Abstract
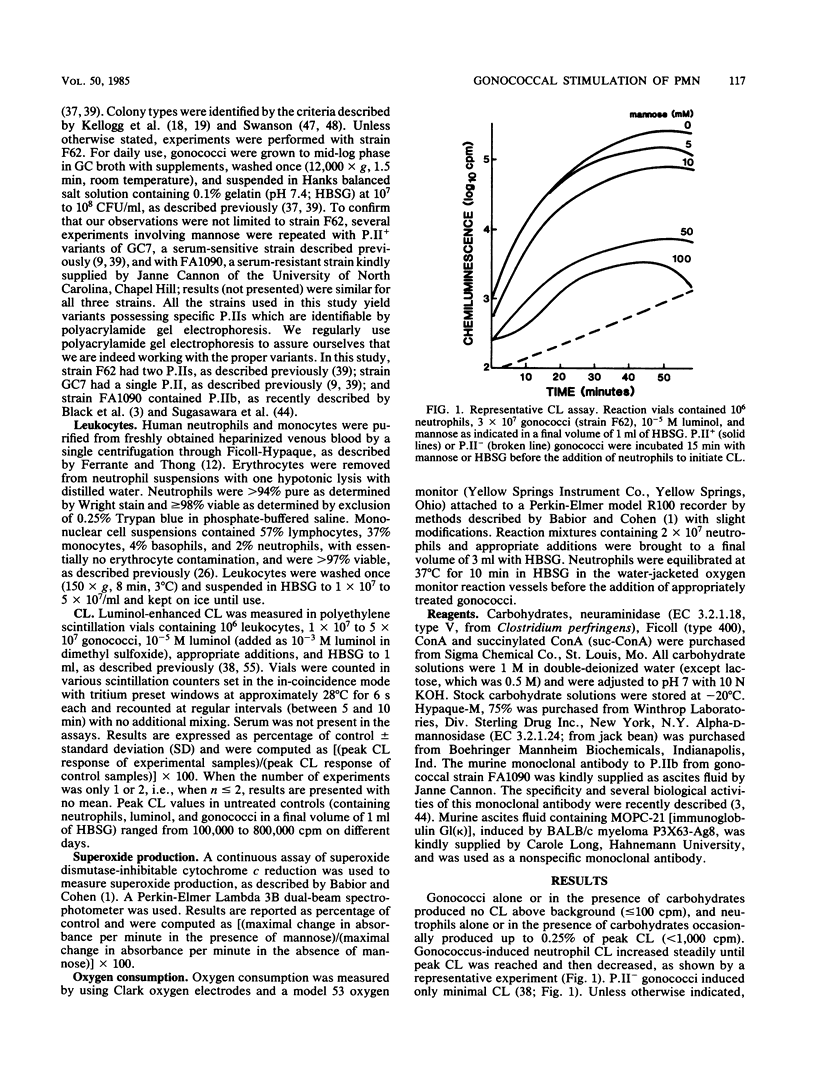
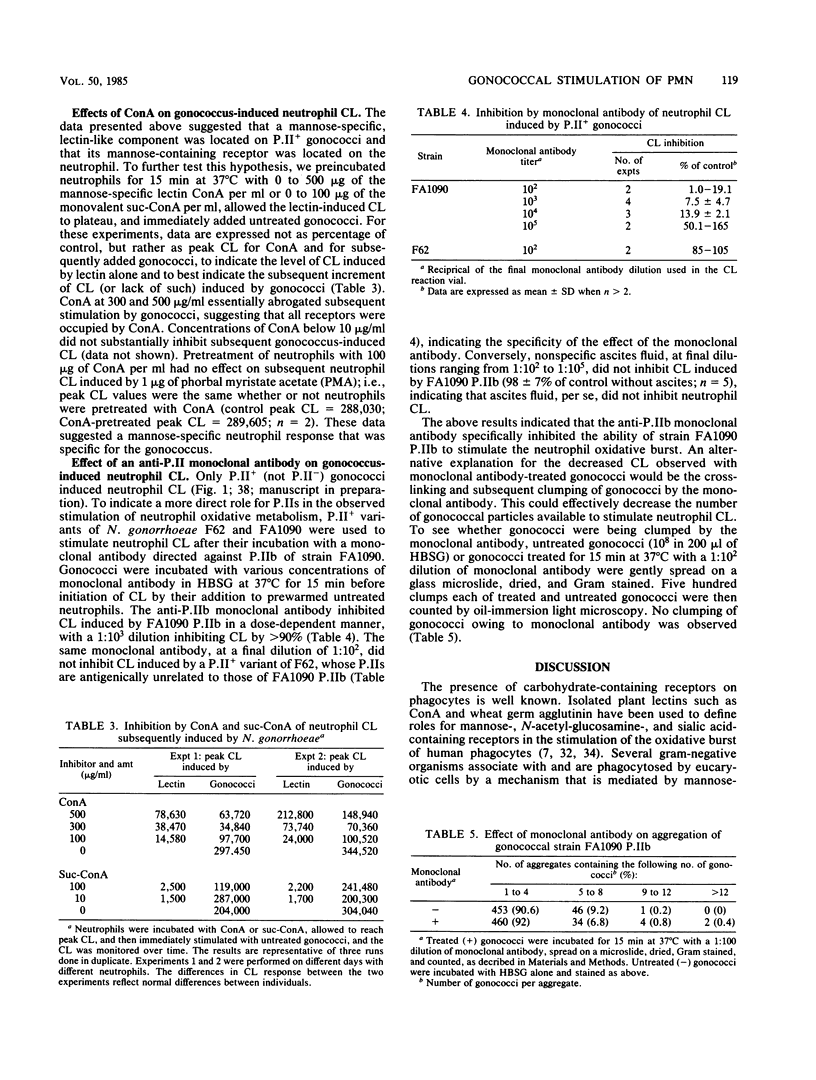
We investigated the ability of carbohydrates, glycosidases, and concanavalin A to inhibit the stimulation of the human leukocyte oxidative burst by gonococci in the absence of serum. The gonococci used in this study contained protein II (P.II) outer membrane proteins, and neutrophil oxidative burst was measured by luminol-enhanced chemiluminescence (CL). The following carbohydrates inhibited CL induced by nonpiliated P.II+ gonococci: beta-D-glucosamine greater than N-acetylneuraminic acid (sialic acid) greater than mannose greater than alpha-methylmannoside greater than N-acetyl-beta-D-glucosamine greater than or equal to glucose greater than or equal to lactose. Fucose, galactose, or beta-D-galactosamine (all 100 mM) did not inhibit or slightly increased CL, indicating a specificity for the observed effects. Mannose and alpha-methylmannoside also inhibited induction of monocyte CL by P.II+ gonococci. Incubation of neutrophils with concanavalin A inhibited subsequent gonococcus-induced CL but not phorbol myristate acetate-induced CL. Treatment of neutrophils with alpha-mannosidase reduced subsequent gonococcus-induced CL greater than 99%, whereas such treatment of gonococci had no effect on their ability to induce neutrophil CL. Incubation of a P.IIb-containing variant of Neisseria gonorrhoeae FA1090 with anti-P.IIb monoclonal antibody inhibited subsequent stimulation of neutrophil CL in a dose-responsive manner, indicating a specific role for P.IIb in the stimulatory process. The data suggest that one or more lectin-like components on the surface of P.II+ gonococci mediate their ability to stimulate the oxidative burst of human phagocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Black W. J., Schwalbe R. S., Nachamkin I., Cannon J. G. Characterization of Neisseria gonorrhoeae protein II phase variation by use of monoclonal antibodies. Infect Immun. 1984 Aug;45(2):453–457. doi: 10.1128/iai.45.2.453-457.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C. Gonococcal membrane proteins: speculation on their role in pathogenesis. Prog Allergy. 1983;33:298–313. [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C. Purification and partial characterization of the opacity-associated proteins of Neisseria gonorrhoeae. J Exp Med. 1984 Feb 1;159(2):452–462. doi: 10.1084/jem.159.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E., Wilson M. K., Newburger P. E. Con-A-stimulated superoxide production by granulocytes: reversible activation of NADPH oxidase. Blood. 1982 Nov;60(5):1188–1194. [PubMed] [Google Scholar]
- Cohen M. S., Metcalf J. A., Root R. K. Regulation of oxygen metabolism in human granulocytes: relationship between stimulus binding and oxidative response using plant lectins as probes. Blood. 1980 Jun;55(6):1003–1010. [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Gonococcal interactions with polymorphonuclear neutrophils: importance of the phagosome for bactericidal activity. J Clin Invest. 1978 Dec;62(6):1161–1171. doi: 10.1172/JCI109235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton L. J., Rest R. F. In vivo degradation of gonococcal outer membrane proteins within human leukocyte phagolysosomes. Infect Immun. 1983 Dec;42(3):1034–1040. doi: 10.1128/iai.42.3.1034-1040.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y., Ofek I., Yashouv-Gan Y., Sharon N., Mirelman D. Isolation of a mannose-specific lectin from Escherichia coli and its role in the adherence of the bacteria to epithelial cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1551–1559. doi: 10.1016/0006-291x(78)91179-8. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36(2):109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- Forslin L., Danielsson D. In vitro studies of the adherence of Neisseria gonorrhoeae and other urogenital bacteria to vaginal and uroepithelial cells, with special regard to the menstrual cycle. Gynecol Obstet Invest. 1980;11(6):327–340. doi: 10.1159/000299854. [DOI] [PubMed] [Google Scholar]
- Francioli P., Shio H., Roberts R. B., Müller M. Phagocytosis and killing of Neisseria gonorrhoeae by Trichomonas vaginalis. J Infect Dis. 1983 Jan;147(1):87–94. doi: 10.1093/infdis/147.1.87. [DOI] [PubMed] [Google Scholar]
- Gibbs D. L., Roberts R. B. The interaction in vitro between human polymorphonuclear leukocytes and Neisseria gonorrhoeae cultivated in the chick embryo. J Exp Med. 1975 Jan 1;141(1):155–171. doi: 10.1084/jem.141.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARKNESS A. H. The pathology of gonorrhoea. Br J Vener Dis. 1948 Dec;24(4):137–147. [PMC free article] [PubMed] [Google Scholar]
- James J. F., Zurlinden E., Lammel C. J., Brooks G. F. Relation of protein I and colony opacity to serum killing of Neisseria gonorrhoeae. J Infect Dis. 1982 Jan;145(1):37–44. doi: 10.1093/infdis/145.1.37. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane D. F., Weir D. M., Blackwell C. C., Winstanley F. P. Binding of Neisseria gonorrhoeae by lectin-like receptors on human phagocytes. J Clin Lab Immunol. 1984 Mar;13(3):107–110. [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koransky J. R., Scales R. W., Kraus S. J. Bacterial hemagglutination by Neisseria gonorrhoeae. Infect Immun. 1975 Sep;12(3):495–498. doi: 10.1128/iai.12.3.495-498.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer D. L., Dreyfus L. A., Robertson D. C. Interaction of polymorphonuclear leukocytes with smooth and rough strains of Brucella abortus. Infect Immun. 1979 Mar;23(3):737–742. doi: 10.1128/iai.23.3.737-742.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Mezzatesta J. R., Rest R. F. Phagocytic killing of Neisseria gonorrhoeae by human monocytes. Infect Immun. 1983 Oct;42(1):99–105. doi: 10.1128/iai.42.1.99-105.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. M., Garbus J., Hornick R. B. Lack of enhanced oxygen consumption by polymorphonuclear leukocytes on phagocytosis of virulent Salmonella typhi. Science. 1972 Mar 3;175(4025):1010–1011. doi: 10.1126/science.175.4025.1010. [DOI] [PubMed] [Google Scholar]
- Novotny P., Short J. A., Walker P. D. An electron-microscope study of naturally occurring and cultured cells of Neisseria Gonorrhoeae. J Med Microbiol. 1975 Aug;8(3):413–427. doi: 10.1099/00222615-8-3-413. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Ozaki Y., Iwata J., Ohashi T. Mechanism of neutrophil chemiluminescence induced by wheat germ agglutinin: partial characterization of the antigens recognized by wheat germ agglutinin. Blood. 1984 Nov;64(5):1094–1102. [PubMed] [Google Scholar]
- Perez H. D., Ong R. R. Degranulation of polymorphonuclear leukocytes is induced by multivalent cross-linking of wheat germ agglutinin binding site(s) on cell membrane. Inflammation. 1984 Sep;8(3):277–285. doi: 10.1007/BF00916416. [DOI] [PubMed] [Google Scholar]
- Pruzzo C., Debbia E., Satta G. Mannose-inhibitable adhesins and T3-T7 receptors of Klebsiella pneumoniae inhibit phagocytosis and intracellular killing by human polymorphonuclear leukocytes. Infect Immun. 1982 Jun;36(3):949–957. doi: 10.1128/iai.36.3.949-957.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Fischer S. H., Ingham Z. Z., Jones J. F. Interactions of Neisseria gonorrhoeae with human neutrophils: effects of serum and gonococcal opacity on phagocyte killing and chemiluminescence. Infect Immun. 1982 May;36(2):737–744. doi: 10.1128/iai.36.2.737-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F. Killing of Neisseria gonorrhoeae by human polymorphonuclear neutrophil granule extracts. Infect Immun. 1979 Aug;25(2):574–579. doi: 10.1128/iai.25.2.574-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Pretzer E. Degradation of gonococcal outer membrane proteins by human neutrophil lysosomal proteases. Infect Immun. 1981 Oct;34(1):62–68. doi: 10.1128/iai.34.1.62-68.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Fulbright R. S., Eads M. E., Sawyer W. D. Ethylenediaminetetraacetic acid-sensitive antiphagocytic activity of Neisseria gonorrhoeae. Infect Immun. 1977 Mar;15(3):817–827. doi: 10.1128/iai.15.3.817-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P., Eftekhar F., Puterman M. L. Nonopsonic phagocytosis of strains of Pseudomonas aeruginosa from cystic fibrosis patients. Infect Immun. 1984 Mar;43(3):1006–1011. doi: 10.1128/iai.43.3.1006-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawara R. J., Cannon J. G., Black W. J., Nachamkin I., Sweet R. L., Brooks G. F. Inhibition of Neisseria gonorrhoeae attachment to HeLa cells with monoclonal antibody directed against a protein II. Infect Immun. 1983 Dec;42(3):980–985. doi: 10.1128/iai.42.3.980-985.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg Edén C., Bjursten L. M., Hull R., Hull S., Magnusson K. E., Moldovano Z., Leffler H. Influence of adhesins on the interaction of Escherichia coli with human phagocytes. Infect Immun. 1984 Jun;44(3):672–680. doi: 10.1128/iai.44.3.672-680.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Colony opacity and protein II compositions of gonococci. Infect Immun. 1982 Jul;37(1):359–368. doi: 10.1128/iai.37.1.359-368.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., King G., Zeligs B. Studies on gonococcus infection. VII. In vitro killing of gonococci by human leukocytes. Infect Immun. 1975 Jan;11(1):65–68. doi: 10.1128/iai.11.1.65-68.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Young D., King G. Studies on Gonococcus infection. X. Pili and leukocyte association factor as mediators of interactions between gonococci and eukaryotic cells in vitro. Infect Immun. 1975 Jun;11(6):1352–1361. doi: 10.1128/iai.11.6.1352-1361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Zeligs B., Siam M. A., Parrott C. Studies on gonococcus infection. V. Observations on in vitro interactions of gonococci and human neutrophils. Infect Immun. 1974 Sep;10(3):633–644. doi: 10.1128/iai.10.3.633-644.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. W., Hill J. C., Tyeryar F. J., Jr Interaction of gonococci with phagocytic leukocytes from men and mice. Infect Immun. 1973 Jul;8(1):98–104. doi: 10.1128/iai.8.1.98-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M., McIntyre P. A. The requirement for membrane sialic acid in the stimulation of superoxide production during phagocytosis by human polymorphonuclear leukocytes. J Exp Med. 1976 Jun 1;143(6):1308–1316. doi: 10.1084/jem.143.6.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Robertson J. N. The human fallopian tube: a laboratory model for gonococcal infection. J Infect Dis. 1974 Jun;129(6):650–659. doi: 10.1093/infdis/129.6.650. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman G. M., Martin C. F., McNicol P. J. Inhibition of anion transport in human erythrocytes by pilated Neisseria gonorrhoeae. Can J Microbiol. 1984 Jan;30(1):52–56. doi: 10.1139/m84-009. [DOI] [PubMed] [Google Scholar]


