Abstract
Type III secretion systems (T3SSs) are essential virulence devices for many gram-negative bacteria that are pathogenic for plants, animals, and humans. They serve to translocate virulence effector proteins directly into eukaryotic host cells. T3SSs are composed of a large cytoplasmic bulb and a transmembrane region into which a needle is embedded, protruding above the bacterial surface. The emerging antibiotic resistance of bacterial pathogens urges the development of novel strategies to fight bacterial infections. Therapeutics that rather than kill bacteria only attenuate their virulence may reduce the frequency or progress of resistance emergence. Recently, a group of salicylidene acylhydrazides were identified as inhibitors of T3SSs in Yersinia, Chlamydia, and Salmonella species. Here we show that these are also effective on the T3SS of Shigella flexneri, where they block all related forms of protein secretion so far known, as well as the epithelial cell invasion and induction of macrophage apoptosis usually demonstrated by this bacterium. Furthermore, we show the first evidence for the detrimental effect of these compounds on T3SS needle assembly, as demonstrated by increased numbers of T3S apparatuses without needles or with shorter needles. Therefore, the compounds generate a phenocopy of T3SS export apparatus mutants but with incomplete penetrance. We discuss why this would be sufficient to almost completely block the later secretion of effector proteins and how this begins to narrow the search for the molecular target of these compounds.
Type III secretion systems (T3SSs) are key determinants of virulence in many gram-negative bacteria (10) that are pathogenic for plants, animals, and humans. These systems mediate a complex membrane transport process that directly delivers bacterial proteins across three biological membranes into host cells to enable bacterial infection. Major human pathogens that employ T3SSs for infection include Shigella, Salmonella, Chlamydia, Yersinia, and Pseudomonas species and enteropathogenic Escherichia coli.
We study the pathogenesis of Shigella flexneri. Shigella causes bacillary dysentery in humans, a disease characterized by invasion of, massive inflammation in, and destruction of the colonic mucosa. The genes required for invasion are clustered on a 31-kb region of a large virulence plasmid. There, the mxi and spa operons encode the T3SS apparatus and the ipa and ipg operons encode proteins known as effectors, five of which, IpaA to -D and IpgD, are abundantly secreted early in invasion (33). The T3SS apparatus comprises three distinct substructures: a cytoplasmic bulb, a region spanning both bacterial membranes and a hollow “needle” protruding from the bacterial surface. These last two portions can be purified together and are termed the needle complex (NC) (5, 21, 44). The central 25-Å channel of the NC (9) is thought to serve as a conduit for effector protein secretion.
T3SSs are activated only upon direct physical contact with host cells. A protein complex at the tip of the needle, consisting of IpaB and IpaD, is directly involved in sensing host cells and regulating secretion (12, 31, 36, 42). Upon activation of the T3SS, IpaC is recruited to the needle tip, and this leads to insertion of the IpaBC translocon, which is required for translocation of the other early effectors into the plasma membrane of the host cell (42). The Shigella T3SS displays three different functional states of protein secretion: (i) a burst of protein secretion, known as induction, upon host cell contact (25) or addition of an artificial inducer, the small amphipathic dye molecule Congo red (CR) (2); (ii) a low rate of protein secretion, termed leakage, prior to host cell contact (23); and (iii) “constitutive secretion,” which represents an unregulated and higher rate of secretion than leakage that occurs in some needle mutants (19) and IpaB or IpaD deletion strains (6, 34, 35, 42).
To battle the increasing antibiotic resistance of pathogenic bacteria, it will be crucial to develop new antimicrobial agents. Strategies that rely on existing drugs and targets, which affect the viability of bacteria, all face the same problem, i.e., that bacteria quickly develop drug resistance under the huge selective pressure applied. The generation of drugs that specifically target pathogenic properties without killing bacteria is a strategy that might decrease the chance of bacterial resistance emerging against these drugs (20). Previously, in a large screening assay, small molecules that inhibit the expression of a virulence factor of the Yersinia T3SS without affecting the viability of the bacterial cell were identified (18). In subsequent years, these and other related salicylidene acylhydrazides were found to inhibit secretion of T3SS-related proteins and to affect host cell infection by Yersinia, Chlamydia, and Salmonella (3, 15, 28-30, 43). An overview of the T3SS-related inhibitory effects on studied pathogens for the compounds used in this work is given in Table 1. Results are shown for T3SS-mediated protein secretion and in vitro virulence (pathogen replication, host cell infection, and macrophage survival), which represent key assays to study T3SS activity. These T3SS inhibitors were also found to reduce the motility of Yersinia and Salmonella (18, 29) and to inhibit secretion and surface localization of flagellin in Salmonella (29). Taken together, these findings indicate that the inhibitors act on a conserved target, since they interfere with both the T3SS virulence determinant and the genetically related bacterial flagellar export system that mediates motility.
TABLE 1.
Overview of inhibitory effects of compounds in studied T3SS-carrying pathogens
| Compound | Alternative name(s) (reference) | Effect on (reference)a:
|
Other effects (reference) | |
|---|---|---|---|---|
| T3SS protein secretion/expression | In vitro virulence | |||
| INP0007 | Compound 2 (18), compound 1 (30) | Yersinia, yes (18, 30); Salmonella, yes (15, 29) | Yersinia, yes (30); Salmonella, yes (15, 29) | Yersinia motility (18) |
| INP0010 | Compound 8 (30) | Yersinia, yes (30); Salmonella, yes (29); Chlamydia, yes (3) | Yersinia, yes (3); Salmonella, yes (29); Chlamydia, yes (3) | |
| INP0400 | Salmonella, yes (29); Shigella, yes (this study) | Yersinia, yes (3); Salmonella, yes (29); Chlamydia, yes (28); Shigella, yes (this study) | Shigella T3SS needle assembly (this study) | |
| INP0401 | Compound 10 (30) | Yersinia, yes (30); Salmonella, yes (29); Shigella, yes (this study) | Salmonella, yes (29); Shigella, no (this study) | Shigella T3SS needle assembly (this study) |
| INP0402 | Compound 15 (30) | Yersinia, yes (30); Salmonella, yes (29); Shigella, yes (this study) | Salmonella, yes (29); Shigella, yes (this study) | Shigella T3SS needle assembly (this study) |
| INP0403 | Compound 11 (30) | Yersinia, yes (30); Salmonella, yes (15, 29) | Salmonella, yes (15, 29) | Salmonella induced in vivo inflammatory response (15) |
| INP0404 | Compound 20 (30) | Yersinia, yes (30); Salmonella, yes (29); Shigella, yes (this study) | Salmonella, yes (29); Shigella, yes (this study) | Salmonella motility and flagellin expression (29), Shigella T3SS needle assembly (this study) |
| INP0405 | Compound 9 (30) | Yersinia, yes (30); Salmonella, yes (29); Shigella, yes (this study) | Salmonella, yes (29); Shigella, yes (this study) | Salmonella motility and flagellin expression (29) |
| INP0406 | Yersinia, no (C. Sundin, unpublished data); Salmonella, yes (29); Shigella, no (this study) | Yersinia, no (3); Salmonella, yes (29); Chlamydia, no (3); Shigella, no (this study) | ||
T3SS-mediated protein secretion or expression (the latter only for reference 31) and in vitro virulence represent key assays for studying T3SS activity.
In this study we have investigated the effects of the small-molecule inhibitors on the Shigella flexneri T3SS. The extensive assays to analyze the Shigella T3SS-related protein secretion and virulence and the ultrastructure of its apparatus should be of great value in better understanding the general mode of action of these T3SS inhibitors. In addition, knowledge on how the inhibitors act on the T3SS should help us increase our understanding of the molecular mechanism of this complex biological system. Our results show that the T3SS inhibitors affect T3SS protein secretion and consequently Shigella virulence. Analysis of the secreton structure reveals that the inhibitors act on secreton assembly, leading to a twofold increase in needleless secretons and to a population of secretons with significantly shorter needles. MxiH, the major component of the Shigella T3SS needle, is itself one of the first T3SS substrates secreted during secreton biogenesis. This suggests that the molecular target of these T3SS-inhibitory molecules lies within the highly conserved cytoplasmic or inner membrane export apparatus.
MATERIALS AND METHODS
Compounds.
All small-molecule compounds used in this study were provided by Innate Pharmaceuticals AB (Umeå, Sweden). While some compounds have been used in previous studies under different names, they are now designated according to a new naming convention (INPxxxx). The same set of compounds was used and their structural formulas shown in a study performed by Negrea and coworkers (29); the formulas are reprinted in Fig. S1 in the supplemental material. Stock solutions (20 mM) of the compounds were prepared in dimethyl sulfoxide (DMSO), stored in the dark, and kept for no more than 4 weeks. The compounds were added at 50 μM except when indicated otherwise. DMSO was used as a control, and the same volume as for the stock solutions was added.
Bacterial strains and growth conditions.
The S. flexneri strains used in this study are listed in Table 2. Bacteria were grown in TCSB medium as previously described (4), except when indicated otherwise. To assess the effect of the compounds on Shigella growth in culture, overnight cultures were diluted in fresh TCSB medium to an optical density at 600 nm (OD600) of 0.1, and then DMSO or small-molecule compounds were added. The cultures were incubated on a shaker at 37°C for 8 h. The growing cultures were sampled at 2-h intervals to determine their cell densities (OD600).
TABLE 2.
Shigella strains used in this study
Secretion assays.
The analyses of secretion of T3SS effectors were performed as described previously (19), except for the following modifications. For leakage and constitutive secretion assays, overnight cultures were diluted in fresh TCSB medium to an OD600 of 0.1, and then small-molecule compounds were added. The cultures were incubated on a shaker at 30°C for 30 min and then further grown on a shaker at 37°C for 8 h. The culture supernatants were analyzed for protein content. For CR induction, an overnight culture of wild-type Shigella was diluted 1:100 in fresh TCSB medium and grown on a shaker at 37°C to an OD600 of 0.7. The small-molecule compounds were added and growth of the cultures was continued for another 30 min, after which cells pellets were harvested and resuspended in phosphate-buffered saline (PBS) to an OD600 of 5. Small-molecule compounds were added again at 100 μM to compensate for the higher cell density, and the CR induction assay was performed. Fast constitutive secretion of the ΔipaD strain (42) was assayed as in the CR induction protocol but with the CR dye left out.
HeLa cell invasion.
HeLa cell invasion by Shigella strain M90T-AfaE was monitored using a modified gentamicin protection assay (1). In short, HeLa cells were grown to confluence in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum and penicillin-streptomycin in a 24-well plate. The small-molecule compounds were added at the start of exponential growth of Shigella cultures. Bacterial cultures were grown to an OD600 of 0.8 and spun down, and cell pellets were resuspended in PBS. HeLa cells were washed twice with PBS and then incubated in DMEM with 20 mM HEPES (pH 7.4) just before infection. Shigella cells were added to the HeLa cells to a multiplicity of infection of 2 and the plate centrifuged for 10 min at 900 × g and 20°C. Shigella M90T-AfaE expresses the E. coli afimbrial adhesin AfaE (22). This results in faster and more efficient binding of Shigella to eukaryotic cells and allows a reduction of the incubation time for HeLa cell infection from the usual 30 min to 5 min (8) at 37°C. The latter is important to prevent further Shigella growth during the incubation time and thus the formation of bacterial cells in the absence of the small-molecule compounds. After infection, the medium was aspirated, and HeLa cells were washed four times with cold PBS to remove free bacterial cells and incubated with DMEM supplemented with 100 μg/ml gentamicin for 45 min at 37°C to kill noninvading bacteria. The gentamicin solution was aspirated, and 0.1% Triton X-100 in PBS was added and incubated for 10 min at room temperature to lyse the HeLa cells. Finally, the lysate mix was plated out in serial dilutions, and CFU were determined on the following day.
Macrophage infection.
A single red colony and a single white colony of Shigella flexneri strain CCUG 29416 were picked from an LB plate containing 0.01% CR and incubated overnight in LB on a shaker at 37°C. The white colony was obtained by repeated growth in liquid culture and plating on a CR plate. The white color of the colony reflects the loss of the virulence plasmid (24), and this was checked by the absence of a PCR product for ipaC and the absence of IpaC and IpaB proteins on a Western blot. The macrophage cell line, J774A.1, was seeded into a 96-well plate at a density of 7 × 104 cells/well in DMEM (100 μl/well) plus 10% fetal calf serum and gentamicin (3 μg/ml) and grown for 16 h at 37°C in 5% CO2. After 16 h the cells were washed once with PBS, and fresh DMEM (50 μl) containing the different compounds at 100 μM and bacteria (50 μl; OD600 = 0.002) were added to give a final OD600 of 0.001 and a compound concentration of 50 μM. Prior to infection, the overnight culture of Shigella was diluted 10-fold in DMEM and incubated on a shaker for 1.5 h at 37°C. After 5 h of infection, the medium was removed and 100 μl fresh PBS was added to the wells, and then a stock solution containing CalceinAM (Invitrogen) and Sytox Orange (Invitrogen) in PBS (20 μl; final concentrations of 1 μM and 0.05 μM for CalceinAM and Sytox Orange, respectively) was added and the plate incubated for 40 min at 37°C in a CO2 incubator. The fluorescence was then read in a microplate reader (TECAN GENios; Calcein at 485/535 nm and Sytox Orange at 535/595 nm), and the results were confirmed by fluorescence microscopy.
Western blotting for secreton component level determination.
To analyze whole-cell levels of secreton components, overnight cultures were diluted into fresh TCSB medium to an OD600 of 0.1 and then small-molecule compounds were added. The cultures were incubated on a shaker at 30°C for 30 min and then further grown on a shaker at 37°C to an OD600 of 0.8. The cell pellets were harvested and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting and immunodetection using mouse monoclonal and rabbit polyclonal antibodies directed against MxiJ (44) and MxiH (23), respectively.
Shigella osmotic shock treatment.
Overnight cultures were diluted into fresh TCSB medium to an OD600 of 0.1, and then the compounds were added. The cultures were incubated on a shaker at 30°C for 30 min and then further grown on a shaker at 37°C to an OD600 of 1. Ghost cells were prepared by osmotic shock treatment of bacterial cultures using glass beads as described previously (19).
NC purification.
NCs were purified as described previously (44). Overnight cultures were diluted in fresh TCSB medium to an OD600 of 0.1, small-molecule compounds were added, and then the cultures were grown on a shaker at 37°C to an OD600 of 1. The compounds were maintained until bacterial lysis, which preceded the affinity purification step. This results in a total time for incubation with the compounds of approximately 5 h. In the control sample, a culture was grown in the absence of any compounds and INP0400 was added just before cell lysis.
Electron microscopy.
Samples containing Shigella ghost cells or purified NCs were deposited onto 300-mesh Formvar and carbon-coated glow-discharged copper grids and subsequently stained for 1 min with either 1% (ghost cells) or 2% (NCs) phosphotungstic acid (pH 7.0). Electron microscopy images were taken on a Philips Tecnai12 or Zeiss Leo 912 transmission electron microscope at 80 kV. Micrographs were recorded at a magnification of ×20,000 on ProScan charge-coupled device cameras using the SIS software. Quantification of complete secretons and of bases was performed by hand on enlarged prints of the micrographs. Only secretons and bases at the edges of osmotically shocked cells, where the presence or absence of needles can be unambiguously discerned, were used. Needle lengths in NC preparations were measured in the same way. The measured needle length was converted to actual length using the thickness of the needle (7 nm) as a scale. The significance of the differences in the distribution of the needle length frequencies was assessed using a G test. The expected frequencies were set at 11% below 35 nm, 79% within 35 to 63 nm, 9% within 63 to 91 nm, and 1% within 91 to 112 nm using the average of the mean percentages obtained for the INP0400 control, INP0406, DMSO, and no addition, the mean frequencies and standard deviations of which were comparable (see Table 3). The statistical analysis was performed using the data sets pooled from either two or three independent experiments.
TABLE 3.
Effects of inhibitors on secreton needle length
| Needle length (nm) | % of needles (mean ± SD)a after the following addition:
|
||||
|---|---|---|---|---|---|
| INP0400 | INP0400Cb | INP0406 | DMSO | None | |
| <35 | 18 ± 4 | 11 ± 7 | 9 ± 4 | 11 ± 1 | 13 ± 1 |
| 35-63 | 66 ± 10 | 73 ± 4 | 79 ± 5 | 81 ± 9 | 81 ± 6 |
| 63-91 | 15 ± 13 | 15 ± 8 | 10 ± 8 | 8 ± 10 | 5 ± 4 |
| 91-112 | 1 ± 1 | 1 ± 1 | 1 ± 0 | 0 ± 0 | 1 ± 1 |
Data derived are from either two or three independent experiments.
Control in which Shigella cultures were grown in the absence of compounds and INP0400 was added just prior to the cell lysis step.
RESULTS
The small-molecule compounds inhibit secretion of Shigella T3SS proteins.
The compounds were analyzed for their effect on protein secretion by the Shigella T3SS. First, their effect on the viability of Shigella cultures was tested. The compounds were added at 50 μM at the start of the exponential growth of wild-type Shigella cultures. An equivalent amount of DMSO was added as a control and showed no effect on Shigella culture growth (data not shown). INP0401, INP0402, INP0405, and INP0406 had no significant effect on cell growth, while INP0400 and INP0404 had only a mild effect (Fig. 1). The presence of INP0007, INP0010, and INP0403 led to severe growth defects (Fig. 1) and often led to cell lysis as observed by SDS-PAGE analysis of culture supernatants (data not shown). Similar effects of the compounds on culture growth were observed for the mxiH/D73A and ΔipaD mutant strains (data not shown). In light of these results, INP0007, INP0010, and INP0403 were excluded from subsequent experiments. The effects of the remaining compounds on the different type III secretion phenotypes were assessed. For the leakage and constitutive secretion assays, all drugs were added at the beginning of culture growth. For the fast constitutive secretion and CR induction assays, the drugs were added 30 min before the end of culture growth and maintained throughout the secretion assay. INP0400, and to a lesser extent INP0401 and INP0402, inhibited T3SS “leakage” by wild-type Shigella (Fig. 2A). INP0400, as well as INP0404, also inhibited constitutive secretion of the mxiH/D73A (Fig. 2B) and ΔipaD (data not shown) mutant strains. Similarly, both INP0400 and INP0405 inhibited the fast constitutive secretion phenotype (42) of the ΔipaD strain (Fig. 2C). INP0400 is the only compound that effectively inhibited CR-induced secretion (Fig. 3). Of note, no inhibitory effect on CR-induced secretion was observed when the compounds were added only after the cells were resuspended in PBS buffer (data not shown). In conclusion, while the variety of secretion phenotypes that the Shigella T3SS displays were affected by different compounds, INP0400 was the strongest and the only consistent inhibitor, while INP0406 showed no effect in any of the secretion assays. INP0406 was therefore used as a control throughout all experiments.
FIG. 1.
Effect of the compounds on Shigella growth in broth culture. An overnight culture of wild-type Shigella was diluted to an OD600 of 0.1 and then grown in the presence of DMSO or 50 μM of the compounds for 8 h. The cultures were sampled at 2-h intervals for cell density measurements at 600 nm.
FIG. 2.
Inhibition of leakage and constitutive secretion of T3SS proteins. Shigella cultures were grown in the presence of DMSO or compounds, and then culture supernatants were isolated. Samples were separated by SDS-PAGE, and the 12% gels were silver stained. The gels show “leakage” of wild-type Shigella (A), constitutive secretion of the mxiH/D73A mutant, (B) and fast constitutive secretion of the ΔipaD strain (C). The positions of the abundant T3SS proteins are indicated on the right. The SepA protein, which is secreted by an autotransporter pathway, acted as a loading control.
FIG. 3.
Inhibition of CR-induced secretion. Wild-type Shigella cultures were grown in the presence of DMSO or compounds, and then culture supernatants were isolated. Samples were separated by SDS-PAGE, and the 12% gel was silver stained. The positions of the major T3SS proteins are indicated on the right.
The secretion inhibitors affect host cell infection.
The compounds were tested for their ability to inhibit invasion of HeLa cells and killing of macrophages by Shigella. For the HeLa cell invasion assay, the selection of compounds was limited to the inhibitors INP0400 and INP0402, while INP0406 was used as a negative control. Although INP0400 had a significant impact on the ability of Shigella to invade HeLa cells, INP0402 appeared to be the strongest inhibitor in this assay (invasion, relative to the result for the DMSO control set to 100%, was 40% ± 13%, 17% ± 11%, and 81% ± 13% [means ± standard deviations from duplicate experiments] for INP0400, INP0402, and INP0406, respectively). No inhibitory effect was observed when the compounds were added just 30 min before the end instead of at the start of cell growth (data not shown).
The inhibitors affect the induction of macrophage apoptosis.
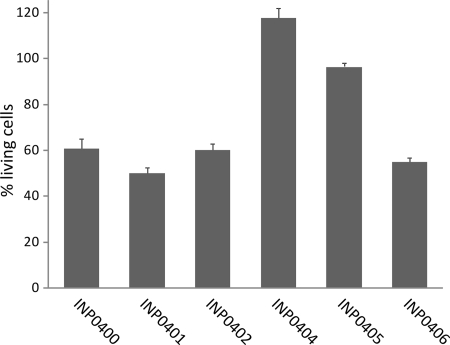
To further investigate the effect of the compounds on Shigella flexneri, an ex vivo assay in which the effect of the compounds was measured during cell infection was developed. In these experiments the mouse macrophage cell line J774 was infected with the virulent and plasmid-cured Shigella flexneri strain CCUG 29416. After 5 h of infection, the fluorescent dyes CalceinAM and Sytox Orange were added, at which point CalceinAM is taken up by the cells. If the cells are alive, an enzyme converts nonfluorescent CalceinAM to intensely green fluorescent Calcein (3). If the membranes of the eukaryotic cells have been damaged, Sytox Orange is taken up and will bind DNA, which increases the red fluorescence of Sytox Orange 400 times. The wild-type Shigella strain caused cell death by injection of effector proteins into the macrophage cytosol via the T3SS (45), and a reduced green fluorescence signal (Fig. 4) and an increased red fluorescence signal were observed. In contrast, the virulence plasmid-cured strain had no or little effect on macrophage viability compared to uninfected cells (not shown). The Sytox Orange results are not shown since they display similar but opposite trends. However, they are a fast indicator of toxicity of the compounds for macrophages and show that the compounds were not toxic to macrophages (reference 3 and data not shown). INP0404 and INP0405 showed the strongest inhibitory effect on the T3SS-mediated macrophage killing, and they restored the green fluorescence signal to the same level as for uninfected cells at 20 μM (not shown) and 50 μM (Fig. 4), respectively. INP0400, INP0401, and INP0402 had less of an effect on inhibiting cell death, while the negative control, INP0406, had little inhibiting effect (Fig. 4). This assay provides information on virulence inhibition and toxicity and shows that this class of compounds clearly inhibits T3SS-mediated virulence and helps the macrophages clear the infection.
FIG. 4.
Survival of Shigella-infected macrophages. Cells of macrophage cell line J774 were infected with wild-type and plasmid-cured Shigella flexneri strain CCUG 29416 grown in the presence of the compounds. The fluorescence intensity was measured in a microplate reader (Calcein, 485/535 nm; Sytox Orange, 535/595 nm), and the relative amounts of living cells and cells with the cell membrane destroyed, respectively, were calculated. The bars show the mean values from three samples, and the standard deviations were calculated according to a Gauss formula suitable for percentage values. Cells infected by wild-type Shigella without addition showed a value of around 40% living cells. The value for uninfected cells was set to 100% living cells.
The secretion inhibitors affect assembly of the T3SS needle.
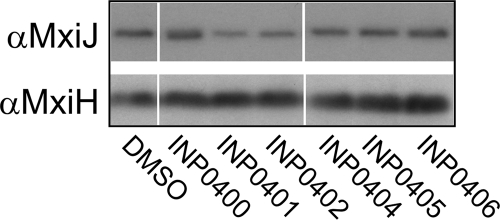
The results described so far show that the compounds have inhibitory effects on Shigella T3SS activity. This could result from an effect of the inhibitors on the assembly of the T3SS machinery. To investigate whether the drugs prevented expression of or destabilized T3S components, we examined the levels of MxiJ, an inner membrane protein of the NC, and MxiH, the needle subunit, in whole-cell extracts of bacteria exposed to the compounds. Western blotting showed that the levels of these proteins remained generally similar in all drug-treated samples and in particular compared to control compound INP0406 (Fig. 5) or a DMSO control. However, the possibility remained that the compounds affected assembly of the T3SS without an apparent effect on the levels of its protein components. To test this hypothesis, the macromolecular T3SS structure was analyzed by electron microscopy. Shigella cells from cultures grown in the presence of the inhibitors were osmotically shocked to produce ghost cells. In these cells, the cytoplasm is washed out to allow the visualization of the secretons at the cell periphery. The number of secretons per cell was decreased by 30 to 40% in all compound-treated samples, although the sample with the control compound INP0406 was much less affected (Fig. 6). The DMSO and INP0406 controls showed that 20% of secretons in each cell lacked a detectable needle. We term these needleless secretons “bases.” About half of these can be attributed to assembly of immature and thus incomplete secretons (A. J. Blocker, unpublished data), while the rest are likely caused by the mechanical force (vortexing with glass beads) applied during the preparation of ghost cells. More importantly, the percentage of secretons without a detectable needle (percentage of bases/secreton) increased significantly for inhibitors INP0400 to INP0404 (twofold increase) (Fig. 6). This increase in needleless secretons is probably an underestimate because bases are harder to identify with certainty. Indeed, in view of the fact that the levels of bacterial MxiJ and MxiH remain constant, nondetection of bases may also explain the apparent decrease in total secreton numbers in the samples treated with the active drugs.
FIG. 5.
Whole-cell levels of T3SS component proteins. Wild-type Shigella cultures were grown in the presence of DMSO or compounds, and then whole-cell extracts were isolated. Samples were separated by SDS-PAGE and analyzed by Western blotting. The antibodies used for the blots are indicated on the left. Lanes were removed for consistency of display of the samples. For each blot, the same exposure was used to make the composite image, and the remaining lanes are shown in their initial loading order.
FIG. 6.
Analysis of secreton assembly. Quantification of complete secretons and needleless bases from electron micrographs of ghost cells prepared from wild-type Shigella cultures grown in the presence of DMSO or compounds is shown. Data are derived from experiments in duplicate or triplicate (n = 10 to 13 images per sample per experiment). Micrographs of the DMSO control showed ∼5 secretons/image and 19% ± 5% bases/secretons (not shown). Percentages of secretons (dark gray bars) are relative to the DMSO control (set to 100%). Percentages of bases/secretons (light gray bars) are normalized to the DMSO control for each experiment separately. The errors given are standard deviations.
To assess the effect of the inhibitors on the assembly of the T3SS needle in greater detail, NCs were purified from Shigella cultures grown in the presence of DMSO, INP0400, or INP0406 (see “NC purification” in Materials and Methods). For this, the mxiG/pSZ3 Shigella strain, which produces secretons with a His-tagged MxiG inner membrane component that allows affinity purification of NCs (44), was used. The proportion of NCs that lacked the needle structure was markedly increased in these samples (data not shown) relative to the percentage of needleless bases observed in osmotically shocked cells. As this proportion did not change in the different samples, it was assumed to result primarily from mechanical damage occurring to NCs during isolation. Therefore, in this assay we have discounted the needleless structures and instead focused on those that retained the needle. The needle lengths of the latter NCs were measured from electron microscopy images and the length distribution determined. The needles were assigned to four different classes of needle length, i.e., less than 35, 35 to 63, 63 to 91, and 91 to 112 nm (Table 3). For all samples, the majority of needles were in the 35- to 63-nm range. However, NCs purified from cultures treated with the inhibitor INP0400 had significantly increased numbers of needles shorter than 35 nm (and consequently decreased numbers of needles in the 35- to 63-nm class) than the DMSO and INP0406 controls. To exclude the possibility of a destabilization or depolymerization effect of INP0400 on the needles, NCs were purified from a Shigella culture grown in the absence of any compound and INP0400 was added after the spheroplasting step, just before cell lysis. These NCs displayed a needle length distribution similar to that of the DMSO and INP0406 controls (Table 3, INP0400C). When the statistical significance of these findings was tested, the distribution of needle lengths in the INP0400 sample was found to differ from those of all other samples at a P value of 7.6 × 10−9, whereas the distributions of needle lengths in all other samples were similar to the expected frequencies in the control samples (P values were all above 0.02). Taken together, these results show that the inhibitors affect assembly of the needle.
DISCUSSION
We have shown that a variety of tested small-molecule compounds (INP0400 to INP0402, INP0404, and INP0405) inhibit all known forms of T3SS secretion in Shigella, as well as this bacterium's ability to invade HeLa cells and cause apoptosis of macrophages. We further showed that these compounds partially block the assembly of the Shigella T3SS. The block was found to occur at the level of export of the first substrate(s) in the secretion hierarchy, the needle protein component(s). This step is a highly conserved part of the T3SS/flagellar assembly pathway, as is further supported by a previous finding that these drugs also inhibit motility/flagellar protein surface presentation. In light of these observations, we propose that the inhibition of T3SS assembly is the primary effect of these drugs in gram-negative bacteria.
The results from the variety of secretion assays performed show differences in the spectra of activity of the tested compounds. They may result from the different functional states of the T3SS and the different set of substrates under conditions of leakage and induced secretion in wild-type cells and (fast) constitutive secretion in T3SS mutants (2, 6, 11, 13, 23, 25, 27, 34, 35, 39, 42). Thus, we propose that the different T3SS activity states are differentially sensitive to the action of the drugs. Such differences, together with the divergence in T3SSs, may explain why the groups of compounds that are most effective against the tested species of T3SS-carrying bacteria differ (3, 15, 18, 28-30, 38, 43). There are also differences in the spectra of activity of the tested compounds in the HeLa cell invasion and macrophage cytotoxicity assays. In contrast to the case for the similarly set up protein secretion assays, the drugs were added at the start of bacterial growth in the HeLa cell invasion assay, while they were added just prior to infection in the macrophage cytotoxicity assay. Thus, the drugs can be taken up by the bacterial cell prior to HeLa cell invasion, while they have to cross the macrophage membrane first to reach the intracellular bacteria. Therefore, these differences may reflect differences in properties of uptake of the compounds across the bacterial and macrophage cell envelope. In addition, altered solubility of the compounds in the different media when added in these assays may play a role. However, the compounds all belong to the same chemical class of hydrazones, and given their strong similarity in chemical structure, it is likely that the effective compounds have the same molecular target(s), albeit with expected different binding affinities. This is supported by the fact that INP0400 was found to be the most effective compound in all but the macrophage survival assay, which first requires drug uptake by eukaryotic cells.
While all forms of T3SS-mediated secretion in Shigella were inhibited, the strongest effect was observed when the compounds were added during growth. No inhibition of CR-induced secretion was seen when the compounds were added less than 30 min prior to cell harvesting (not shown). Invasion of cells was also not inhibited when the drugs were added only during incubation of Shigella with the HeLa cells instead of during bacterial growth (not shown). Our findings are consistent with a study by Nordfelth and coworkers (30) in which they observed a delay of 30 min in the effect of the inhibitors on the Yersinia T3SS yopE promoter activity. Furthermore, Hudson et al. (16) showed that Salmonella-induced intestinal secretory and inflammatory responses in calves can be inhibited by preincubation of the bacteria with structurally related compounds but not by treatment of the bacteria with these chemicals at the time of inoculation. Interestingly, drug pretreatment had no effect on Chlamydia elementary bodies, which are unable to grow in vitro, whereas addition of the compounds after elementary body infection of eukaryotic cells leads to strong inhibition of bacterial replication within intracellular vacuoles, a phenomenon known to involve a T3SS (38). After overnight growth, it is during renewed exponential growth at 37°C that Shigella secretons are assembled de novo (Blocker, unpublished data). Hence, these data are consistent with those we obtained by electron microscopy showing that the compounds affect needle elongation, a key step in secreton maturation. Indeed, it is unlikely that the effects of the drugs are due to destabilization of preassembled secretons or depolymerization of needle subunits because (i) they were ineffective when added after bacterial growth, (ii) the levels of MxiJ and MxiH in whole-cell extracts were unaffected by addition of any of the compounds, and (iii) addition of INP0400 prior to the NC purification step, rather than during growth, did not lead to significantly shorter needles.
Our electron microscopy data demonstrate that the T3SS assembly block is not complete. Nevertheless, the assembly defect generated by the drugs is very reminiscent of the phenotype of many mutants with mutations in the cytoplasmic or inner membrane T3S export apparatus (40). This is why we propose that the compounds generate a phenocopy of T3SS export apparatus mutants with incomplete penetrance. The incomplete penetrance is probably due to the weak binding affinity of these (early-generation) drugs for their target(s). That would explain why the drugs act efficaciously only when added during growth of bacteria or during long incubation times when the bacteria are within host cells (as in the macrophage killing assay). However, the drugs significantly slow or even stochastically abolish needle maturation. Needle assembly must be complete before the secreton becomes capable of host cell interaction and effector protein secretion (reviewed in reference 10). This would explain the strong inhibitory effect of the drugs in our T3SS-related secretion and virulence assays. Drug removal would allow rapid completion of needle assembly and consequently the recovery of T3SS activity (30).
These conclusions are also largely consistent with the work of Negrea et al. (29), who showed that a partially overlapping set of salicylidene acylhydrazides compounds inhibit secretion of SipB (the IpaB homolog) by the Salmonella SPI1 T3SS, intracellular growth of this bacterium (which is mediated by the SPI2 T3SS), and flagellum-mediated motility in this organism. Of particular relevance, they showed that the compounds reduce surface presentation of flagellin. This would be expected if the compounds led to a partial block in hook assembly, which is the morphological equivalent of the T3SS needle, as there is a known transcriptional feedback mechanism that prevents filament biogenesis when the hook is incomplete (7). Negrea et al. (29) also showed that treatment with the compounds led to transcriptional silencing of the SPI1 region that would contribute to the effect of the drugs. That could imply the existence of a negative regulatory mechanism that leads to SPI1 transcriptional silencing following inhibition of T3S, even though such a mechanism has not been observed to date.
In summary, our data suggest that the primary effect of these compounds is on T3SS export apparatuses. Thus, their direct or indirect target(s) probably lies within the inner membrane or cytoplasmic export apparatus components of both flagella and the T3SS. This explains why an incomplete block in T3SS assembly results in a clear inhibition of T3SS activity. As these components are the most conserved ones between the two related families of protein secretion machines, it is not surprising that the compounds exhibit such a broad efficacy spectrum (3, 15, 18, 28-30, 38, 43) (Table 1).
Future work should focus on the elucidation of the exact molecular target(s) of these exciting compounds within T3SSs. This work is urgently needed, as target identification is the only path toward rational improvement of the affinity of these compounds for their target(s) and hence of their efficacy. Such an effort is justified by the fact that these compounds are presently the only type well-known to broadly inhibit T3SS assembly and hence function (although two other families of T3SS inhibitors have been identified recently) (14, 16, 17, 32), representing an entirely new therapeutic opportunity.
Supplementary Material
Acknowledgments
We thank Pia Keyser (Innate Pharmaceuticals AB, Umeå, Sweden) for her help and stimulating discussions.
A.K.J.V. was funded by an E.C. Marie Curie postdoctoral fellowship (MEIF-CT-2005-023694), and A.J.B. was funded by the Guy G. F. Newton Senior Research Fellowship.
Footnotes
Published ahead of print on 7 November 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Adam, T., M. Arpin, M. C. Prevost, P. Gounon, and P. J. Sansonetti. 1995. Cytoskeletal rearrangements and the functional role of T-plastin during entry of Shigella flexneri into HeLa cells. J. Cell Biol. 129367-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahrani, F. K., P. J. Sansonetti, and C. Parsot. 1997. Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect. Immun. 654005-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey, L., A. Gylfe, C. Sundin, S. Muschiol, M. Elofsson, P. Nordstrom, B. Henriques-Normark, R. Lugert, A. Waldenstrom, H. Wolf-Watz, and S. Bergstrom. 2007. Small molecule inhibitors of type III secretion in Yersinia block the Chlamydia pneumoniae infection cycle. FEBS Lett. 581587-595. [DOI] [PubMed] [Google Scholar]
- 4.Blocker, A., P. Gounon, E. Larquet, K. Niebuhr, V. Cabiaux, C. Parsot, and P. Sansonetti. 1999. The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blocker, A., N. Jouihri, E. Larquet, P. Gounon, F. Ebel, C. Parsot, P. Sansonetti, and A. Allaoui. 2001. Structure and composition of the Shigella flexneri “needle complex,” a part of its type III secreton. Mol. Microbiol. 39652-663. [DOI] [PubMed] [Google Scholar]
- 6.Blocker, A., K. Komoriya, and S. Aizawa. 2003. Type III secretion systems and bacterial flagella: insights into their function from structural similarities. Proc. Natl. Acad. Sci. USA 1003027-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chevance, F. F., and K. T. Hughes. 2008. Coordinating assembly of a bacterial macromolecular machine. Nat. Rev. Microbiol. 6455-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerc, P., and P. J. Sansonetti. 1987. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect. Immun. 552681-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordes, F. S., K. Komoriya, E. Larquet, S. Yang, E. H. Egelman, A. Blocker, and S. M. Lea. 2003. Helical structure of the needle of the type III secretion system of Shigella flexneri. J. Biol. Chem. 27817103-17107. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis, G. R. 2006. The type III secretion injectisome. Nature reviews. 4811-825. [DOI] [PubMed] [Google Scholar]
- 11.Deane, J. E., S. C. Graham, E. P. Mitchell, D. Flot, S. Johnson, and S. M. Lea. 2008. Crystal structure of Spa40, the specificity switch for the Shigella flexneri type III secretion system. Mol. Microbiol. 69267-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espina, M., A. J. Olive, R. Kenjale, D. S. Moore, S. F. Ausar, R. W. Kaminski, E. V. Oaks, C. R. Middaugh, W. D. Picking, and W. L. Picking. 2006. IpaD localizes to the tip of the type III secretion system needle of Shigella flexneri. Infect. Immun. 744391-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferris, H. U., and T. Minamino. 2006. Flipping the switch: bringing order to flagellar assembly. Trends Microbiol. 14519-526. [DOI] [PubMed] [Google Scholar]
- 14.Gauthier, A., M. L. Robertson, M. Lowden, J. A. Ibarra, J. L. Puente, and B. B. Finlay. 2005. Transcriptional inhibitor of virulence factors in enteropathogenic Escherichia coli. Antimicrob. Agents Chemother. 494101-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson, D. L., A. N. Layton, T. R. Fierhprrld, A. J. Bowen, H. Wolf-Watz, M. Elofsson, M. P. Stevens, and E. E. Galyov. 2007. Inhibition of type III secretion in Salmonella enterica serovar Typhimurium by small-molecule inhibitors. Antimicrob. Agents Chemother. 512631-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwatsuki, M., R. Uchida, H. Yoshijima, H. Ui, K. Shiomi, Y. P. Kim, T. Hirose, T. Sunazuka, A. Abe, H. Tomoda, and S. Omura. 2008. Guadinomines, type III secretion system inhibitors, produced by Streptomyces sp. K01-0509. II. Physico-chemical properties and structure elucidation. J. Antibiot. (Tokyo) 61230-236. [DOI] [PubMed] [Google Scholar]
- 17.Iwatsuki, M., R. Uchida, H. Yoshijima, H. Ui, K. Shiomi, A. Matsumoto, Y. Takahashi, A. Abe, H. Tomoda, and S. Omura. 2008. Guadinomines, type III secretion system inhibitors, produced by Streptomyces sp. K01-0509. I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. (Tokyo). 61222-229. [DOI] [PubMed] [Google Scholar]
- 18.Kauppi, A. M., R. Nordfelth, H. Uvell, H. Wolf-Watz, and M. Elofsson. 2003. Targeting bacterial virulence: inhibitors of type III secretion in Yersinia. Chem. Biol. 10241-249. [DOI] [PubMed] [Google Scholar]
- 19.Kenjale, R., J. Wilson, S. F. Zenk, S. Saurya, W. L. Picking, W. D. Picking, and A. Blocker. 2005. The needle component of the type III secreton of Shigella regulates the activity of the secretion apparatus. J. Biol. Chem. 28042929-42937. [DOI] [PubMed] [Google Scholar]
- 20.Keyser, P., M. Elofsson, S. Rosell, and H. Wolf-Watz. 2008. Virulence blockers as alternatives to antibiotics: type III secretion inhibitors against Gram-negative bacteria. J. Intern. Med. 26417-29. [DOI] [PubMed] [Google Scholar]
- 21.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galan, and S. I. Aizawa. 1998. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280602-605. [DOI] [PubMed] [Google Scholar]
- 22.Le Bouguenec, C., L. Lalioui, L. du Merle, M. Jouve, P. Courcoux, S. Bouzari, R. Selvarangan, B. J. Nowicki, Y. Germani, A. Andremont, P. Gounon, and M. I. Garcia. 2001. Characterization of AfaE adhesins produced by extraintestinal and intestinal human Escherichia coli isolates: PCR assays for detection of Afa adhesins that do or do not recognize Dr blood group antigens. J. Clin. Microbiol. 391738-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magdalena, J., A. Hachani, M. Chamekh, N. Jouihri, P. Gounon, A. Blocker, and A. Allaoui. 2002. Spa32 regulates a switch in substrate specificity of the type III secreton of Shigella flexneri from needle components to Ipa proteins. J. Bacteriol. 1843433-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurelli, A. T., B. Blackmon, and R. Curtiss III. 1984. Loss of pigmentation in Shigella flexneri 2a is correlated with loss of virulence and virulence-associated plasmid. Infect. Immun. 43397-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menard, R., P. Sansonetti, and C. Parsot. 1994. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 135293-5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 1755899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minamino, T., R. Chu, S. Yamaguchi, and R. M. Macnab. 2000. Role of FliJ in flagellar protein export in Salmonella. J. Bacteriol. 1824207-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muschiol, S., L. Bailey, A. Gylfe, C. Sundin, K. Hultenby, S. Bergstrom, M. Elofsson, H. Wolf-Watz, S. Normark, and B. Henriques-Normark. 2006. A small-molecule inhibitor of type III secretion inhibits different stages of the infectious cycle of Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 10314566-14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negrea, A., E. Bjur, S. E. Ygberg, M. Elofsson, H. Wolf-Watz, and M. Rhen. 2007. Salicylidene acylhydrazides that affect type III protein secretion in Salmonella enterica serovar typhimurium. Antimicrob. Agents Chemother. 512867-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordfelth, R., A. M. Kauppi, H. A. Norberg, H. Wolf-Watz, and M. Elofsson. 2005. Small-molecule inhibitors specifically targeting type III secretion. Infect. Immun. 733104-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olive, A. J., R. Kenjale, M. Espina, D. S. Moore, W. L. Picking, and W. D. Picking. 2007. Bile salts stimulate recruitment of IpaB to the Shigella flexneri surface, where it colocalizes with IpaD at the tip of the type III secretion needle. Infect. Immun. 752626-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan, N., C. Lee, and J. Goguen. 2007. High throughput screening for small-molecule inhibitors of type III secretion in Yersinia pestis. Adv. Exp. Med. Biol. 603367-375. [DOI] [PubMed] [Google Scholar]
- 33.Parsot, C. 1994. Shigella flexneri: genetics of entry and intercellular dissemination in epithelial cells. Curr. Top. Microbiol. Immunol. 192217-241. [DOI] [PubMed] [Google Scholar]
- 34.Parsot, C., R. Menard, P. Gounon, and P. J. Sansonetti. 1995. Enhanced secretion through the Shigella flexneri Mxi-Spa translocon leads to assembly of extracellular proteins into macromolecular structures. Mol. Microbiol. 16291-300. [DOI] [PubMed] [Google Scholar]
- 35.Picking, W. L., L. Coye, J. C. Osiecki, A. Barnoski Serfis, E. Schaper, and W. D. Picking. 2001. Identification of functional regions within invasion plasmid antigen C (IpaC) of Shigella flexneri. Mol. Microbiol. 39100-111. [DOI] [PubMed] [Google Scholar]
- 36.Sani, M., A. Botteaux, C. Parsot, P. Sansonetti, E. J. Boekema, and A. Allaoui. 2007. IpaD is localized at the tip of the Shigella flexneri type III secretion apparatus. Biochim. Biophys. Acta 1770307-311. [DOI] [PubMed] [Google Scholar]
- 37.Sansonetti, P. J., D. J. Kopecko, and S. B. Formal. 1982. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect. Immun. 35852-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slepenkin, A., P. A. Enquist, U. Hagglund, L. M. de la Maza, M. Elofsson, and E. M. Peterson. 2007. Reversal of the antichlamydial activity of putative type III secretion inhibitors by iron. Infect. Immun. 753478-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorg, I., S. Wagner, M. Amstutz, S. A. Muller, P. Broz, Y. Lussi, A. Engel, and G. R. Cornelis. 2007. YscU recognizes translocators as export substrates of the Yersinia injectisome. EMBO J. 263015-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sukhan, A., T. Kubori, J. Wilson, and J. E. Galan. 2001. Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex. J. Bacteriol. 1831159-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran Van Nhieu, G., A. Ben-Ze'ev, and P. J. Sansonetti. 1997. Modulation of bacterial entry into epithelial cells by association between vinculin and the Shigella IpaA invasin. EMBO J. 162717-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veenendaal, A. K., J. L. Hodgkinson, L. Schwarzer, D. Stabat, S. F. Zenk, and A. J. Blocker. 2007. The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol. Microbiol. 631719-1730. [DOI] [PubMed] [Google Scholar]
- 43.Wolf, K., H. J. Betts, B. Chellas-Gery, S. Hower, C. N. Linton, and K. A. Fields. 2006. Treatment of Chlamydia trachomatis with a small molecule inhibitor of the Yersinia type III secretion system disrupts progression of the chlamydial developmental cycle. Mol. Microbiol. 611543-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zenk, S. F., D. Stabat, J. L. Hodgkinson, A. K. J. Veenendaal, S. Johnson, and A. J. Blocker. 2007. Identification of minor inner-membrane components of the Shigella type III secretion system ‘needle complex’. Microbiology 1532405-2415. [DOI] [PubMed] [Google Scholar]
- 45.Zychlinsky, A., M. C. Prevost, and P. J. Sansonetti. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358167-169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.