Abstract
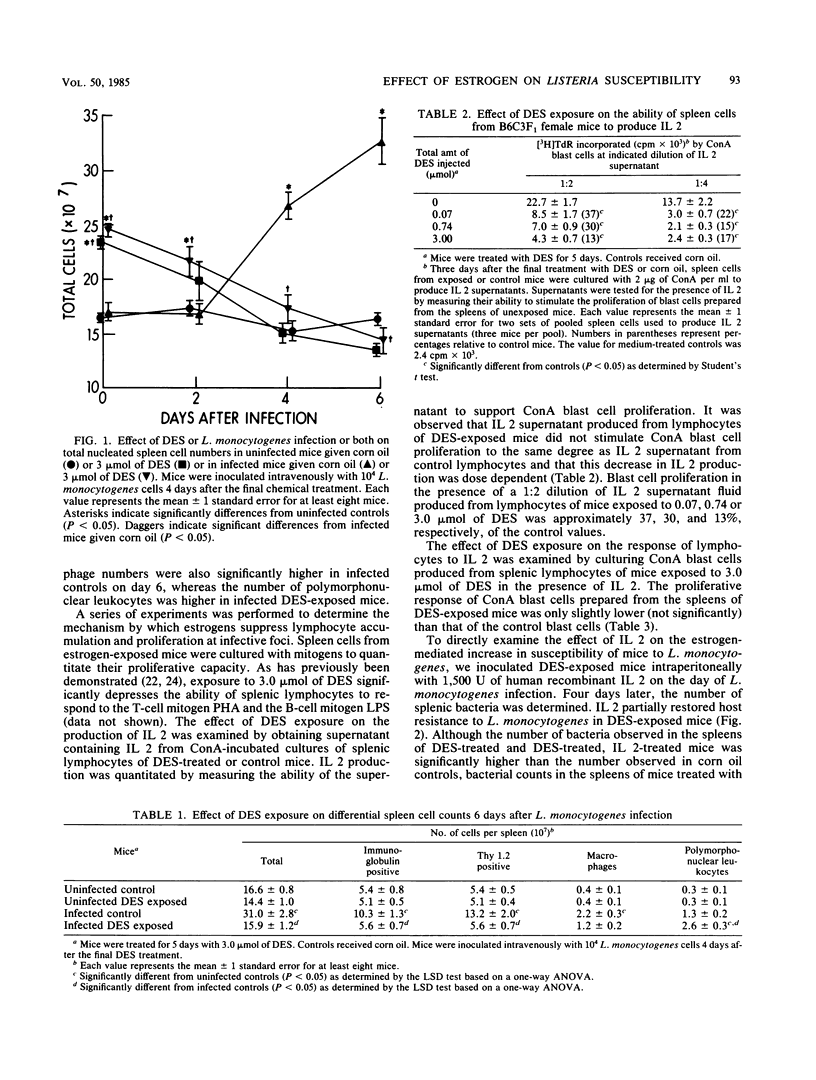
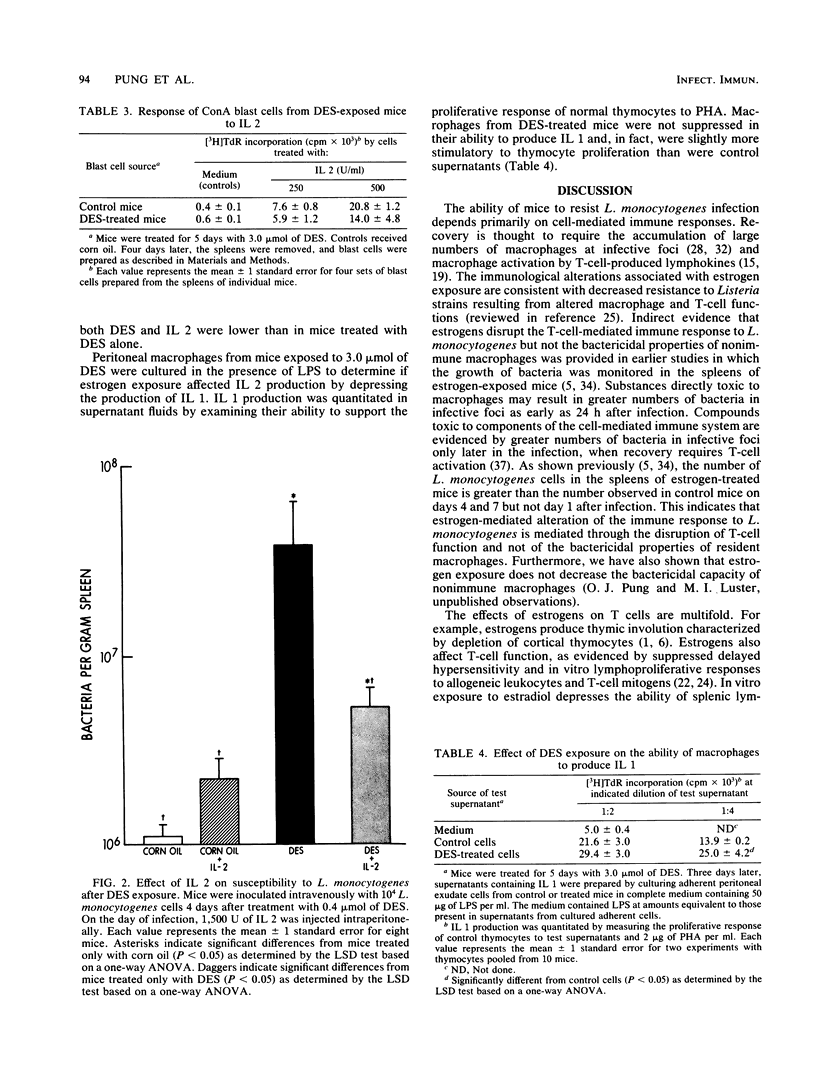
Mice given pharmacological levels of the synthetic estrogen diethylstilbestrol demonstrated a marked increase in susceptibility to infection with Listeria monocytogenes. Experiments were performed in an effort to determine the mechanism(s) by which estrogen treatment increases the susceptibility of mice to L. monocytogenes infection. Estrogen exposure depressed the in vivo proliferative response of splenic lymphocytes to L. monocytogenes, which correlated with the decreased in vitro response of these cells to phytohemagglutinin. Interleukin 2 (IL 2) production by splenic lymphocytes from estrogen-treated mice was decreased, although these cells were capable of proliferating normally in response to exogenous IL 2. Interleukin 1 production by peritoneal macrophages was not depressed by estrogen exposure. The number of bacteria observed in the spleens of estrogen-exposed mice challenged with L. monocytogenes was reduced by IL 2 administration. Thus, estrogens may decrease host resistance to L. monocytogenes by inhibiting IL 2 production and the subsequent proliferation of antigen-sensitized T lymphocytes required for recovery.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boorman G. A., Luster M. I., Dean J. H., Wilson R. E. The effect of adult exposure to diethylstilbestrol in the mouse on macrophage function and numbers. J Reticuloendothel Soc. 1980 Dec;28(6):547–560. [PubMed] [Google Scholar]
- Cole P. Activation of mouse peritoneal cells to kill Listeria monocytogenes by T-lymphocyte products. Infect Immun. 1975 Jul;12(1):36–41. doi: 10.1128/iai.12.1.36-41.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler B. S., Forbes A. P., Ingersoll F. M., Scully R. E. Endometrial carcinoma after stilbestrol therapy in gonadal dysgenesis. N Engl J Med. 1972 Sep 28;287(13):628–631. doi: 10.1056/NEJM197209282871302. [DOI] [PubMed] [Google Scholar]
- Dean J. H., Luster M. I., Boorman G. A., Luebke R. W., Lauer L. D. The effect of adult exposure to diethylstilbestrol in the mouse: alterations in tumor susceptibility and host resistance parameters. J Reticuloendothel Soc. 1980 Dec;28(6):571–583. [PubMed] [Google Scholar]
- Fugmann R. A., Aranyi C., Barbera P. W., Bradof J. N., Gibbons R. D., Fenters J. D. The effect of diethylstilbestrol as measured by host resistance and tumor susceptibility assays in mice. J Toxicol Environ Health. 1983 Apr-Jun;11(4-6):827–841. doi: 10.1080/15287398309530387. [DOI] [PubMed] [Google Scholar]
- Grossman C. J., Sholiton L. J., Nathan P. Rat thymic estrogen receptor--I. Preparation, location and physiochemical properties. J Steroid Biochem. 1979 Sep;11(3):1233–1240. doi: 10.1016/0022-4731(79)90190-0. [DOI] [PubMed] [Google Scholar]
- Henriksen O., Frey J. R. Control of the expression of interleukin-2 activity. Cell Immunol. 1982 Oct;73(1):106–114. doi: 10.1016/0008-8749(82)90439-7. [DOI] [PubMed] [Google Scholar]
- Herbst A. L., Ulfelder H., Poskanzer D. C. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971 Apr 15;284(15):878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- Hoffenbach A., Lagrange P. H., Bach M. A. Deficit of interleukin 2 production associated with impaired T-cell proliferative responses in Mycobacterium lepraemurium infection. Infect Immun. 1983 Jan;39(1):109–116. doi: 10.1128/iai.39.1.109-116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalland T. Decreased and disproportionate T-cell population in adult mice after neonatal exposure to diethylstilbestrol. Cell Immunol. 1980 Apr;51(1):55–63. doi: 10.1016/0008-8749(80)90237-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Effective antibacterial protection induced by a Listeria monocytogenes-specific T cell clone and its lymphokines. Infect Immun. 1983 Mar;39(3):1265–1270. doi: 10.1128/iai.39.3.1265-1270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H., Simon M. M., Röllinghoff M., Wagner H. Interleukin 2 induction in Lyt 1+ 23- T cells from Listeria monocytogenes-immune mice. Infect Immun. 1982 Sep;37(3):1292–1294. doi: 10.1128/iai.37.3.1292-1294.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns R. J., Campbell P. A. Production of migration inhibitory factor by Listeria-immune mouse T lymphocytes, but not B lymphocytes. Int Arch Allergy Appl Immunol. 1983;70(1):59–64. doi: 10.1159/000233274. [DOI] [PubMed] [Google Scholar]
- Kearns R. J., DeFreitas E. C. In vitro propagation of antigen-specific T lymphocytes that adoptively transfer resistance to Listeria monocytogenes. Infect Immun. 1983 May;40(2):713–719. doi: 10.1128/iai.40.2.713-719.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson E. L., Iscove N. N., Coutinho A. Two distinct factors are required for induction of T-cell growth. Nature. 1980 Feb 14;283(5748):664–666. doi: 10.1038/283664a0. [DOI] [PubMed] [Google Scholar]
- Luft B. J., Remington J. S. Effect of pregnancy on resistance to Listeria monocytogenes and Toxoplasma gondii infections in mice. Infect Immun. 1982 Dec;38(3):1164–1171. doi: 10.1128/iai.38.3.1164-1171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster M. I., Boorman G. A., Dean J. H., Luebke R. W., Lawson L. D. The effect of adult exposure to diethylstilbestrol in the mouse: alterations in immunological functions. J Reticuloendothel Soc. 1980 Dec;28(6):561–569. [PubMed] [Google Scholar]
- Luster M. I., Boorman G. A., Korach K. S., Dieter M. P., Hong L. Mechanisms of estrogen-induced myelotoxicity: evidence of thymic regulation. Int J Immunopharmacol. 1984;6(4):287–297. doi: 10.1016/0192-0561(84)90045-6. [DOI] [PubMed] [Google Scholar]
- Luster M. I., Hayes H. T., Korach K., Tucker A. N., Dean J. H., Greenlee W. F., Boorman G. A. Estrogen immunosuppression is regulated through estrogenic responses in the thymus. J Immunol. 1984 Jul;133(1):110–116. [PubMed] [Google Scholar]
- McMartin K. E., Kennedy K. A., Greenspan P., Alam S. N., Greiner P., Yam J. Diethylstilbestrol: a review of its toxicity and use as a growth promotant in food-producing animals. J Environ Pathol Toxicol. 1978 Jan-Feb;1(3):279–313. [PubMed] [Google Scholar]
- Meltzer M. S., Oppenheim J. J. Bidirectional amplification of macrophage-lymphocyte interactions: enhanced lymphocyte activation factor production by activated adherent mouse peritoneal cells. J Immunol. 1977 Jan;118(1):77–82. [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Morahan P. S., Bradley S. G., Munson A. E., Duke S., Fromtling R. A., Marciano-Cabral F. Immunotoxic effects of diethylstilbestrol on host resistance: comparison with cyclophosphamide. J Leukoc Biol. 1984 Mar;35(3):329–341. doi: 10.1002/jlb.35.3.329. [DOI] [PubMed] [Google Scholar]
- NICOL T., BILBEY D. L., CHARLES L. M., CORDINGLEY J. L., VERNON-ROBERTS B. OESTROGEN: THE NATURAL STIMULANT OF BODY DEFENCE. J Endocrinol. 1964 Oct;30:277–291. doi: 10.1677/joe.0.0300277. [DOI] [PubMed] [Google Scholar]
- Noller K. L., Kurland L. T. Study of prenatal DES exposure and its consequences in Rochester, Minnesota. J Toxicol Environ Health Suppl. 1976;1:1–11. [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pung O. J., Luster M. I., Hayes H. T., Rader J. Influence of steroidal and nonsteroidal sex hormones on host resistance in mice: increased susceptibility to Listeria monocytogenes after exposure to estrogenic hormones. Infect Immun. 1984 Nov;46(2):301–307. doi: 10.1128/iai.46.2.301-307.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind D., Frey J. A. Sex difference in antibody response of CFW mice to Candida albicans. Infect Immun. 1972 May;5(5):695–698. doi: 10.1128/iai.5.5.695-698.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimson W. H., Crilly P. J. Effects of steroids on the secretion of immunoregulatory factors by thymic epithelial cell cultures. Immunology. 1981 Oct;44(2):401–407. [PMC free article] [PubMed] [Google Scholar]
- Tripathy S. P., Mackaness G. B. The effect of cytotoxic agents on the primary immune response to Listeria monocytogenes. J Exp Med. 1969 Jul 1;130(1):1–16. doi: 10.1084/jem.130.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umiel T., Pecht M., Trainin N. THF, a thymic hormone, promotes interleukin-2 production in intact and thymus-deprived mice. J Biol Response Mod. 1984 Aug;3(4):423–434. [PubMed] [Google Scholar]
- Watson S. R., Redington T. J., Miller T. B., Bullock W. E. Flow microfluorometry analysis of alterations in T-lymphocyte subsets during murine listeriosis. Infect Immun. 1984 Aug;45(2):372–377. doi: 10.1128/iai.45.2.372-377.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


