Abstract
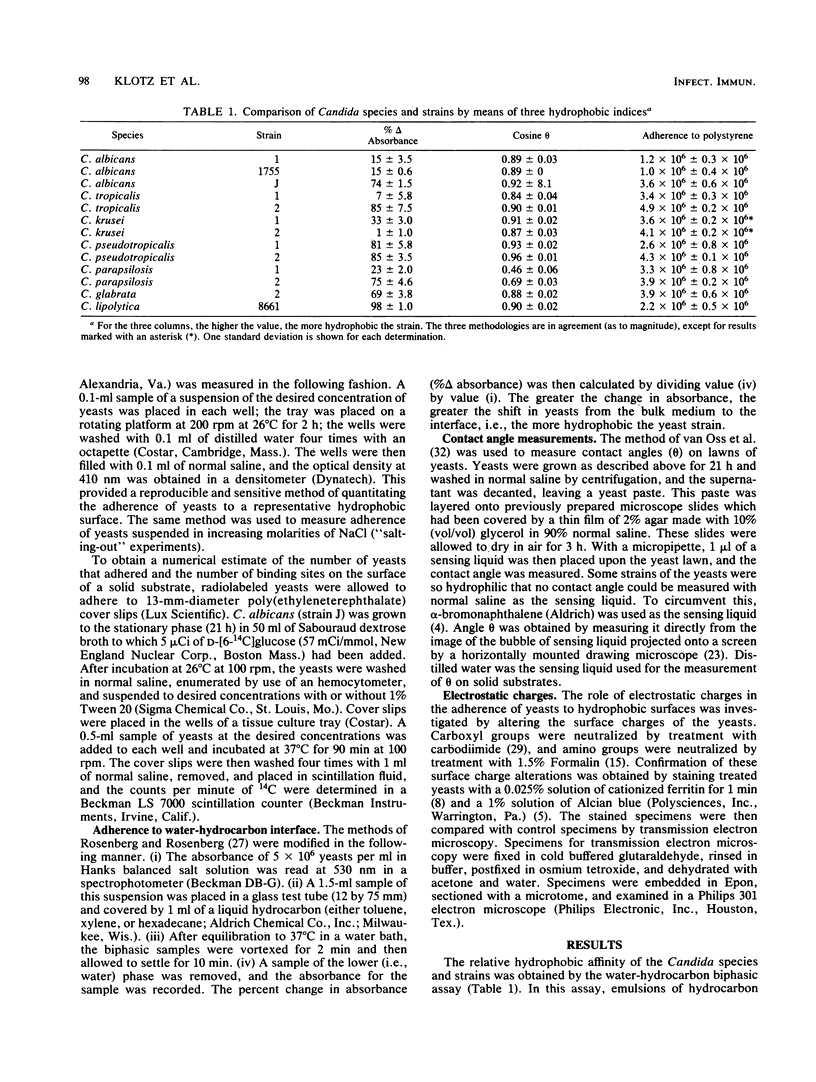
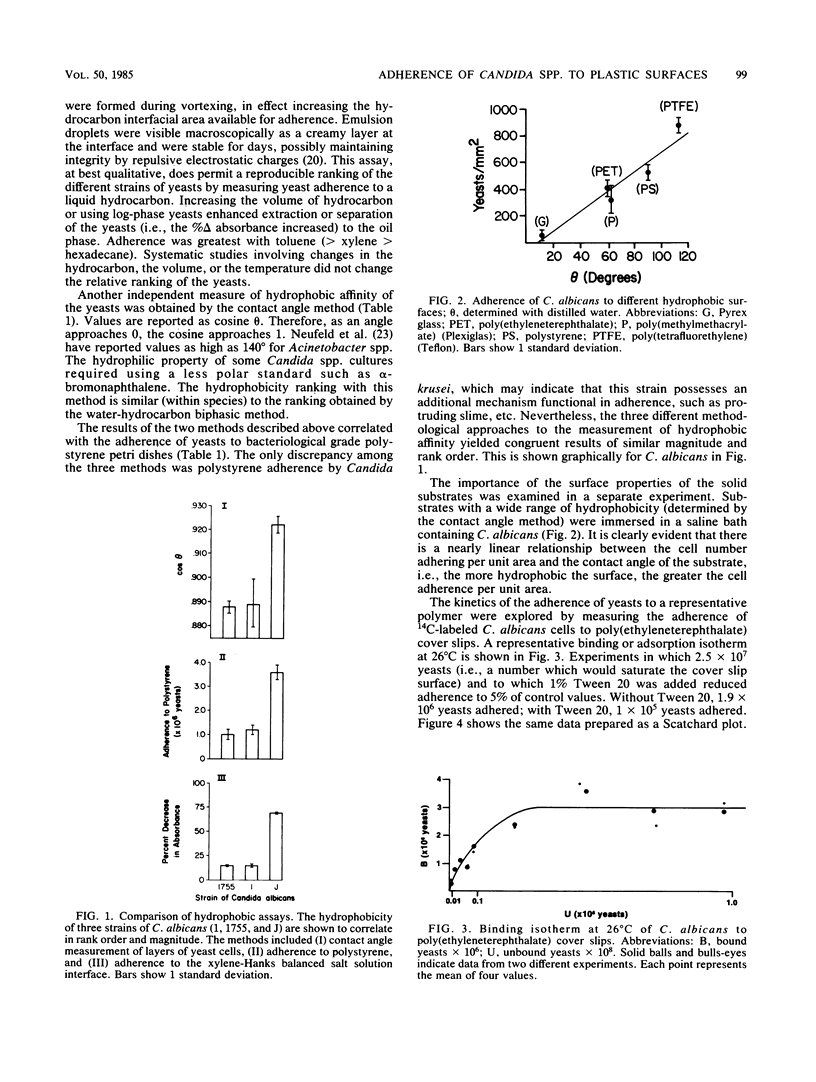
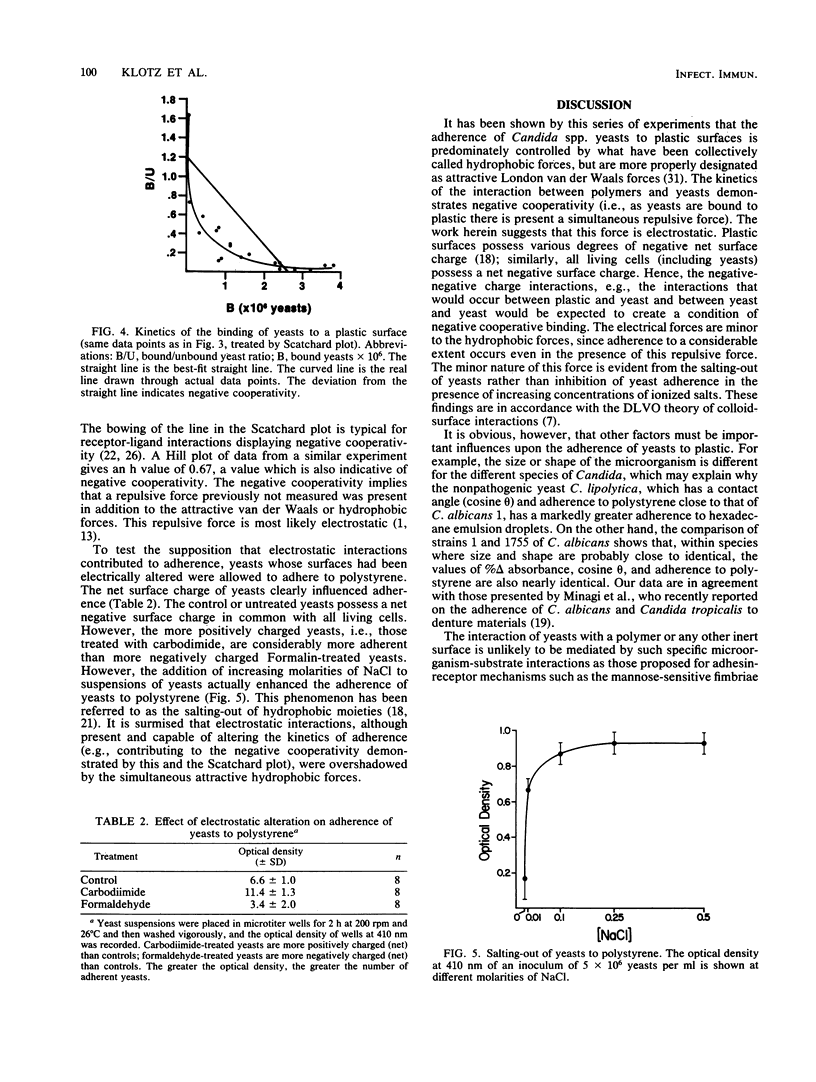
The ability of Candida albicans and Candida spp. to adhere to inert polymeric surfaces may allow these organisms direct ingress into the human host. Biophysical characterization of this adherence shows that the forces responsible for such adherence are attractive London-van der Waals forces (or hydrophobic forces) and electrostatic forces. The hydrophobic affinity of yeasts was determined by (i) a water-hydrocarbon two-phase assay and by (ii) measurement of the contact angle (theta) of a liquid droplet on a monolayer of yeast cells. The hydrophobicity of the yeasts correlated with the tendency of yeasts to adhere to polystyrene and was reduced in the presence of Tween 20. The adherence of yeasts to polymers of increasing hydrophobicity (determined by the contact angle method) was directly proportional to theta. Yeast surface charges were altered by selectively blocking amino and carboxyl groups. The more positively charged yeasts adhered in greater numbers. Increasing the molarity of NaCl increased yeast adherence. These forces probably contribute to the negative cooperativity (determined by Scatchard and Hill plot) that characterizes the adherence of yeasts to polymers.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Absolom D. R., van Oss C. J., Genco R. J., Francis D. W., Neumann A. W. Surface thermodynamics of normal and pathological human granulocytes. Cell Biophys. 1980 Jun;2(2):113–126. doi: 10.1007/BF02795838. [DOI] [PubMed] [Google Scholar]
- Andrade J. D. Interfacial phenomena and biomaterials. Med Instrum. 1973 Mar-Apr;7(2):110–119. [PubMed] [Google Scholar]
- Baier R. E., Gott V. L., Feruse A. Surface chemical evaluation of thromboresistant materials before and after venous implanttion. Trans Am Soc Artif Intern Organs. 1970;16:50–57. [PubMed] [Google Scholar]
- Behnke O., Zelander T. Preservation of intercellular substances by the cationic dye alcian blue in preparative procedures for electron microscopy. J Ultrastruct Res. 1970 Jun;31(5-6):424–428. doi: 10.1016/s0022-5320(70)90159-0. [DOI] [PubMed] [Google Scholar]
- Boyce J. F., Wong P. C., Schürch S., Roach M. R. Rabbit arterial endothelium and subendothelium. A change in interfacial free energy that may promote initial platelet adhesion. Circ Res. 1983 Sep;53(3):372–377. doi: 10.1161/01.res.53.3.372. [DOI] [PubMed] [Google Scholar]
- Danon D., Goldstein L., Marikovsky Y., Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972 Mar;38(5):500–510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Dexter S. C., Sullivan J. D., Williams J., Watson S. W. Influence of substrate wettability on the attachment of marine bacteria to various surfaces. Appl Microbiol. 1975 Aug;30(2):298–308. doi: 10.1128/am.30.2.298-308.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M., Loeb G. I. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl Environ Microbiol. 1979 Jan;37(1):67–72. doi: 10.1128/aem.37.1.67-72.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson D. F., Scheer D. Cell surface energy, contact angles and phase partition. III. Adhesion of bacterial cells to hydrophobic surfaces. Biochim Biophys Acta. 1980 Nov 18;602(3):506–510. doi: 10.1016/0005-2736(80)90329-6. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Russell E., Jr, Remington J. S. The compromised host and infection. II. Deep fungal infection. J Infect Dis. 1969 Aug;120(2):169–191. doi: 10.1093/infdis/120.2.169. [DOI] [PubMed] [Google Scholar]
- Heckels J. E., Blackett B., Everson J. S., Ward M. E. The influence of surface charge on the attachment of Neisseria gonorrhoeae to human cells. J Gen Microbiol. 1976 Oct;96(2):359–364. doi: 10.1099/00221287-96-2-359. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Drutz D. J., Harrison J. L., Huppert M. Adherence and penetration of vascular endothelium by Candida yeasts. Infect Immun. 1983 Oct;42(1):374–384. doi: 10.1128/iai.42.1.374-384.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagi S., Miyake Y., Inagaki K., Tsuru H., Suginaka H. Hydrophobic interaction in Candida albicans and Candida tropicalis adherence to various denture base resin materials. Infect Immun. 1985 Jan;47(1):11–14. doi: 10.1128/iai.47.1.11-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G. Hydrophobic interactions and the adherence of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Nov;38(2):637–644. doi: 10.1128/iai.38.2.637-644.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G., Staat R. H., Arnold R. R. Positive coooperativity in the binding of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Jan;35(1):157–165. doi: 10.1128/iai.35.1.157-165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld R. J., Zajic J. E., Gerson D. F. Cell surface measurements in hydrocarbon and carbohydrate fermentations. Appl Environ Microbiol. 1980 Mar;39(3):511–517. doi: 10.1128/aem.39.3.511-517.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann A. W., Absolom D. R., van Oss C. J., Zingg W. Surface thermodynamics of leukocyte and platelet adhesion to polymer surfaces. Cell Biophys. 1979 Mar;1(1):79–92. doi: 10.1007/BF02785058. [DOI] [PubMed] [Google Scholar]
- Pope L. M., Cole G. T., Guentzel M. N., Berry L. J. Systemic and gastrointestinal candidiasis of infant mice after intragastric challenge. Infect Immun. 1979 Aug;25(2):702–707. doi: 10.1128/iai.25.2.702-707.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Rosenberg E. Role of adherence in growth of Acinetobacter calcoaceticus RAG-1 on hexadecane. J Bacteriol. 1981 Oct;148(1):51–57. doi: 10.1128/jb.148.1.51-57.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbet G. V., Lakshmi M. S. Characterisation of Escherichia coli cell surface by isoelectric equilibrium analysis. Biochim Biophys Acta. 1973 Feb 27;298(1):50–58. doi: 10.1016/0005-2736(73)90008-4. [DOI] [PubMed] [Google Scholar]
- Torres-Rojas J. R., Stratton C. W., Sanders C. V., Horsman T. A., Hawley H. B., Dascomb H. E., Vial L. J., Jr Candidal suppurative peripheral thrombophlebitis. Ann Intern Med. 1982 Apr;96(4):431–435. doi: 10.7326/0003-4819-96-4-431. [DOI] [PubMed] [Google Scholar]


