Abstract
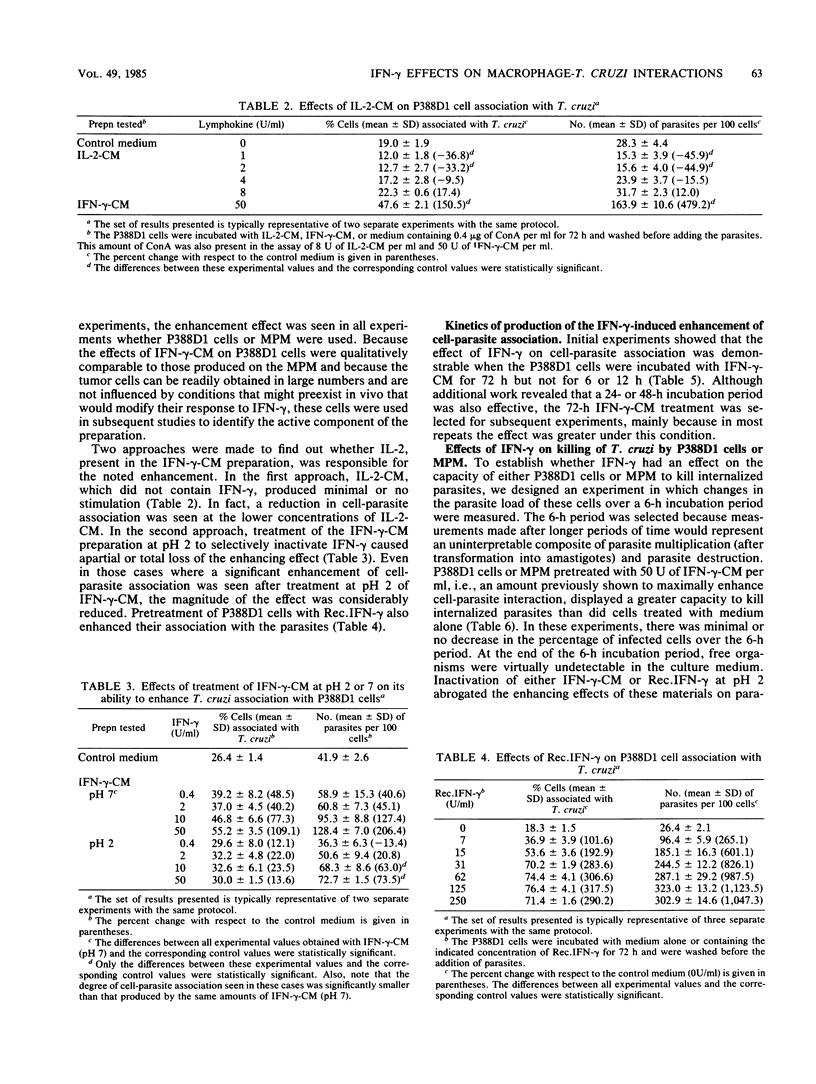
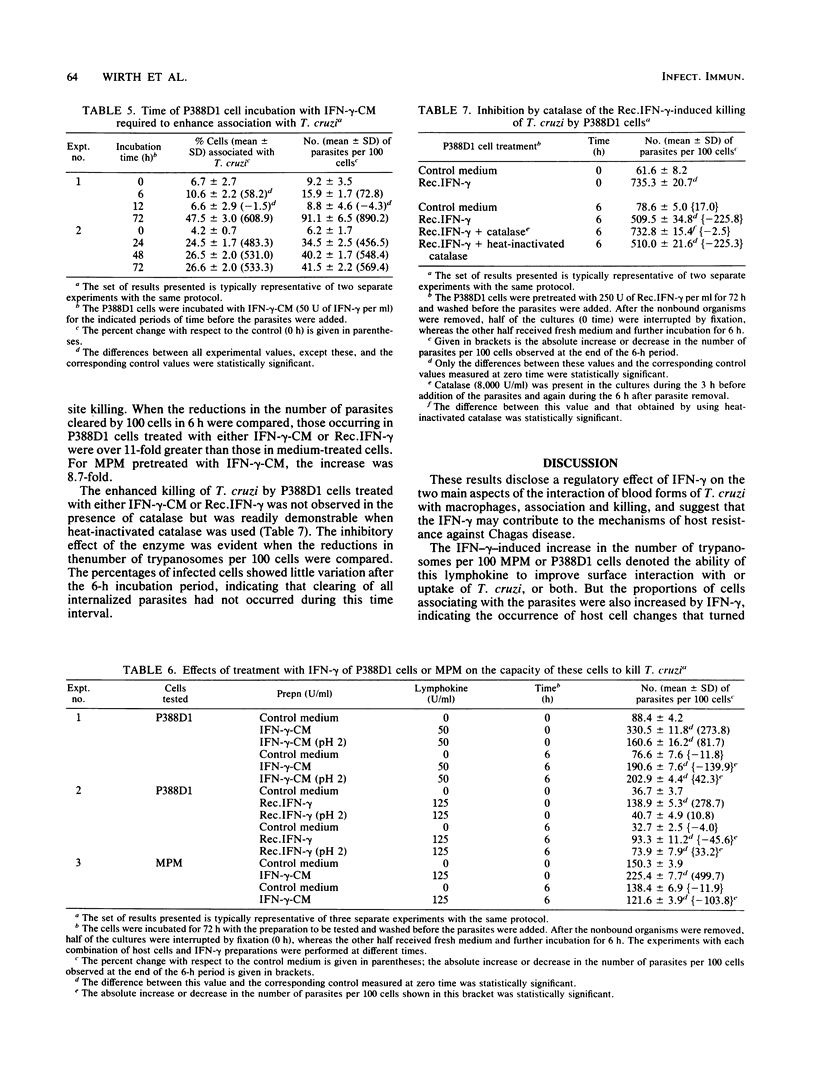
The effects of gamma interferon (IFN-gamma) on P388D1 cell or mouse resident peritoneal macrophage association (i.e., binding and internalization) with the protozoan Trypanosoma cruzi were studied, as well as the effects of this lymphokine on intracellular parasite killing. Incubation of either type of cell with a conditioned medium containing IFN-gamma and traces of interleukin 2 markedly increased the capacities of the cells to associate with virulent blood forms of T. cruzi, as evidenced by significant increases in both the proportion of parasite-associated cells and the number of parasites associated with the cells. Three lines of evidence pointed to IFN-gamma, and not interleukin 2, as the lymphokine responsible for the noted effect. First, a conditioned medium containing interleukin 2 but not IFN-gamma failed to enhance P338D1 cell-parasite association. Second, treatment of the IFN-gamma preparation at pH 2 to selectively inactivate IFN-gamma reduced its enhancing effect. Third, recombinant IFN-gamma, devoid of other lymphokines, also enhanced parasite association with P388D1 cells. Incubation of P388D1 cells with IFN-gamma for 24, 48, or 72 h increased cell association with T. cruzi, whereas a 12-h incubation period was insufficient, suggesting that IFN-gamma triggered time-dependent cellular events leading to the enhancement. Treatment of mouse resident peritoneal macrophages with the IFN-gamma-containing conditioned medium also increased the capacity of these cells to kill internalized trypanosomes. P388D1 cells, which showed minimal or no cytotoxicity after mock treatment with medium, displayed cytotoxicity after incubation with the IFN-gamma-containing conditioned medium; similar results were obtained with recombinant IFN-gamma. Catalase prevented parasite killing by P388D1 cells, indicating that H2O2 mediated the cytotoxicity. These results, underscoring the regulatory effects of IFN-gamma on macrophage-parasite interactions, suggest a possible role for this lymphokine in the mechanisms of host defense active against T. cruzi infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Budzko D. B., Kierszenbaum F. Isolation of Trypanosoma cruzi from blood. J Parasitol. 1974 Dec;60(6):1037–1038. [PubMed] [Google Scholar]
- Golgher R. R., Bertelli M. S., Petrillo-Peixoto M. L., Brener Z. Effect of interferon on the development of Trypanosoma cruzi in tissue culture "vero" cells. Mem Inst Oswaldo Cruz. 1980;75(1-2):157–160. doi: 10.1590/s0074-02761980000100015. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel-Bellan A., Joskowicz M., Fradelizi D., Eisen H. Modification of T-cell proliferation and interleukin 2 production in mice infected with Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3466–3469. doi: 10.1073/pnas.80.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F., Saavedra L. E. The effects of bacterial endotoxin on the infection of mice with Trypanosoma cruzi. J Protozool. 1972 Nov;19(4):655–657. doi: 10.1111/j.1550-7408.1972.tb03552.x. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F., Sonnenfeld G. Beta-interferon inhibits cell infection by Trypanosoma cruzi. J Immunol. 1984 Feb;132(2):905–908. [PubMed] [Google Scholar]
- Kierszenbaum F., Sonnenfeld G. Characterization of the antiviral activity produced during Trypanosoma cruzi infection and protective effects of exogenous interferon against experimental Chagas' disease. J Parasitol. 1982 Apr;68(2):194–198. [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. A. Trypanosoma cruzi: in vitro induction of macrophage microbicidal activity. J Exp Med. 1978 Jul 1;148(1):288–300. doi: 10.1084/jem.148.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata F., Wietzerbin J., Pons F. G., Falcoff E., Eisen H. Synergistic protection by specific antibodies and interferon against infection by Trypanosoma cruzi in vitro. Eur J Immunol. 1984 Oct;14(10):930–935. doi: 10.1002/eji.1830141013. [DOI] [PubMed] [Google Scholar]
- Postan M., Dvorak J. A., McDaniel J. P. Studies of Trypanosoma cruzi clones in inbred mice. I. A comparison of the course of infection of C3H/HEN- mice with two clones isolated from a common source. Am J Trop Med Hyg. 1983 May;32(3):497–506. doi: 10.4269/ajtmh.1983.32.497. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld G., Kierszenbaum F. Increased serum levels of an interferon-like activity during the acute period of experimental infection with different strains of Trypanosoma cruzi. Am J Trop Med Hyg. 1981 Nov;30(6):1189–1191. doi: 10.4269/ajtmh.1981.30.1189. [DOI] [PubMed] [Google Scholar]
- Villalta F., Kierszenbaum F. Role of inflammatory cells in Chagas' disease. II. Interactions of mouse macrophages and human monocytes with intracellular forms of Trypanosoma cruzi: uptake and mechanism of destruction. J Immunol. 1984 Dec;133(6):3338–3343. [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F. Fibronectin enhances macrophage association with invasive forms of Trypanosoma cruzi. J Immunol. 1984 Jul;133(1):460–464. [PubMed] [Google Scholar]
- Wu-Hsieh B., Zlotnik A., Howard D. H. T-cell hybridoma-produced lymphokine that activates macrophages to suppress intracellular growth of Histoplasma capsulatum. Infect Immun. 1984 Jan;43(1):380–385. doi: 10.1128/iai.43.1.380-385.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A., Roberts W. K., Vasil A., Blumenthal E., Larosa F., Leibson H. J., Endres R. O., Graham S. D., Jr, White J., Hill J. Coordinate production by a T cell hybridoma of gamma interferon and three other lymphokine activities: multiple activities of a single lymphokine? J Immunol. 1983 Aug;131(2):794–800. [PubMed] [Google Scholar]
- Zlotnik A., Shimonkevitz R. P., Gefter M. L., Kappler J., Marrack P. Characterization of the gamma-interferon-mediated induction of antigen-presenting ability in P388D1 cells. J Immunol. 1983 Dec;131(6):2814–2820. [PubMed] [Google Scholar]


