Abstract
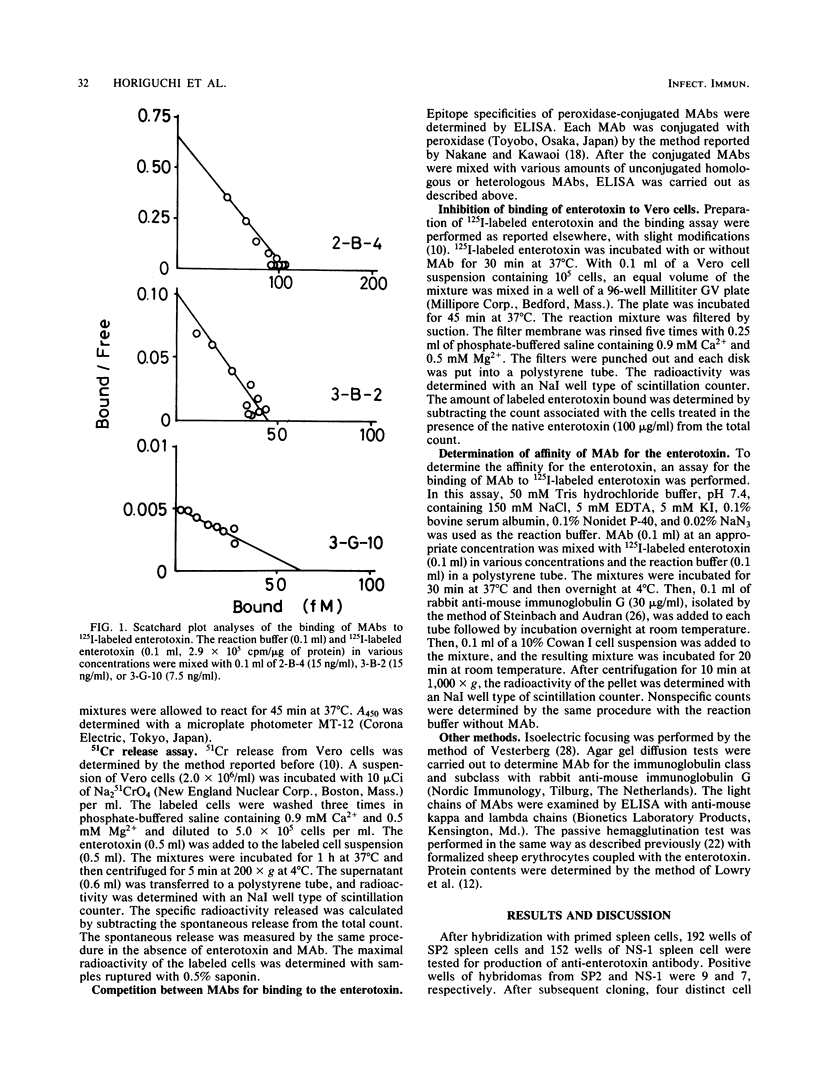
Four hybridoma cell lines producing monoclonal antibodies to Clostridium perfringens enterotoxin were established by fusion of mouse myeloma and spleen cells obtained from mice immunized with the enterotoxin and its toxoid. An enzyme-linked immunosorbent assay indicated that the two antibodies, 2-B-4 and 3-G-10, bound to those regions that were located close each other; the others, 3-B-2 and 2-H-2, bound to other independent regions on the enterotoxin. Release of 51Cr from Vero cells with the enterotoxin was inhibited by either 2-B-4 or 3-G-10, both of which inhibited the binding of 125I-labeled enterotoxin to the cells. Neither binding nor cytotoxicity of the enterotoxin was affected by 2-H-2; 3-B-2 only barely inhibited the binding but neutralized the enterotoxin shown by 51Cr release. It seems justified to conclude that 3-B-2 blocks the toxic action after the enterotoxin has bound to Vero cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Clostridium perfringens Type A Food Poisoning I. Response of the Rabbit Ileum as an Indication of Enteropathogenicity of Strains of Clostridium perfringens in Monkeys. Infect Immun. 1971 Jan;3(1):167–170. doi: 10.1128/iai.3.1.167-170.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. W., Parker C. D. Interaction of monoclonal antibodies with pertussis toxin and its subunits. Infect Immun. 1984 Oct;46(1):195–201. doi: 10.1128/iai.46.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum P. E. Inhibition of protein synthesis by a tryptic polypeptide of Clostridium perfringens type A enterotoxin. Biochim Biophys Acta. 1982 Oct 20;708(1):6–11. doi: 10.1016/0167-4838(82)90196-0. [DOI] [PubMed] [Google Scholar]
- Granum P. E., Whitaker J. R., Skjelkvåle R. Trypsin activation of enterotoxin from Clostridium perfringens type A: fragmentation and some physicochemical properties. Biochim Biophys Acta. 1981 May 29;668(3):325–332. doi: 10.1016/0005-2795(81)90165-3. [DOI] [PubMed] [Google Scholar]
- Hauschild A. H., Hilsheimer R. Purification and characteristics of the enterotoxin of Clostridium perfringens type A. Can J Microbiol. 1971 Nov;17(11):1425–1433. doi: 10.1139/m71-227. [DOI] [PubMed] [Google Scholar]
- Hayakawa S., Uchida T., Mekada E., Moynihan M. R., Okada Y. Monoclonal antibody against diphtheria toxin. Effect on toxin binding and entry into cells. J Biol Chem. 1983 Apr 10;258(7):4311–4317. [PubMed] [Google Scholar]
- Horiguchi Y., Kozaki S., Sakaguchi G. Determination of Clostridium botulinum toxin by reversed passive latex agglutination. Nihon Juigaku Zasshi. 1984 Aug;46(4):487–491. doi: 10.1292/jvms1939.46.487. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mao S. J., Kazmar R. E., Silverfield J. C., Alley M. C., Kluge K., Fathman C. G. Immunochemical properties of human low density lipoproteins as explored by monoclonal antibodies. Binding characteristics distinct from those of conventional serum antibodies. Biochim Biophys Acta. 1982 Nov 12;713(2):365–374. doi: 10.1016/0005-2760(82)90255-7. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Sugimoto N. Calcium-independent and dependent steps in action of Clostridium perfringens enterotoxin on HeLa and Vero cells. Biochem Biophys Res Commun. 1979 Nov 28;91(2):629–636. doi: 10.1016/0006-291x(79)91568-7. [DOI] [PubMed] [Google Scholar]
- McClane B. A., McDonel J. L. Characterization of membrane permeability alterations induced in Vero cells by Clostridium perfringens enterotoxin. Biochim Biophys Acta. 1980 Aug 14;600(3):974–985. doi: 10.1016/0005-2736(80)90499-x. [DOI] [PubMed] [Google Scholar]
- McClane B. A., McDonel J. L. The effects of Clostridium perfringens enterotoxin on morphology, viability, and macromolecular synthesis in Vero cells. J Cell Physiol. 1979 May;99(2):191–200. doi: 10.1002/jcp.1040990205. [DOI] [PubMed] [Google Scholar]
- Moyle W. R., Ehrlich P. H., Canfield R. E. Use of monoclonal antibodies to subunits of human chorionic gonadotropin to examine the orientation of the hormone in its complex with receptor. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2245–2249. doi: 10.1073/pnas.79.7.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Remmers E. F., Colwell R. R., Goldsby R. A. Production and characterization of monoclonal antibodies to cholera toxin. Infect Immun. 1982 Jul;37(1):70–76. doi: 10.1128/iai.37.1.70-76.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M., Granum P. E. Sequence of the amino-terminal part of enterotoxin from Clostridium perfringens type A: identification of points of trypsin activation. Infect Immun. 1983 Jun;40(3):943–949. doi: 10.1128/iai.40.3.943-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M., Granum P. E. The amino acid sequence of the enterotoxin from Clostridium perfringens type A. FEBS Lett. 1985 Mar 25;182(2):479–484. doi: 10.1016/0014-5793(85)80358-6. [DOI] [PubMed] [Google Scholar]
- Sakaguchi G., Sakaguchi S., Kozaki S., Sugii S., Oishi I. Cross reaction in reversed passive hemagglutination between Clostridium botulinum type A and B toxins and its avoidance by the sue of anti-toxic component immunoglobulin isolated by affinity chromatography. Jpn J Med Sci Biol. 1974 Jun;27(3):161–172. doi: 10.7883/yoken1952.27.161. [DOI] [PubMed] [Google Scholar]
- Sakaguchi G., Uemura T., Riemann H. P. Simplified method for purification of Clostridium perfringens type A enterotoxin. Appl Microbiol. 1973 Nov;26(5):762–767. doi: 10.1128/am.26.5.762-767.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard A. J., Cussell D., Hughes M. Production and characterization of monoclonal antibodies to tetanus toxin. Infect Immun. 1984 Feb;43(2):710–714. doi: 10.1128/iai.43.2.710-714.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R. L., Duncan C. L. Purification and biochemical properties of Clostridium perfringens type A enterotoxin. Infect Immun. 1972 Nov;6(5):662–673. doi: 10.1128/iai.6.5.662-673.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Uemura T., Maekawa T., Sakaguchi G. Biological assay for Clostridium perfringens enterotoxin with Vero cells. Nihon Juigaku Zasshi. 1984 Oct;46(5):715–720. doi: 10.1292/jvms1939.46.715. [DOI] [PubMed] [Google Scholar]


