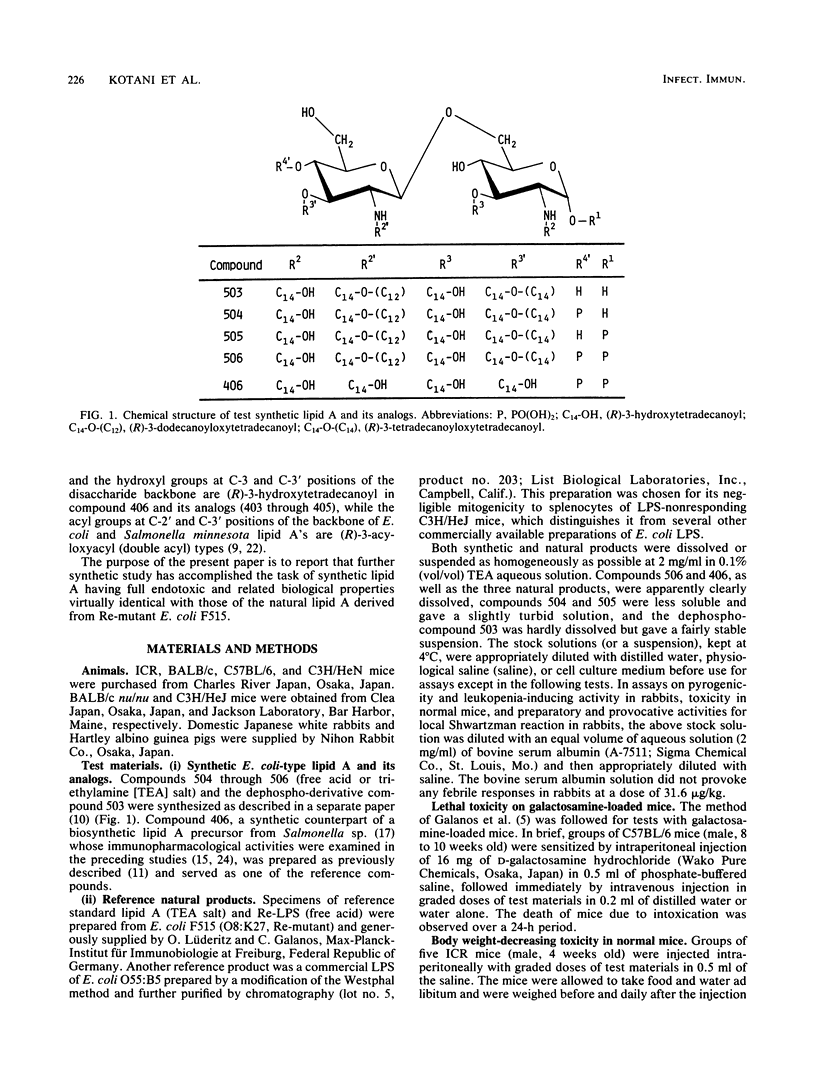
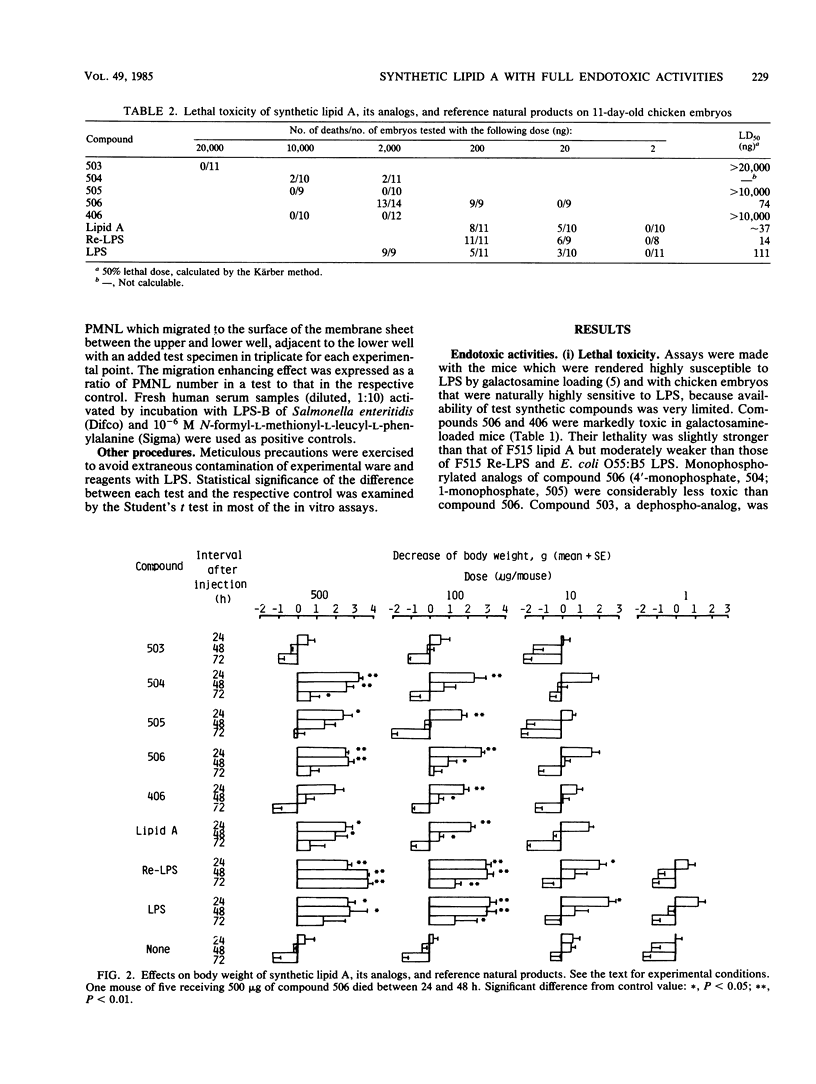
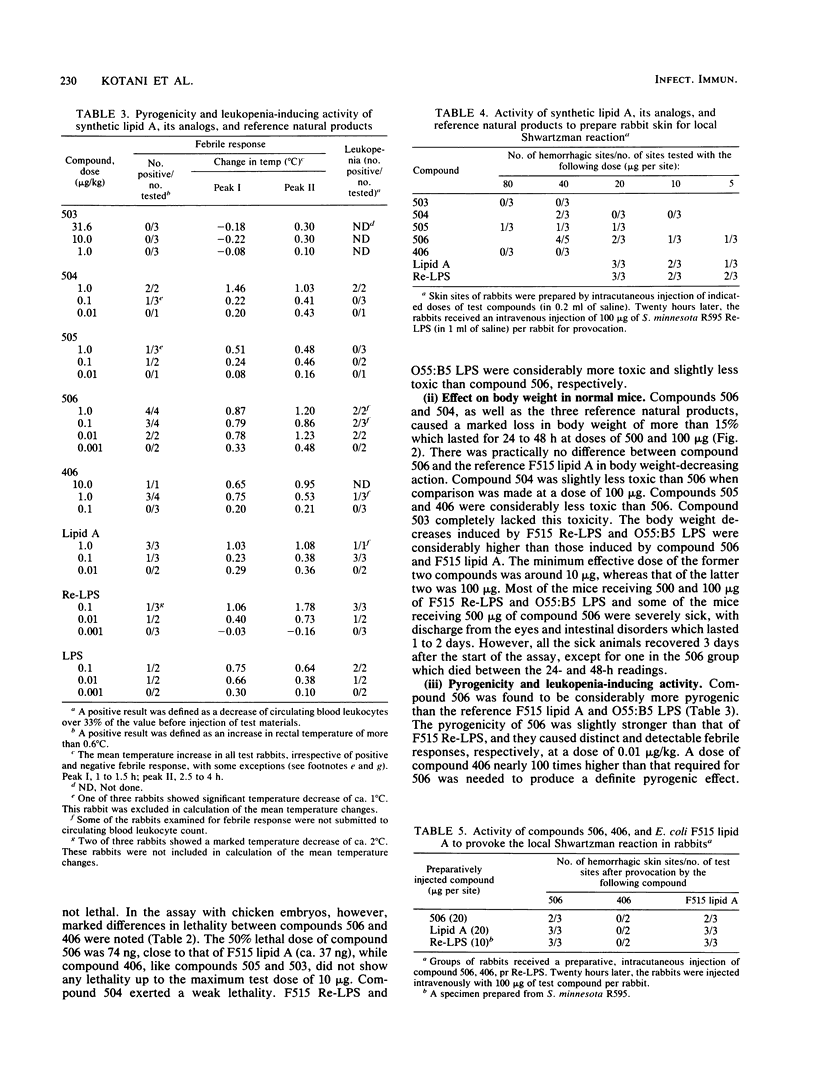
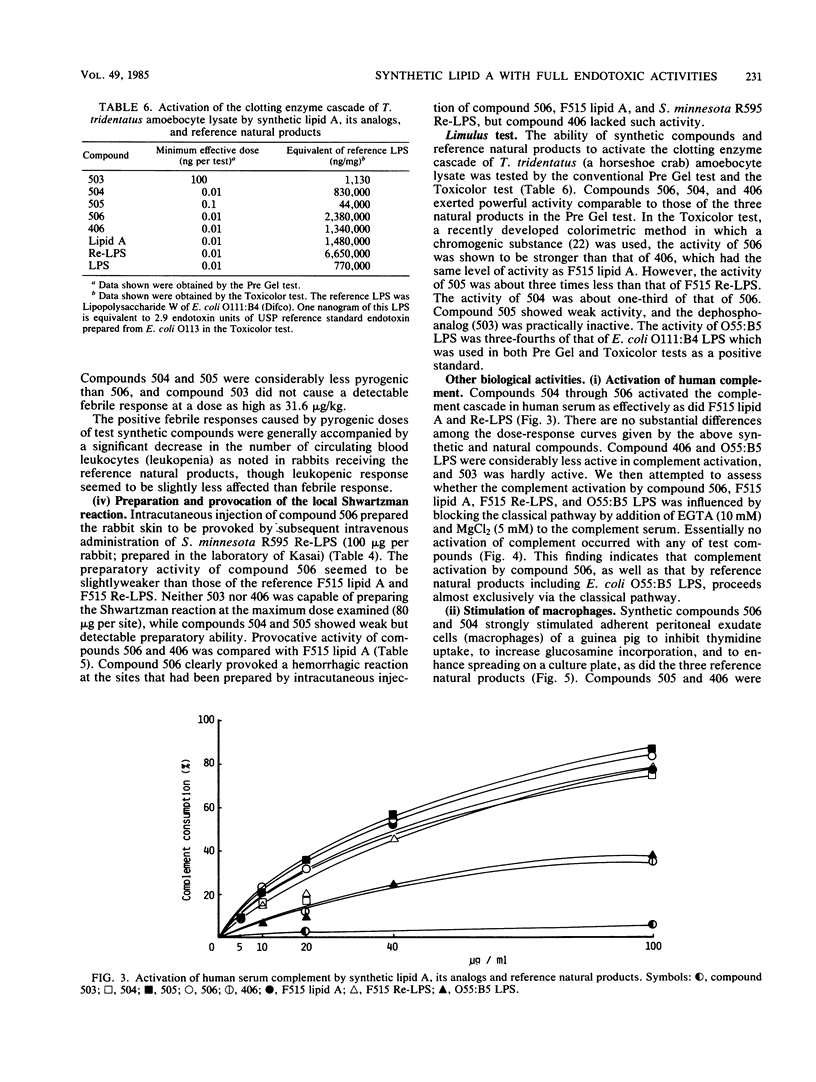
Abstract
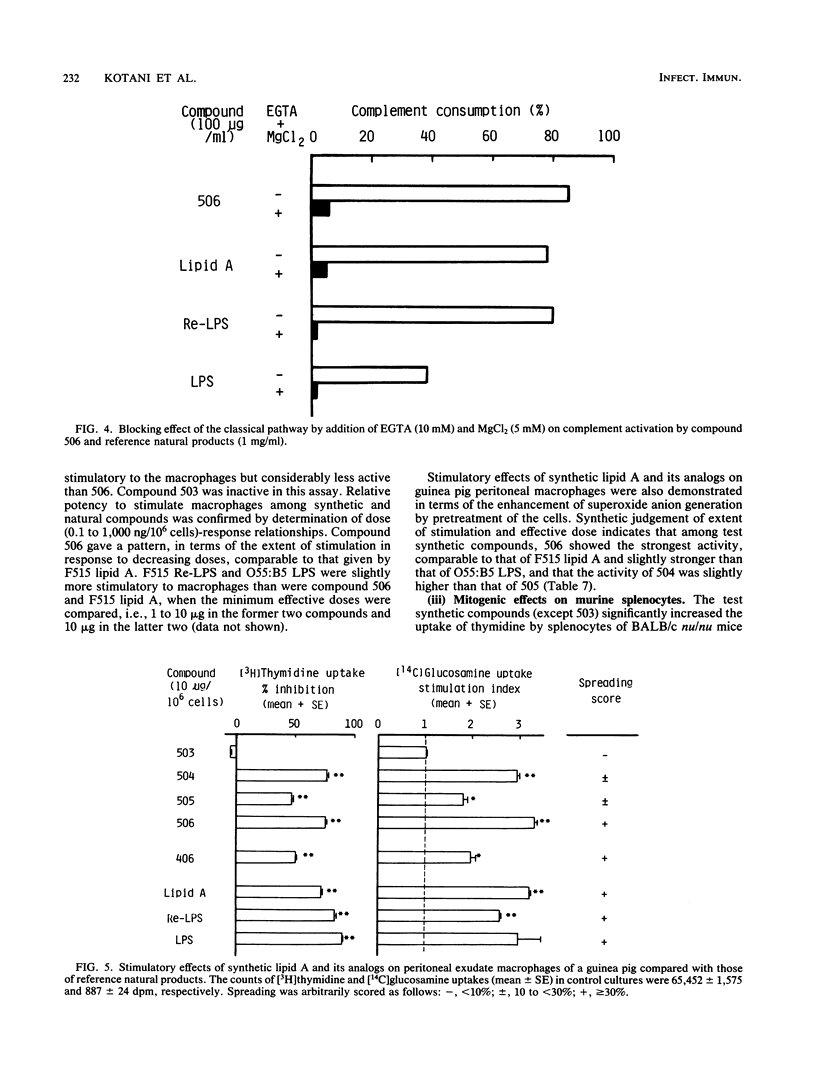
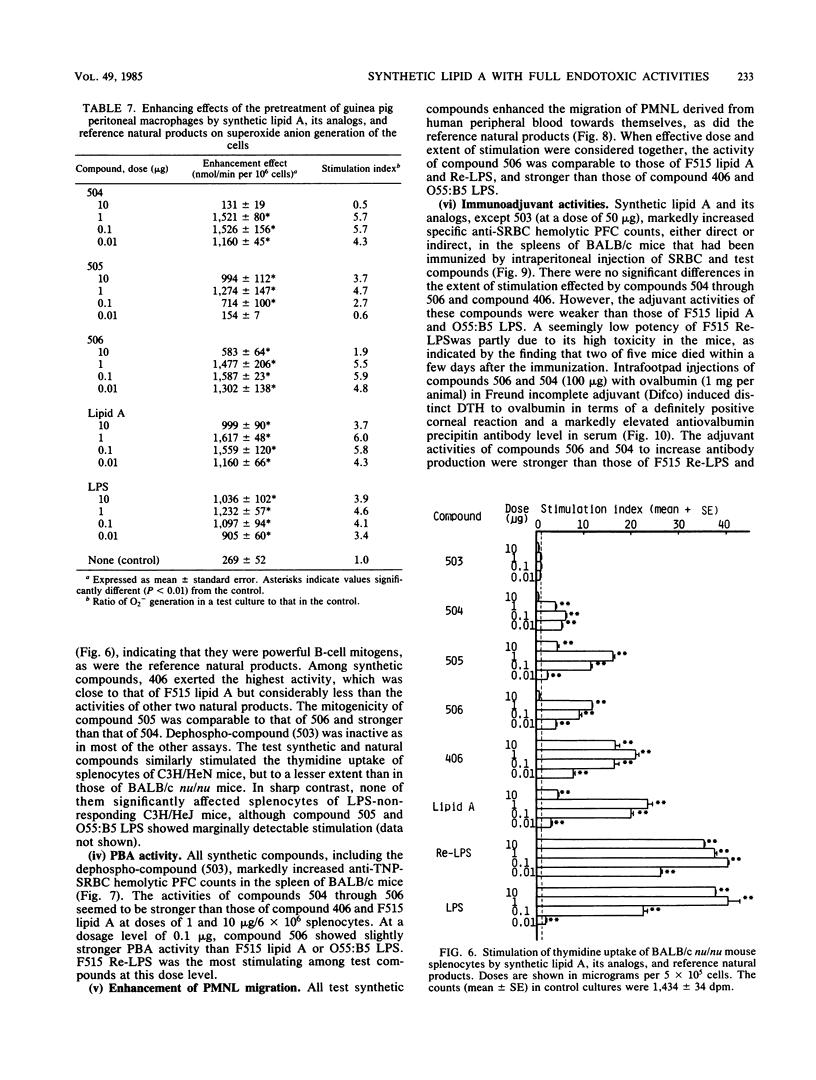
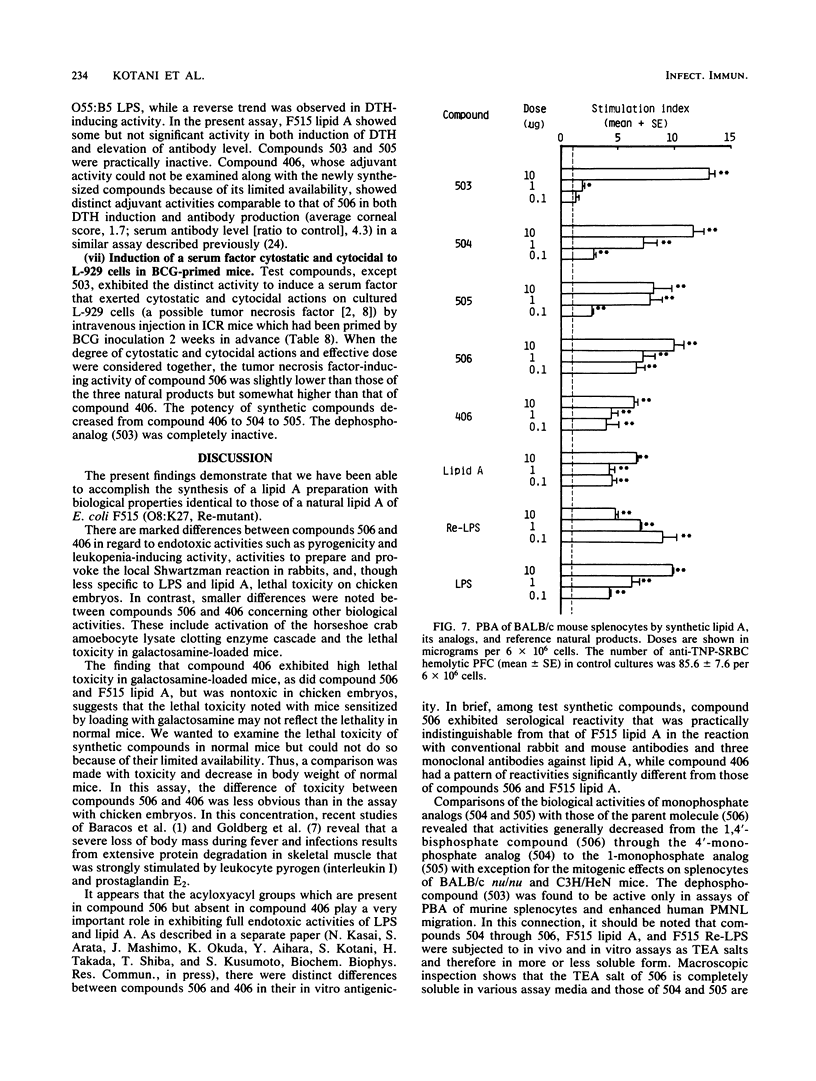
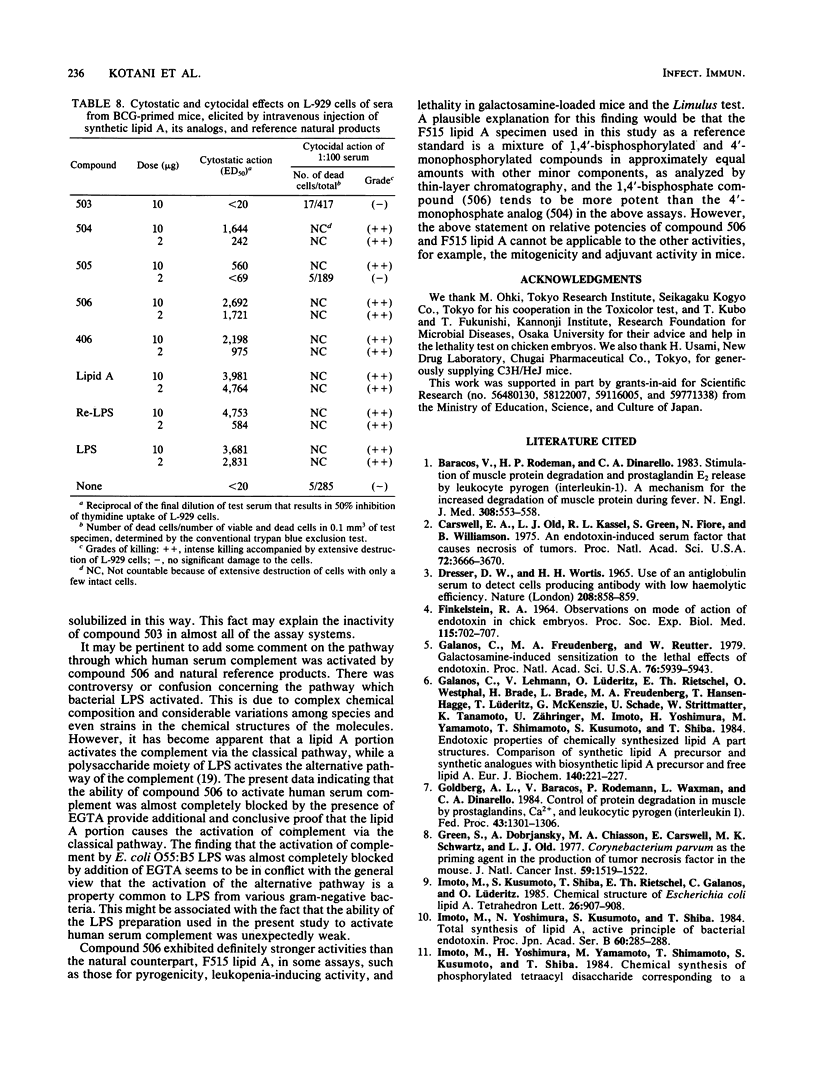
A synthetic compound (506), beta (1-6) D-glucosamine disaccharide 1,4'-bisphosphate, which is acylated at 2'-amino and 3'-hydroxyl groups with (R)-3-dodecanoyloxytetradecanoyl and (R)-3-tetradecanoyloxytetradecanoyl groups, respectively, and has (R)-3-hydroxytetradecanoyl groups at 2-amino and 3-hydroxyl groups, exhibited full endotoxic activities identical to or sometimes stronger than those of a reference lipid A from an Escherichia coli Re-mutant (strain F515). Endotoxic activities tested include pyrogenicity and leukopenia-inducing activity in rabbits, body weight-decreasing toxicity in normal mice, lethal toxicity in galactosamine-sensitized mice and chicken embryos, and the preparation and provocation of the local Shwartzman reaction in rabbits. Compound 406, a synthetic counterpart of a biosynthetic precursor of lipid A molecule, showed by contrast only weak activities in all of the above assay systems except for the lethality in galactosamine-loaded mice. This finding strongly suggests that the presence of acyloxyacyl groups at the C-2' and C-3' positions of the disaccharide backbone is one of the most important determinant structures of the lipid A molecule for exhibition of strong biological activities characteristic of lipopolysaccharide and its lipid A moiety. The activities of the corresponding 4'-monophosphate (compound 504) and 1-monophosphate (505) analogs were considerably less than those of the parent molecule 506 and the reference F515 lipid A. Regarding other biological activities, not only compound 506 but also compounds 504, 505, and 406 showed definite activities, sometimes comparable to those of F515 lipid A and other reference natural products. These are the activation of Tachypleus tridentatus amoebocyte clotting enzyme cascade and human complement via the classical pathway, mitogenic and polyclonal B-cell activation of murine splenocytes, stimulation of peritoneal macrophages in a guinea pig, enhancement of migration of human blood polymorphonuclear leukocytes, and induction of a serum factor that is cytostatic and cytocidal to L-929 cells in Mycobacterium bovis BCG-primed mice. Relative potencies of test synthetic compounds depended on the assay systems and varied from one system to another. Dephospho-compound 503 lacked most of the biological activities that were definitely observed with phosphorylated compounds, probably because of its insolubility. This study demonstrates the successful chemical synthesis of an E. coli-type lipid A.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser D. W., Wortis D. H. Use of an antiglobulin serum to detect cells producing antibody with low haemolytic efficiency. Nature. 1965 Nov 27;208(5013):859–861. doi: 10.1038/208859a0. [DOI] [PubMed] [Google Scholar]
- FINKELSTEIN R. A. OBSERVATIONS ON MODE OF ACTION OF ENDOTOXIN IN CHICK EMBRYOS. Proc Soc Exp Biol Med. 1964 Mar;115:702–707. doi: 10.3181/00379727-115-29012. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Baracos V., Rodemann P., Waxman L., Dinarello C. Control of protein degradation in muscle by prostaglandins, Ca2+, and leukocytic pyrogen (interleukin 1). Fed Proc. 1984 Apr;43(5):1301–1306. [PubMed] [Google Scholar]
- Green S., Dobrjansky A., Chiasson M. A., Carswell E., Schwartz M. K., Old L. J. Corynebacterium parvum as the priming agent in the production of tumor necrosis factor in the mouse. J Natl Cancer Inst. 1977 Nov;59(5):1519–1522. doi: 10.1093/jnci/59.5.1519. [DOI] [PubMed] [Google Scholar]
- Ishida S. Characterization of the body weight-decreasing toxicities in mice by the lymphocytosis-promoting factor and the heat-labile toxin of B. pertussis and endotoxin. Jpn J Med Sci Biol. 1968 Apr;21(2):115–135. doi: 10.7883/yoken1952.21.115. [DOI] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Harada K., Mori Y., Kawasaki A., Tanaka A., Nagao S., Tanaka S. Immunobiologically active lipid A analogs synthesized according to a revised structural model of natural lipid A. Infect Immun. 1984 Jul;45(1):293–296. doi: 10.1128/iai.45.1.293-296.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Mori Y., Sakuta M., Kawasaki A., Inage M., Kusumoto S., Shiba T. Immunobiological activities of synthetic lipid A analogs and related compounds as compared with those of bacterial lipopolysaccharide, re-glycolipid, lipid A, and muramyl dipeptide. Infect Immun. 1983 Aug;41(2):758–773. doi: 10.1128/iai.41.2.758-773.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann V. Isolation, purification and properties of an intermediate in 3-deoxy-D-manno-octulosonic acid--lipid A biosynthesis. Eur J Biochem. 1977 May 2;75(1):257–266. doi: 10.1111/j.1432-1033.1977.tb11525.x. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Lehmann V., Mayer H., Rietschel E. T., Weckesser J. Chemical structure and biological activities of lipid A's from various bacterial families. Naturwissenschaften. 1978 Nov;65(11):578–585. doi: 10.1007/BF00364907. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Nagao S., Tanaka A. Inhibition of macrophage DNA synthesis by immunomodulators. I. Suppression of [3H]thymidine incorporation into macrophages by MDP and LPS. Microbiol Immunol. 1983;27(4):377–387. doi: 10.1111/j.1348-0421.1983.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Obayashi T. Addition of perchloric acid to blood samples for colorimetric limulus test using chromogenic substrate: comparison with conventional procedures and clinical applications. J Lab Clin Med. 1984 Sep;104(3):321–330. [PubMed] [Google Scholar]
- Seydel U., Lindner B., Wollenweber H. W., Rietschel E. T. Structural studies on the lipid A component of enterobacterial lipopolysaccharides by laser desorption mass spectrometry. Location of acyl groups at the lipid A backbone. Eur J Biochem. 1984 Dec 17;145(3):505–509. doi: 10.1111/j.1432-1033.1984.tb08585.x. [DOI] [PubMed] [Google Scholar]
- Takada H., Kotani S., Tsujimoto M., Ogawa T., Takahashi I., Harada K., Katsukawa C., Tanaka S., Shiba T., Kusumoto S. Immunopharmacological activities of a synthetic counterpart of a biosynthetic lipid A precursor molecule and of its analogs. Infect Immun. 1985 Apr;48(1):219–227. doi: 10.1128/iai.48.1.219-227.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Imai K., Mori R. Macrophage activation by muramyl dipeptide as measured by macrophage spreading and attachment. Microbiol Immunol. 1980;24(6):547–557. doi: 10.1111/j.1348-0421.1980.tb02858.x. [DOI] [PubMed] [Google Scholar]
- Yagawa K., Kaku M., Ichinose Y., Nagao S., Tanaka A., Tomoda A. Biphasic effects of muramyl dipeptide or lipopolysaccharide on superoxide anion-generating activities of macrophages. Infect Immun. 1984 Jul;45(1):82–86. doi: 10.1128/iai.45.1.82-86.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


