Abstract
We characterized operons encoding enzymes involved in denitrification, a nitrogen-cycling process involved in nitrogen losses and greenhouse gas emission, using a metagenomic approach which combines molecular screening and pyrosequencing. Screening of 77,000 clones from a soil metagenomic library led to the identification and the subsequent characterization of nine denitrification gene clusters.
Denitrification is a microbial respiratory process within the nitrogen cycle responsible for the return of fixed nitrogen to the atmosphere. This process contributes to the emission of N2O, which is an important greenhouse gas with a global warming potential (ca. 250 times higher than that of carbon dioxide). Denitrifiers, which constitute a taxonomically diverse functional guild with members belonging to more than 60 genera of bacteria and to some archaea and eukaryotes (13), can represent up to 5% of the total soil microbial community (5, 15). However, the study of denitrifying bacteria, like that of others, is hindered by characteristics that can prevent up to 99% of soil bacteria from being cultivated in vitro. The inventory of genes involved in denitrification and the extent of their diversity in bacteria are yet to be fully explored, while characterization of whole denitrification pathways with full-length gene sequences is still restricted to a limited number of denitrifying isolates and a few complete genomes.
New approaches based on the direct extraction of DNA from the natural environment and PCR amplifications can overcome limitations due to bacterial unculturability, but until now their application to denitrification genes has led only to the recovery of partial sequences for some of these genes (12). Our goals in this study were to apply a metagenomic approach (2) characterized by cloning of DNA extracted from soil and screening of metagenomic DNA library clones in order to identify and characterize gene clusters involved in the denitrification process. The soil metagenomic DNA library we used was constructed by Ginolhac et al. (4) with DNA extracted from grassland soil (Montrond, La Batie-Divisin, France) with 35- to 40-kb metagenomic DNA fragments cloned in the pCC1Fos vector and replicated in the Escherichia coli EC10 bacterial host. About 77,000 clones were screened by colony hybridization according to the protocol described previously (2). In order to increase the range of retrievable sequences, [33P]dCTP-labeled probes consisted of PCR products obtained from DNA extracted from Montrond soil as templates by using degenerate primers targeting the nirS, nirK, and nosZ denitrification genes encoding the cytochrome cd1 nitrite reductase, the copper nitrite reductase, and the nitrous oxide reductase, respectively (5, 6, 14). Pyrosequencing (GATC, Konstanz, Germany) was used to sequence DNA from the clones identified as yielding a positive hybridization signal on the membranes (2). Nine recombinant clones were positively identified by hybridization and sequence analysis as carrying genes coding for denitrification functions: four clones contained a nirS-like gene, three clones had a nirK-like gene, one clone had a nosZ-like gene, and one clone contained both nirK-like and nosZ-like genes (Fig. 1). This number of positive clones is in agreement with the estimated proportion of denitrifiers in the soil bacterial community (between 0.5 and 5%) (5, 15) and the calculation of Leveau (9) that estimated that 57,500 clones with 40-kb metagenomic inserts would be required to recover one gene (99% probability) present in 1% of the soil bacteria, considering an average genome size of 5 Mbp for each soil bacterium. Other genes present in these nine clones are described in Tables S1 to S9 in the supplemental material.
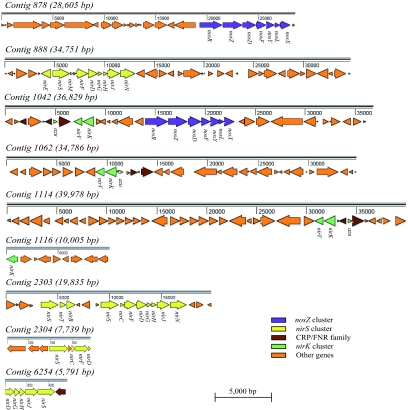
FIG. 1.
Physical maps of environmental gene contigs involved in denitrification processes. Shown are the nosZ clusters (purple), the nirS clusters (yellow), genes from the CRP/FNR family involved in the expression control of the denitrification process (brown), the nirK clusters (green), and other genes not directly involved in the denitrification process (orange), which are described in Tables S1 to S9 in the supplemental material.
The genetic organization of the nirS clusters, with most of the nir gene products presumably involved in the heme D1 biosynthesis (19), was nirESM-FDGHJN, nirSTB---SCFDGHJN, nirS-CFD, and nirDGHJS (each hyphen here indicates an inserted gene) on contigs 888, 2303, 2304, and 6254, respectively. Unfortunately, the assembly of a few contigs could not be completed, and the end of the nirS cluster is missing for contigs 2304 and 6254. The results show a variable gene organization among bacteria, confirming previous data from isolate analysis, and indicate that these clusters are probably subjected to shuffling either by endogenous gene displacement or by horizontal gene transfer between bacteria (11). Two nirS copies were detected in contig 2303 with a 69% similarity, indicating that the original bacterium that provided the DNA fragment contained more than one copy of this gene in its genome. Previous studies reported the presence of multiple copies of nirS in “Magnetospirillum magneticum,” “Dechloromonas aromatica,” and Thiobacillus denitrificans that also exhibited a significant level of divergence within the same genome (3, 8).
In three out of four nirK-containing clones (partial gene sequence in contig 1116), a nirV-like gene was located at a position linked to the nirK gene, as previously observed for several cultivated denitrifiers. The frequent proximity of these two genes on the genome supports the hypothesis of an involvement of a nirV gene product in nitrite reduction (7, 11). In addition to nirV, the azu gene encoding a pseudoazurin electron carrier, the principal electron donor to the copper nitrite reductase (19), was identified 2,503 bp downstream of the nirK gene in contig 1042 and 233 bp and 1,220 bp upstream of nirK in contigs 1062 and 1114, respectively, but with the transcription direction opposite of that of the nirK gene.
The two nos clusters identified in our study contained the nosRZDFYLX genes, with nosR encoding a membrane-bound regulatory protein, nosZ encoding the catalytic subunit of the multicopper nitrous oxide reductase, nosDFY encoding a putative copper insertion complex, nosL encoding a putative outer membrane protein, and nosX encoding a periplasmic component (1, 17, 20). In contrast to the organization of the nirS cluster, the organization of the nosRZDFYL genes observed in our study was identical to that of most cultivated denitrifiers, which indicates a high level of synteny. Interestingly, the nosX gene was located downstream of nosL for both nos contigs. This is commonly observed in Alphaproteobacteria but not in other proteobacteria (11). In contig 1042, the nos genes were located ca. 7,500 bp upstream of the nirK gene. Genetic linkage of the nir and nos genes has also been observed in Brucella melitensis and Bradyrhizobium japonicum USDA110, suggesting that denitrification gene islands are not rare in soil bacteria. Although the nor genes encoding the nitric oxide reductase enzyme were located in the vicinity of the nir genes in several cultivated denitrifiers (11), such linkage was not confirmed in our study.
The metagenomic pyrosequencing approach also detected several genes encoding one-component transcriptional regulators belonging to the superfamily of cyclic AMP receptor protein (CRP)-like proteins and fumarate and nitrate reductase regulatory protein (FNR)-like proteins (Fig. 1) in the vicinity of the denitrification genes. CRP/FNR-like proteins have been established as major transcriptional factors controlling expression of the denitrification process in response to oxygen and nitric oxide presence (16, 18). Putative DNA binding sites of CRP/FNR-like proteins, which consist of inverted and repeated sequences of nucleotides (TTGATNNNATCAA), were identified in the promoter regions of (i) nosR on contigs 878 and 1042, (ii) nirS on contigs 2303, 2304, and 888, and (iii) nirK on contig 1114. CRP/FNR boxes were also found in the promoter regions of genes encoding a nitrate/nitrite antiporter and cytochrome oxidase assembly factor in contig 888 and encoding Fnr protein in contig 1042. Presence of FNR/CRP-like proteins near the denitrification genes and presence of Fnr boxes in their promoter regions support an oxygen-dependent regulation of the denitrification process in the corresponding host strains as commonly observed in cultivated strains (11).
Phylogenetic analysis of the nirS, nirK, and nosZ catalytic subunits revealed that the nirK and nosZ sequences obtained in this study were related to the nirK or nosZ gene from Alphaproteobacteria (up to 84% identity) (Table 1) (see Fig. S1 and S2 in the supplemental material). In addition, gene organization in contigs 1042 and 1062 with the nosX gene downstream of the nosL gene is similar to that found in denitrifier isolates classified in the subclass of the Alphaproteobacteria. Accordingly, assigning contigs to their respective phylogenetic groups using the PhyloPythia software (10) showed that contigs 878, 1042, 1062, 1114, and 1116 were related to Alphaproteobacteria. The four nirS sequences identified in this study were phylogenetically related to the nirS sequences from Betaproteobacteria (Table 1) (see Fig. S3 in the supplemental material). However, phylogenetic affiliation of the full contigs with the PhyloPythia software revealed that contig 6254 was affiliated with Gammaproteobacteria while the three others were affiliated with Betaproteobacteria. This underlined the difficulty of phylogenetic affiliation of the denitrification genes due to the lack of congruence between the denitrification genes and 16S rRNA trees as previously reported by Jones et al. (8).
TABLE 1.
Sequences similar to nirS, nirK, and nosZ found in cultivated microorganisms based on the application of the BLASTN program to the NCBI database
| Environmental gene (contig) | Accession no. | Description of cultivated microorganism | BLASTN score | BLASTN query coverage (%) | E value | Sequence identity (%) |
|---|---|---|---|---|---|---|
| nosZ (878) | CP000301.1 | Rhodopseudomonas palustris BisB18 | 708 | 87 | 0.0 | 75 |
| nirS (888) | AM260480.1 | Ralstonia eutropha H16 chromosome 2 | 876 | 85 | 0.0 | 77 |
| nirK (1042) | CP000390.1 | Mesorhizobium sp. strain BNC1 | 745 | 86 | 0.0 | 80 |
| nosZ (1042) | AE006469.1 | Sinorhizobium meliloti 1021 plasmid pSymA | 1738 | 91 | 0.0 | 84 |
| nirK (1062) | BX572606.1 | Rhodopseudomonas palustris CGA009 | 656 | 86 | 0.0 | 79 |
| nirK (1114) | BA000040.2 | Bradyrhizobium japonicum USDA 110 | 784 | 86 | 0.0 | 81 |
| nirK (1116) | CU234118.1 | Bradyrhizobium sp. strain ORS278 | 865 | 86 | 0.0 | 82 |
| nirS (2303a) | CR555306.1 | Azoarcus sp. strain EbN1 | 1260 | 92 | 0.0 | 81 |
| nirS (2303b) | CP000089.1 | “Dechloromonas aromatica” RCB | 1230 | 87 | 0.0 | 81 |
| nirS (2304) | CP001013.1 | Leptothrix cholodnii SP-6 | 1509 | 89 | 0.0 | 84 |
| nirS (6254) | CP001013.1 | Leptothrix cholodnii SP-6 | 1003 | 92 | 0.0 | 79 |
Our results highlight the potential of the metagenomic approach (2) combined with molecular screening and pyrosequencing to broaden our knowledge of genetic organization and diversity of gene clusters or operons that are distributed in soil microorganisms far beyond the small proportion of cultivable bacteria. Systematic sequencing of the entire soil metagenomic DNA still remains difficult; therefore, an intermediate step of screening a recombinant clone library, such as the hybridization method used in this study, is useful in order to reduce the number of clones to be sequenced. The use of a probe consisting of mixed PCR products allowed us to detect denitrification genes from metagenomic DNA with percentage identities as low as 75% to known genes (Table 1). Use of functional screening in future studies could help detect denitrification genes that would not be detected by hybridization because of their sequence divergence.
Nucleotide sequence accession numbers.
Sequences obtained and annotated in this study have been deposited in GenBank under the accession numbers EU910852 to EU910860.
Supplementary Material
Acknowledgments
This work was supported in part by the Programme National de Recherches sur les Organismes Génétiquement Modifiés from the Agence Nationale de la Recherche for the project Ploben (grant ANR-05-POGM-004-01), the Rhône-Alpes Region, the Bureau des Ressources Génétiques and the Agence Française de Sécurité Sanitaire de l'Environnement et du Travail for the projects AntiReSol EST-2006/1/44 and Gestions Biologique et Sociale de la Dispersion des Résistances aux Antibiotiques EST-2007-1.
Footnotes
Published ahead of print on 14 November 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Chan, Y. K., W. A. McCormick, and R. J. Watson. 1997. A new nos gene downstream from nosDFY is essential for dissimilatory reduction of nitrous oxide by Rhizobium (Sinorhizobium) meliloti. Microbiology 143:2817-2824. [DOI] [PubMed] [Google Scholar]
- 2.Demanèche, S., M. M. David, E. Navarro, P. Simonet, and T. M. Vogel. 2008. Evaluation of functional gene enrichment in a soil metagenomic clone library. J. Microbiol. Methods [Epub ahead of print.] doi: 10.1016/j.mimet.2008.09.009. [DOI] [PubMed]
- 3.Etchebehere, C., and J. Tiedje. 2005. Presence of two different active nirS nitrite reductase genes in a denitrifying Thauera sp. from a high-nitrate-removal-rate reactor. Appl. Environ. Microbiol. 71:5642-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginolhac, A., C. Jarrin, B. Gillet, P. Robe, P. Pujic, K. Tuphile, H. Bertrand, T. M. Vogel, G. Perriere, P. Simonet, and R. Nalin. 2004. Phylogenetic analysis of polyketide synthase I domains from soil metagenomic libraries allows selection of promising clones. Appl. Environ. Microbiol. 70:5522-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry, S., D. Bru, B. Stres, S. Hallet, and L. Philippot. 2006. Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl. Environ. Microbiol. 72:5181-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry, S., E. Baudoin, J. C. Lopez-Gutierrez, F. Martin-Laurent, A. Brauman, and L. Philippot. 2004. Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. J. Microbiol. Methods 59:327-335. [DOI] [PubMed] [Google Scholar]
- 7.Jain, R., and J. P. Shapleigh. 2001. Characterization of nirV and a gene encoding a novel pseudoazurin in Rhodobacter sphaeroides 2.4.3. Microbiology 147:2505-2515. [DOI] [PubMed] [Google Scholar]
- 8.Jones, C. M., B. Stres, M. Rosenquist, and S. Hallin. 2008. Phylogenetic analysis of nitrite, nitric oxide, and nitrous oxide respiratory enzymes reveal a complex evolutionary history for denitrification. Mol. Biol. Evol. 25:1955-1966. [DOI] [PubMed] [Google Scholar]
- 9.Leveau, J. 2007. The magic and menace of metagenomics: prospects for the study of plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 119:279-300. [Google Scholar]
- 10.McHardy, A. C., H. G. Martin, A. Tsirigos, P. Hugenholtz, and I. Rigoutsos. 2007. Accurate phylogenetic classification of variable-length DNA fragments. Nat. Methods 4:63-72. [DOI] [PubMed] [Google Scholar]
- 11.Philippot, L. 2002. Denitrifying genes in bacterial and archaeal genomes. Biochim. Biophys. Acta 1577:355-376. [DOI] [PubMed] [Google Scholar]
- 12.Philippot, L., and S. Hallin. 2005. Finding the missing link between diversity and activity using denitrifying bacteria as a model functional community. Curr. Opin. Microbiol. 8:234-239. [DOI] [PubMed] [Google Scholar]
- 13.Philippot, L., S. Hallin, and M. Schloter. 2007. Ecology of denitrifying prokaryotes in agricultural soil. Adv. Agron. 96:249-305. [Google Scholar]
- 14.Throbäck, I. N., K. Enwall, Å. Jarvis, and S. Hallin. 2004. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 49:401-417. [DOI] [PubMed] [Google Scholar]
- 15.Tiedje, J. M. 1988. Ecology of denitrification and dissimilatory nitrate reduction to ammonium, p. 179-244. In A. J. B. Zehnder (ed.), Biology of anaerobic microorganisms. John Wiley & Sons, Inc., New York, NY.
- 16.Vollack, K. U., E. Hartig, H. Korner, and W. G. Zumft. 1999. Multiple transcription factors of the FNR family in denitrifying Pseudomonas stutzeri: characterization of four fnr-like genes, regulatory responses and cognate metabolic processes. Mol. Microbiol. 31:1681-1694. [DOI] [PubMed] [Google Scholar]
- 17.Wunsch, P., and W. G. Zumft. 2005. Functional domains of NosR, a novel transmembrane iron-sulfur flavoprotein necessary for nitrous oxide respiration. J. Bacteriol. 187:1992-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zumft, W. G. 2002. Nitric oxide signaling and NO dependent transcriptional control in bacterial denitrification by members of the FNR-CRP regulator family. J. Mol. Microbiol. Biotechnol. 4:277-286. [PubMed] [Google Scholar]
- 19.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zumft, W. G., A. Viebrock-Sambale, and C. Braun. 1990. Nitrous oxide reductase from denitrifying Pseudomonas stutzeri. Genes for copper-processing and properties of the deduced products, including a new member of the family of ATP/GTP-binding proteins. Eur. J. Biochem. 192:591-599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.