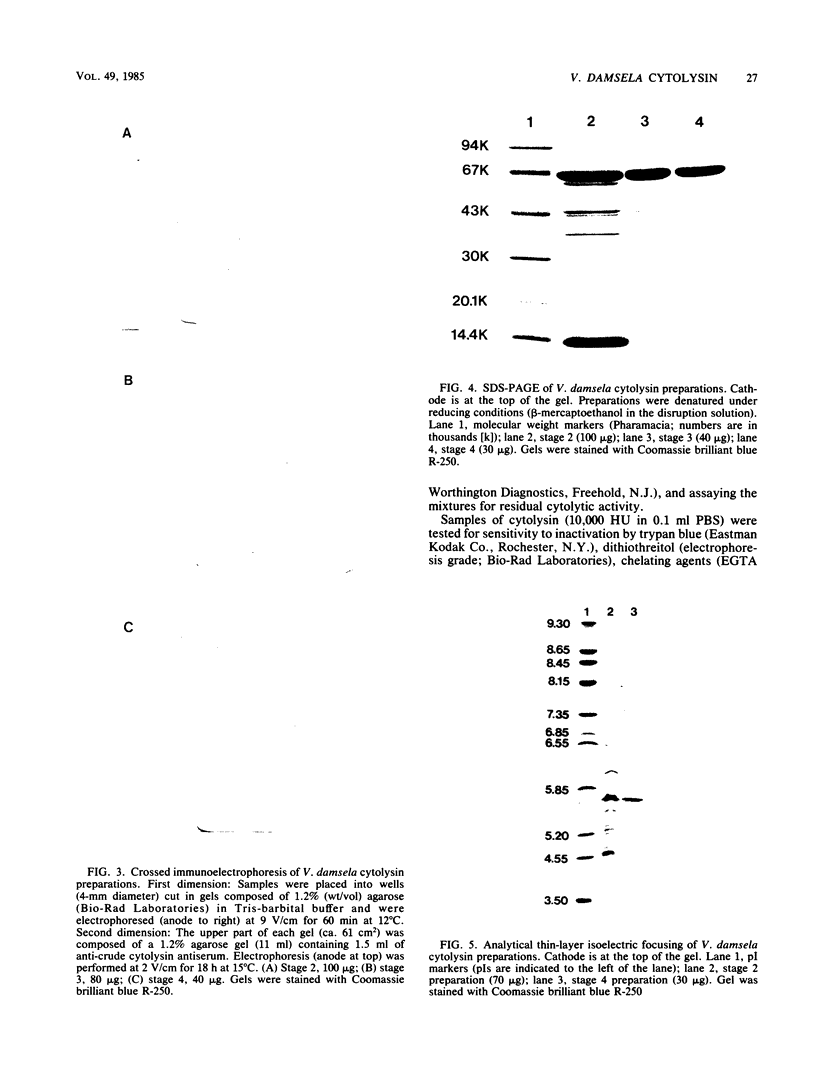
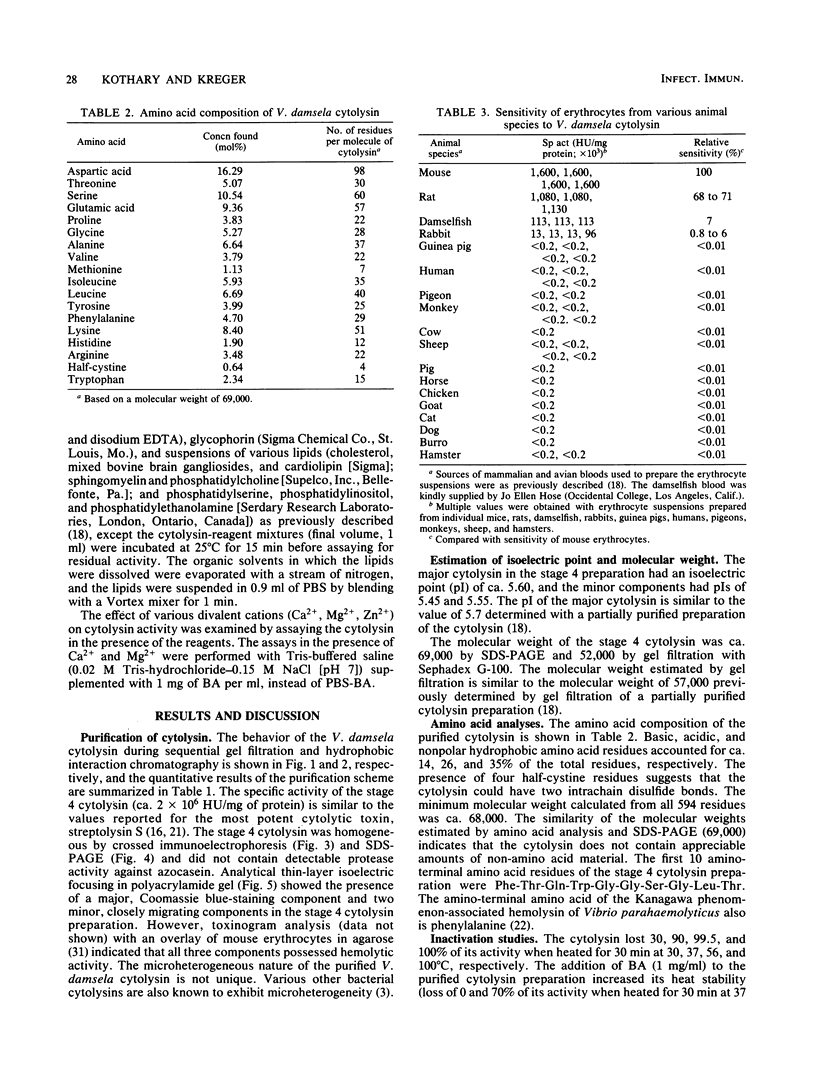
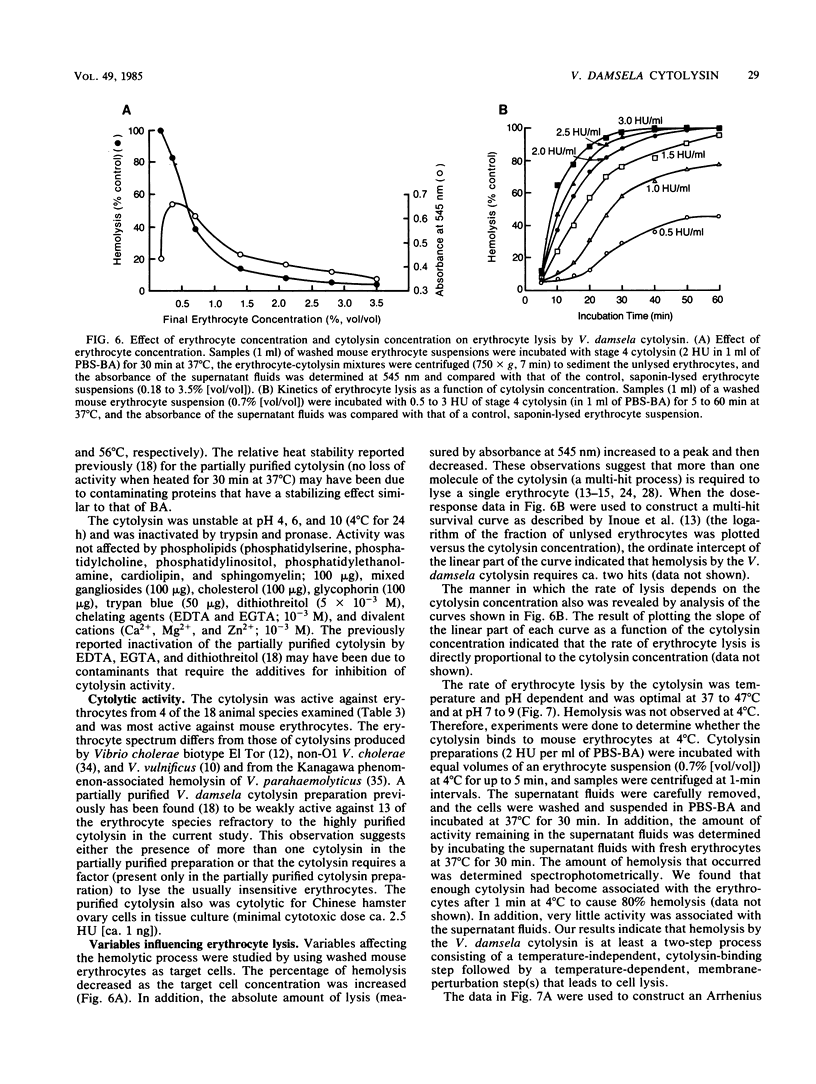
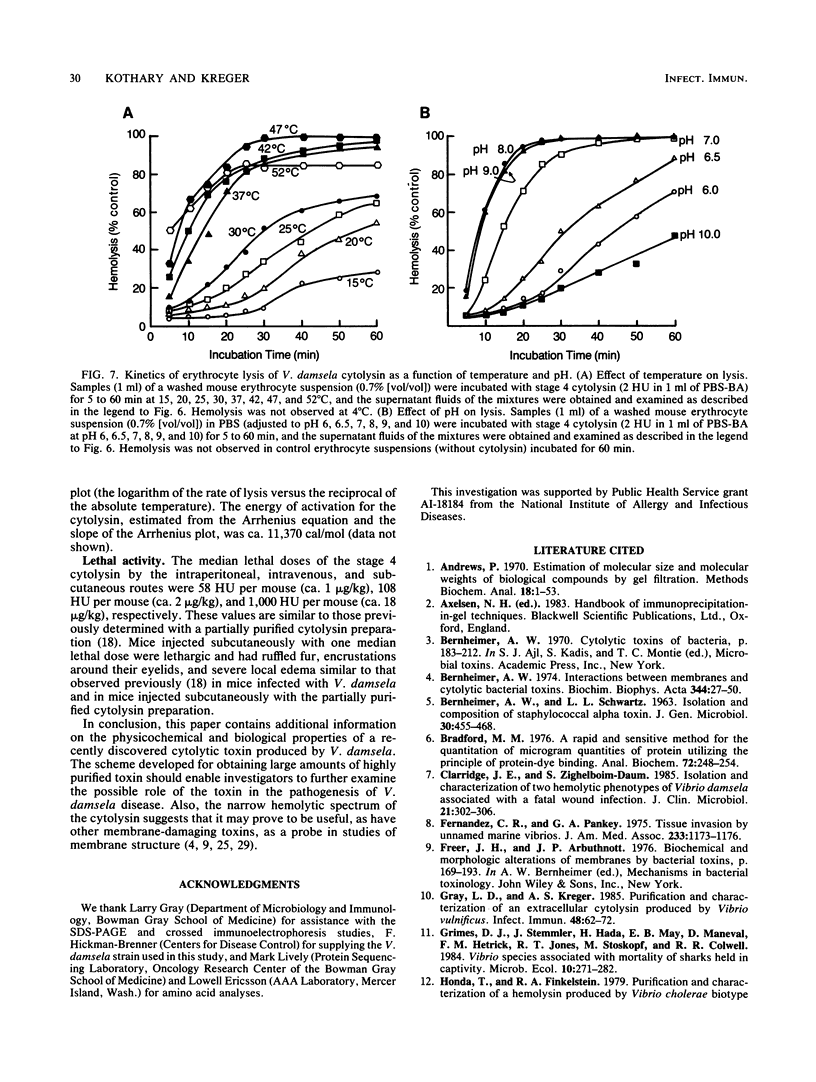
Abstract
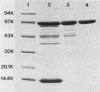
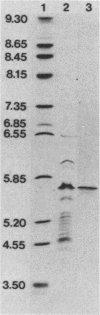
Large amounts of an extremely potent extracellular cytolysin produced by the halophilic bacterium Vibrio damsela were obtained free of detectable contamination with medium constituents and other bacterial products by sequential ammonium sulfate precipitation, gel filtration with Sephadex G-100, and hydrophobic interaction chromatography with phenyl-Sepharose CL-4B. The cytolysin is heat labile and protease sensitive and has a molecular weight (estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis) of ca. 69,000 and an isoelectric point of ca. 5.6. The first 10 amino-terminal amino acid residues of the cytolysin are Phe-Thr-Gln-Trp-Gly-Gly-Ser-Gly-Leu-Thr. The cytolysin was very active against erythrocytes from 4 of the 18 animal species examined (mice, rats, rabbits, damselfish) and against Chinese hamster ovary cells and was lethal for mice (ca. 1 microgram/kg, intraperitoneal median lethal dose). Lysis of mouse erythrocytes by the cytolysin is a multi-hit, at least two-step process consisting of a temperature-independent, toxin-binding step followed by a temperature-dependent, membrane-perturbation step(s).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clarridge J. E., Zighelboim-Daum S. Isolation and characterization of two hemolytic phenotypes of Vibrio damsela associated with a fatal wound infection. J Clin Microbiol. 1985 Mar;21(3):302–306. doi: 10.1128/jcm.21.3.302-306.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C. R., Pankey G. A. Tissue invasion by unnamed marine vibrios. JAMA. 1975 Sep 15;233(11):1173–1176. [PubMed] [Google Scholar]
- Gray L. D., Kreger A. S. Purification and characterization of an extracellular cytolysin produced by Vibrio vulnificus. Infect Immun. 1985 Apr;48(1):62–72. doi: 10.1128/iai.48.1.62-72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Finkelstein R. A. Purification and characterization of a hemolysin produced by Vibrio cholerae biotype El Tor: another toxic substance produced by cholera vibrios. Infect Immun. 1979 Dec;26(3):1020–1027. doi: 10.1128/iai.26.3.1020-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Akiyama Y., Kinoshita T., Higashi Y., Amano T. Evidence for a one-hit theory in the immune bactericidal reaction and demonstration of a multi-hit response for hemolysis by streptolysin O and Clostridium perfringens theta-toxin. Infect Immun. 1976 Feb;13(2):337–344. doi: 10.1128/iai.13.2.337-344.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K., Boese-Marrazzo D. Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect Immun. 1980 Sep;29(3):1028–1033. doi: 10.1128/iai.29.3.1028-1033.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K. Properties of purified pneumococcal hemolysin. Infect Immun. 1972 Nov;6(5):755–760. doi: 10.1128/iai.6.5.755-760.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOYAMA J., EGAMI F. Biochemical studies on streptolysin S' formed in the presence of yeast ribonucleic acid. I. The purification and some properties of the toxin. J Biochem. 1963 Feb;53:147–154. doi: 10.1093/oxfordjournals.jbchem.a127670. [DOI] [PubMed] [Google Scholar]
- Kreger A. S. Cytolytic activity and virulence of Vibrio damsela. Infect Immun. 1984 May;44(2):326–331. doi: 10.1128/iai.44.2.326-331.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger A. S., Gray L. D. Purification of Pseudomonas aeruginosa proteases and microscopic characterization of pseudomonal protease-induced rabbit corneal damage. Infect Immun. 1978 Feb;19(2):630–648. doi: 10.1128/iai.19.2.630-648.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger A., Lockwood D. Detection of extracellular toxin(s) produced by Vibrio vulnificus. Infect Immun. 1981 Aug;33(2):583–590. doi: 10.1128/iai.33.2.583-590.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. Y., Wang M. T., de Faria J. B., Akao T. Streptolysin S: improved purification and characterization. Arch Biochem Biophys. 1978 Dec;191(2):804–812. doi: 10.1016/0003-9861(78)90423-x. [DOI] [PubMed] [Google Scholar]
- Love M., Teebken-Fisher D., Hose J. E., Farmer J. J., 3rd, Hickman F. W., Fanning G. R. Vibrio damsela, a Marine Bacterium, Causes Skin Ulcers on the Damselfish Chromis punctipinnis. Science. 1981 Dec 4;214(4525):1139–1140. doi: 10.1126/science.214.4525.1139. [DOI] [PubMed] [Google Scholar]
- Marchlewicz B. A., Duncan J. L. Lysis of erythrocytes by a hemolysin produced by a group B Streptococcus sp. Infect Immun. 1981 Dec;34(3):787–794. doi: 10.1128/iai.34.3.787-794.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G., Jr, Black R. E. Cholera and other vibrioses in the United States. N Engl J Med. 1985 Feb 7;312(6):343–350. doi: 10.1056/NEJM198502073120604. [DOI] [PubMed] [Google Scholar]
- Morris J. G., Jr, Miller H. G., Wilson R., Tacket C. O., Hollis D. G., Hickman F. W., Weaver R. E., Blake P. A. Illness caused by Vibrio damsela and Vibrio hollisae. Lancet. 1982 Jun 5;1(8284):1294–1297. doi: 10.1016/s0140-6736(82)92853-7. [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Duncan J. L. Characteristics of streptolysin O action. Infect Immun. 1971 Dec;4(6):683–687. doi: 10.1128/iai.4.6.683-687.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tison D. L., Kelly M. T. Vibrio species of medical importance. Diagn Microbiol Infect Dis. 1984 Sep;2(4):263–276. doi: 10.1016/0732-8893(84)90057-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Al-Omani M., Honda T., Takeda Y., Miwatani T. Non-O1 Vibrio cholerae hemolysin: purification, partial characterization, and immunological relatedness to El Tor hemolysin. Infect Immun. 1984 Jul;45(1):192–196. doi: 10.1128/iai.45.1.192-196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen-Yoji H., Hitokoto H., Morozumi S., Le Clair R. A. Purification and characterization o;f a hemolysin produced by Vibrio parahaemolyticus. J Infect Dis. 1971 Jun;123(6):665–667. doi: 10.1093/infdis/123.6.665. [DOI] [PubMed] [Google Scholar]