Abstract
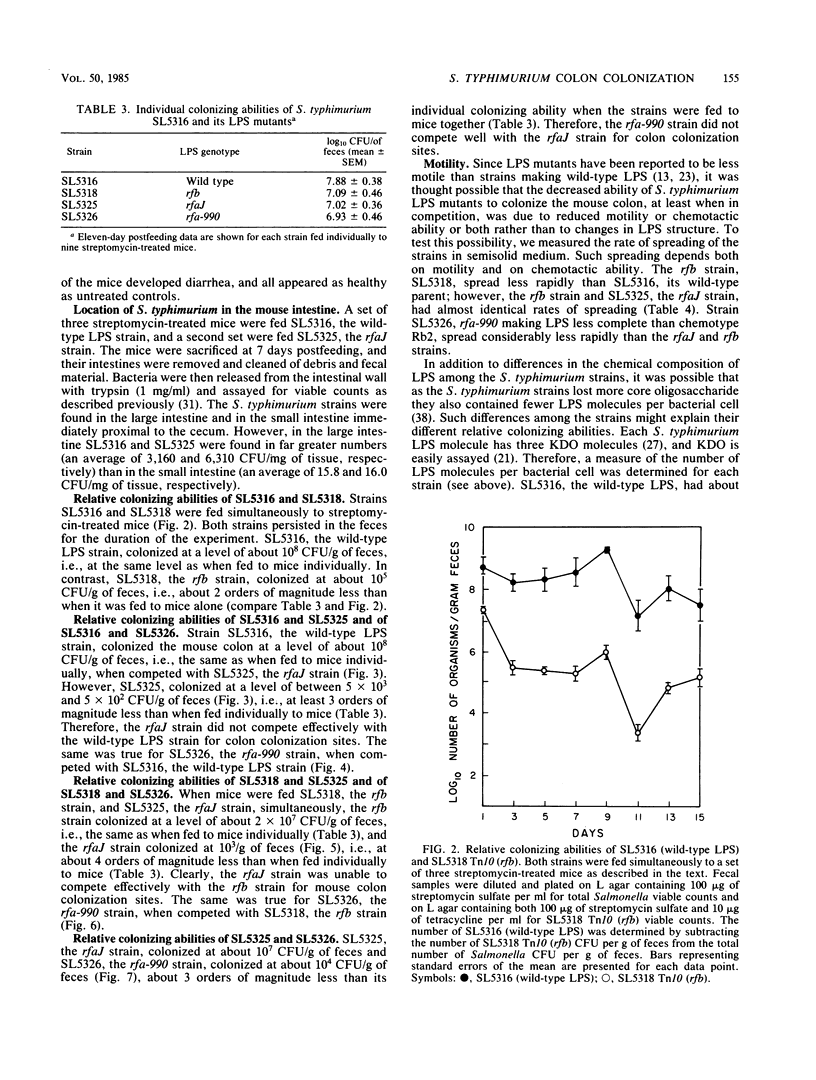
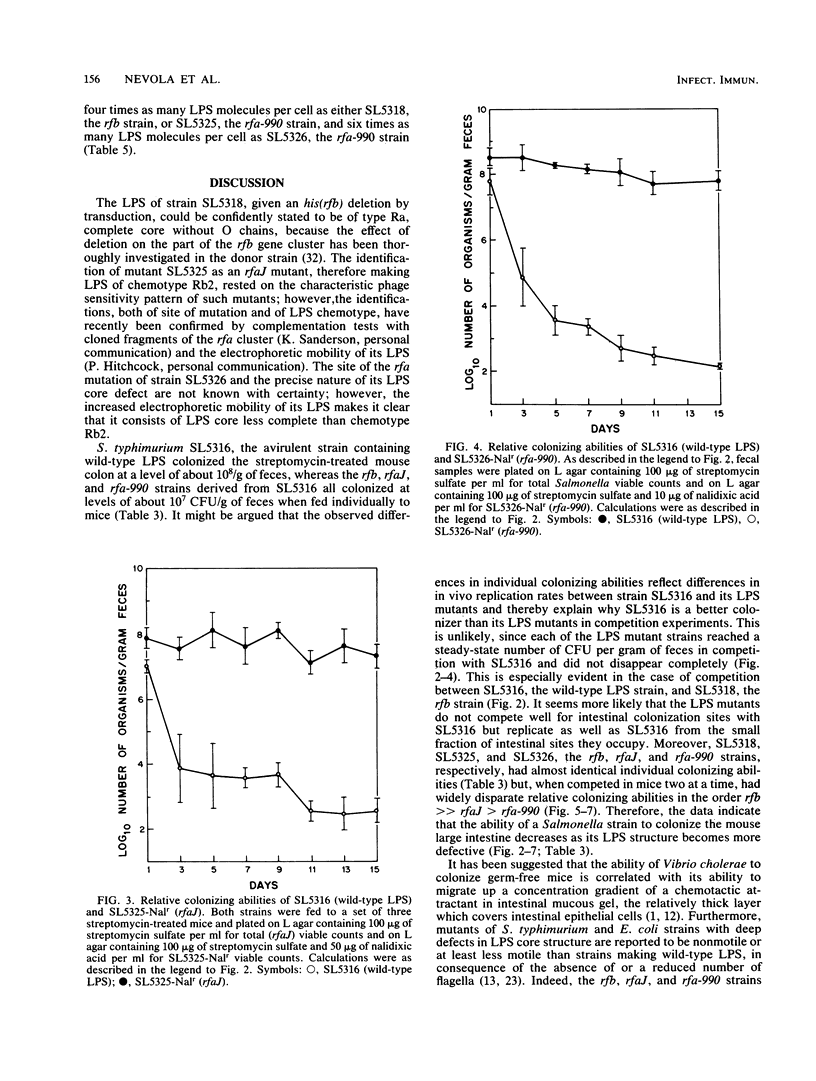
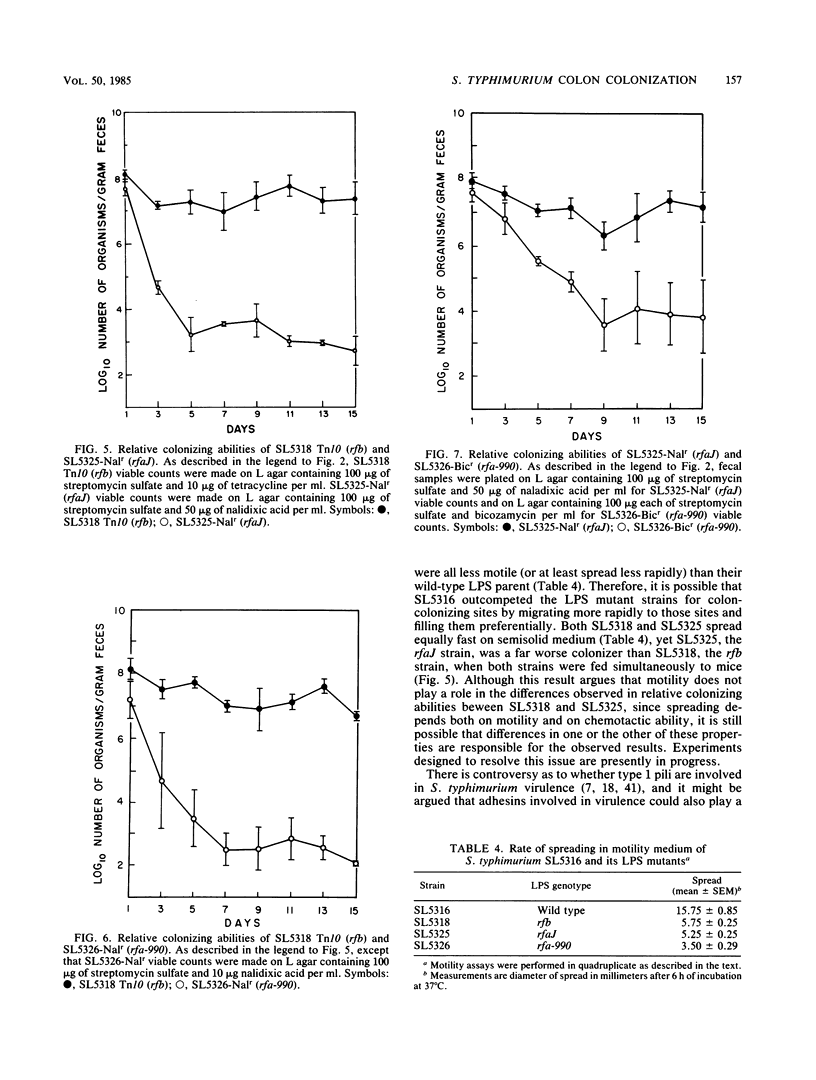
For study of the role of lipopolysaccharide (LPS) character in colonization of the mouse large intestine, use was made of S. typhimurium strain SL5316, which is streptomycin resistant and smooth (wild-type LPS) but nonvirulent because it is Aro- (aromatic dependent). Several rough variants of different LPS chemotype derived from strain SL5316 comprised: an rfb deletion transductant making type Ra (complete core) LPS; an rfaJ mutant making incomplete core of type Rb2; and an rfa-990 mutant making LPS core less complete than chemotype Rb2. We tested these strains for colon-colonizing ability by feeding them to male CD-1 mice receiving streptomycin sulfate (5 g/liter) in their drinking water. Each strain, if fed alone, was found in the feces throughout the 15 days of the experiment at about 10(8) CFU/g for the smooth strain or 10(7) CFU/g for each of its rough derivatives. However, when mice were fed equal numbers of two strains (with differentiating antibiotic resistance characters), the strain with the more complete LPS was found in the feces in great excess, 1000- to 100,000-fold, according to the pair. Thus, when strains were placed in direct competition with one another, their relevant colon-colonizing abilities were found to be wild type greater than rfb much greater than rfaJ greater than rfa-990, showing that the ability of a Salmonella strain to colonize the mouse large intestine decreases as its LPS structure becomes more defective.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Snary D. The structure and function of gastric mucus. Gut. 1972 Aug;13(8):666–672. doi: 10.1136/gut.13.8.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Arruda J. C., Williams T. J., Laux D. C. Adhesion of a human fecal Escherichia coli strain to mouse colonic mucus. Infect Immun. 1985 Apr;48(1):139–145. doi: 10.1128/iai.48.1.139-145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Rossoll R., Cabelli V. J., Yang S. L., Laux D. C. Relationship between the mouse colonizing ability of a human fecal Escherichia coli strain and its ability to bind a specific mouse colonic mucous gel protein. Infect Immun. 1983 Apr;40(1):62–69. doi: 10.1128/iai.40.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Alfredsson G. A., Barker R., Old D. C. A new biotyping scheme for Salmonella typhimurium and its phylogenetic significance. J Med Microbiol. 1975 Feb;8(1):149–166. doi: 10.1099/00222615-8-1-149. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Darekar M. R., Wheater D. W. Fimbriae and infectivity in Salmonella typhimurium. J Med Microbiol. 1976 Nov;9(4):459–473. doi: 10.1099/00222615-9-4-459. [DOI] [PubMed] [Google Scholar]
- Eidels L., Rick P. D., Stimler N. P., Osborn M. J. Transport of D-arabinose-5-phosphate and D-sedoheptulose-7-phosphate by the hexose phosphate transport system of Salmonella typhimurium. J Bacteriol. 1974 Jul;119(1):138–143. doi: 10.1128/jb.119.1.138-143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr New surface-associated heat-labile colonization factor antigen (CFA/II) produced by enterotoxigenic Escherichia coli of serogroups O6 and O8. Infect Immun. 1978 Aug;21(2):638–647. doi: 10.1128/iai.21.2.638-647.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Satterwhite T. K., Evans D. J., Jr, DuPont H. L. Differences in serological responses and excretion patterns of volunteers challenged with enterotoxigenic Escherichia coli with and without the colonization factor antigen. Infect Immun. 1978 Mar;19(3):883–888. doi: 10.1128/iai.19.3.883-888.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner J. F. Intestinal mucins in health and disease. Digestion. 1978;17(3):234–263. doi: 10.1159/000198115. [DOI] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Isaacson R. E. K99 surface antigen of Escherichia coli: antigenic characterization. Infect Immun. 1978 Nov;22(2):555–559. doi: 10.1128/iai.22.2.555-559.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhar M., Nuchamowitz Y., Mirelman D. Adherence of Shigella flexneri to guinea pig intestinal cells is mediated by a mucosal adhesion. Infect Immun. 1982 Mar;35(3):1110–1118. doi: 10.1128/iai.35.3.1110-1118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rabert D. K., Svinarich D. M., Whitfield H. J. Association of adhesive, invasive, and virulent phenotypes of Salmonella typhimurium with autonomous 60-megadalton plasmids. Infect Immun. 1982 Nov;38(2):476–486. doi: 10.1128/iai.38.2.476-486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S. K., Rehemtulla A., Sanderson K. E. Cloning of rfaG, B, I, and J genes for glycosyltransferase enzymes for synthesis of the lipopolysaccharide core of Salmonella typhimurium. J Bacteriol. 1985 Jan;161(1):277–284. doi: 10.1128/jb.161.1.277-284.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Klemm P. Fimbrial colonization factor CFA/1 protein from human enteropathogenic Escherichia coli strains. Purification, characterization and N-terminal sequence. FEBS Lett. 1979 Dec 1;108(1):107–110. doi: 10.1016/0014-5793(79)81188-6. [DOI] [PubMed] [Google Scholar]
- Komeda Y., Icho T., Iino T. Effects of galU mutation on flagellar formation in Escherichia coli. J Bacteriol. 1977 Feb;129(2):908–915. doi: 10.1128/jb.129.2.908-915.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T. T., Stocker B. A. ES18, a general transducing phage for smooth and nonsmooth Salmonella typhimurium. Virology. 1970 Nov;42(3):621–632. doi: 10.1016/0042-6822(70)90308-9. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Hellerqvist C. G. Rough mutants of Salmonella typhimurium: immunochemical and structural analysis of lipopolysaccharides from rfaH mutants. J Gen Microbiol. 1980 Jan;116(1):25–32. doi: 10.1099/00221287-116-1-25. [DOI] [PubMed] [Google Scholar]
- Myhal M. L., Cohen P. S., Laux D. C. Altered colonizing ability for mouse large intestine of a surface mutant of a human faecal isolate of Escherichia coli. J Gen Microbiol. 1983 May;129(5):1549–1558. doi: 10.1099/00221287-129-5-1549. [DOI] [PubMed] [Google Scholar]
- Myhal M. L., Laux D. C., Cohen P. S. Relative colonizing abilities of human fecal and K 12 strains of Escherichia coli in the large intestines of streptomycin-treated mice. Eur J Clin Microbiol. 1982 Jun;1(3):186–192. doi: 10.1007/BF02019621. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Levinthal M., Nikaido K., Nakane K. Extended deletions in the histidine-rough-B region of the Salmonella chromosome. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1825–1832. doi: 10.1073/pnas.57.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M., Mine Y., Nonoyama S., Kamimura T., Fukada S. Therapeutic efficacy of bicyclomycin for shigellosis experimentally induced in rhesus monkeys. J Antibiot (Tokyo) 1974 Dec;27(12):976–983. doi: 10.7164/antibiotics.27.976. [DOI] [PubMed] [Google Scholar]
- Revel H. R. Restriction of nonglucosylated T-even bacteriophage: properties of permissive mutants of Escherichia coli B and K12. Virology. 1967 Apr;31(4):688–701. doi: 10.1016/0042-6822(67)90197-3. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Stocker B. A. Gene rfaH, which affects lipopolysaccharide core structure in Salmonella typhimurium, is required also for expression of F-factor functions. J Bacteriol. 1981 May;146(2):535–541. doi: 10.1128/jb.146.2.535-541.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. P., Reina-Guerra M., Hoiseth S. K., Stocker B. A., Habasha F., Johnson E., Merritt F. Aromatic-dependent Salmonella typhimurium as modified live vaccines for calves. Am J Vet Res. 1984 Jan;45(1):59–66. [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Observations on the pathogenic properties of the K88, Hly and Ent plasmids of Escherichia coli with particular reference to porcine diarrhoea. J Med Microbiol. 1971 Nov;4(4):467–485. doi: 10.1099/00222615-4-4-467. [DOI] [PubMed] [Google Scholar]
- Tannock G. W., Blumershine R. V., Savage D. C. Association of Salmonella typhimurium with, and its invasion of, the ileal mucosa in mice. Infect Immun. 1975 Feb;11(2):365–370. doi: 10.1128/iai.11.2.365-370.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll M. J. Derivation of an F-merogenote and a phi-80 high-frequency transducing phage carrying the histidine operon os Salmonella. J Bacteriol. 1972 Feb;109(2):741–750. doi: 10.1128/jb.109.2.741-750.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Klemm P., Gaastra W. Purification, characterization, and partial covalent structure of Escherichia coli adhesive antigen K99. Infect Immun. 1981 Sep;33(3):877–883. doi: 10.1128/iai.33.3.877-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


