Abstract
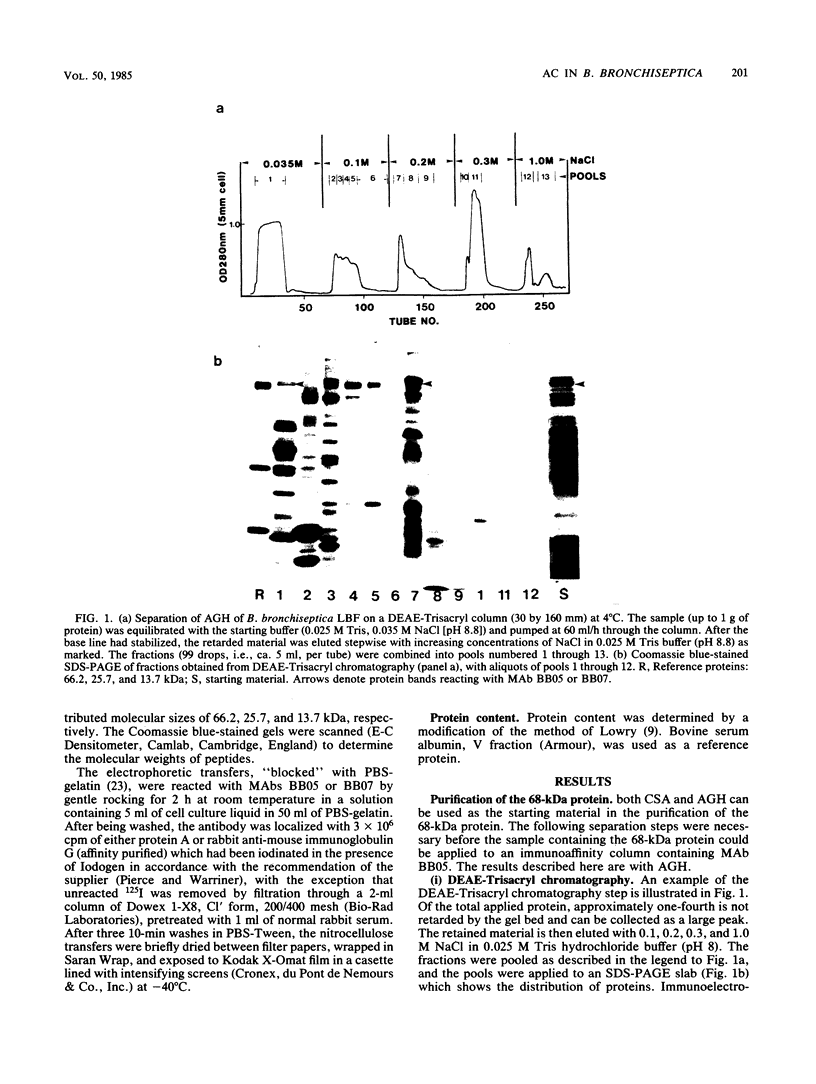
A method was developed which is suitable for the isolation of substantial quantities of outer membrane proteins of Bordetella species in a water-soluble form. The extracted material may then be further fractionated in the absence of detergents by ion-exchange chromatography and preparative flat-bed isoelectrofocusing. These procedures facilitated the isolation of one of the proteins, of molecular weight 68,000, for which the antibody titer correlated with the degree of protection against nasal changes induced in specific-pathogen-free piglets by Bordetella bronchiseptica infection (P. Novotny, M. Kobisch, K. Cownley, A. P. Chubb, and J. A. Montaraz, Infect. Immun. 50:190-198). This protein, which banded between 7.0 and 7.6 pH in preparative isoelectrofocusing, may be further purified with a monoclonal immunosorbent. Immunopurified protein showed adenylate cyclase activity. The enzymatic activity was found to be unstable during processing; i.e., although the crude extract showed up to 150 nmol of cyclic AMP per mg/min, the immunopurified protein showed a maximum of only 200 nmol of cyclic AMP per mg/min. Two strains of B. bronchiseptica, isolated from herds of healthy pigs showing no signs of atrophic rhinitis, did not produce the 68,000-molecular-weight protein and were negative for adenylate cyclase. However, it is not known whether the 68,000-molecular-weight protein is a component of adenylate cyclase or whether it is an unrelated protein associated with this enzyme in some unknown way. Adenylate cyclase activity from culture supernatants of B. bronchiseptica, B. pertussis, and B. parapertussis can be absorbed equally to the same monoclonal immunosorbent.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayme G., Caroff M., Chaby R., Haeffner-Cavaillon N., Le Dur A., Moreau M., Muset M., Mynard M. C., Roumiantzeff M., Schulz D. Biological activities of fragments derived from Bordetella pertussis endotoxin: isolation of a nontoxic, Shwartzman-negative lipid A possessing high adjuvant properties. Infect Immun. 1980 Mar;27(3):739–745. doi: 10.1128/iai.27.3.739-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M., Takezawa T., Nakase Y. Adenylate cyclase activity of Bordetella organisms. I. Its production in liquid medium. Microbiol Immunol. 1980;24(2):95–104. doi: 10.1111/j.1348-0421.1980.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Ezzell J. W., Dobrogosz W. J., Kloos W. E., Manclark C. R. Phase-shift markers in Bordetella: alterations in envelope proteins. J Infect Dis. 1981 Apr;143(4):562–569. doi: 10.1093/infdis/143.4.562. [DOI] [PubMed] [Google Scholar]
- Goodnow R. A. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980 Dec;44(4):722–738. doi: 10.1128/mr.44.4.722-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee D. V., Andreasen T. J., Storm D. R. Calcium-independent stimulation of Bordetella pertussis adenylate cyclase by calmodulin. Biochemistry. 1982 May 25;21(11):2759–2764. doi: 10.1021/bi00540a028. [DOI] [PubMed] [Google Scholar]
- Görg A., Postel W., Westermeier R. Ultrathin-layer isoelectric focusing in polyacrylamide gels on cellophane. Anal Biochem. 1978 Aug 15;89(1):60–70. doi: 10.1016/0003-2697(78)90726-1. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hewlett E. L., Underhill L. H., Cook G. H., Manclark C. R., Wolff J. A protein activator for the adenylate cyclase of Bordetella pertussis. J Biol Chem. 1979 Jul 10;254(13):5602–5605. [PubMed] [Google Scholar]
- Hewlett E., Wolff J. Soluble adenylate cyclase from the culture medium of Bordetella pertussis: purification and characterization. J Bacteriol. 1976 Aug;127(2):890–898. doi: 10.1128/jb.127.2.890-898.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra H., Dankert J. Porin from the outer membrane of Escherichia coli: immunological characterization of native and heat-dissociated forms. J Gen Microbiol. 1981 Aug;125(2):285–292. doi: 10.1099/00221287-125-2-285. [DOI] [PubMed] [Google Scholar]
- Kilhoffer M. C., Cook G. H., Wolff J. Calcium-independent activation of adenylate cyclase by calmodulin. Eur J Biochem. 1983 Jun 1;133(1):11–15. doi: 10.1111/j.1432-1033.1983.tb07423.x. [DOI] [PubMed] [Google Scholar]
- Kumazawa N. H., Yoshikawa M. Conversion of Bordetella pertussis to Bordetella parapertussis. J Hyg (Lond) 1978 Aug;81(1):15–23. doi: 10.1017/s0022172400053729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Mason M. A. The spheroplasts of bordetella pertussis. Can J Microbiol. 1966 Jun;12(3):539–545. doi: 10.1139/m66-077. [DOI] [PubMed] [Google Scholar]
- McPheat W. L., Wardlaw A. C., Novotny P. Modulation of Bordetella pertussis by nicotinic acid. Infect Immun. 1983 Aug;41(2):516–522. doi: 10.1128/iai.41.2.516-522.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaraz J. A., Novotny P., Ivanyi J. Identification of a 68-kilodalton protective protein antigen from Bordetella bronchiseptica. Infect Immun. 1985 Mar;47(3):744–751. doi: 10.1128/iai.47.3.744-751.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny P., Brookes J. E. The use of Bordetella pertussis preserved in liquid nitrogen as a challenge suspension in the Kendrick mouse protection test. J Biol Stand. 1975;3(1):11–29. doi: 10.1016/0092-1157(75)90003-7. [DOI] [PubMed] [Google Scholar]
- Novotny P., Kobisch M., Cownley K., Chubb A. P., Montaraz J. A. Evaluation of Bordetella bronchiseptica vaccines in specific-pathogen-free piglets with bacterial cell surface antigens in enzyme-linked immunosorbent assay. Infect Immun. 1985 Oct;50(1):190–198. doi: 10.1128/iai.50.1.190-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A., Hawkins D. C. Structure and biological properties of solubilized envelope proteins of Bordetella pertussis. Infect Immun. 1983 Feb;39(2):590–598. doi: 10.1128/iai.39.2.590-598.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- WITTLER R. G. The L-form of haemophilus pertussis. J Gen Microbiol. 1951 Nov;5(5 Suppl):1024–1031. doi: 10.1099/00221287-5-5-1024. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984 Aug;150(2):219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]