Abstract
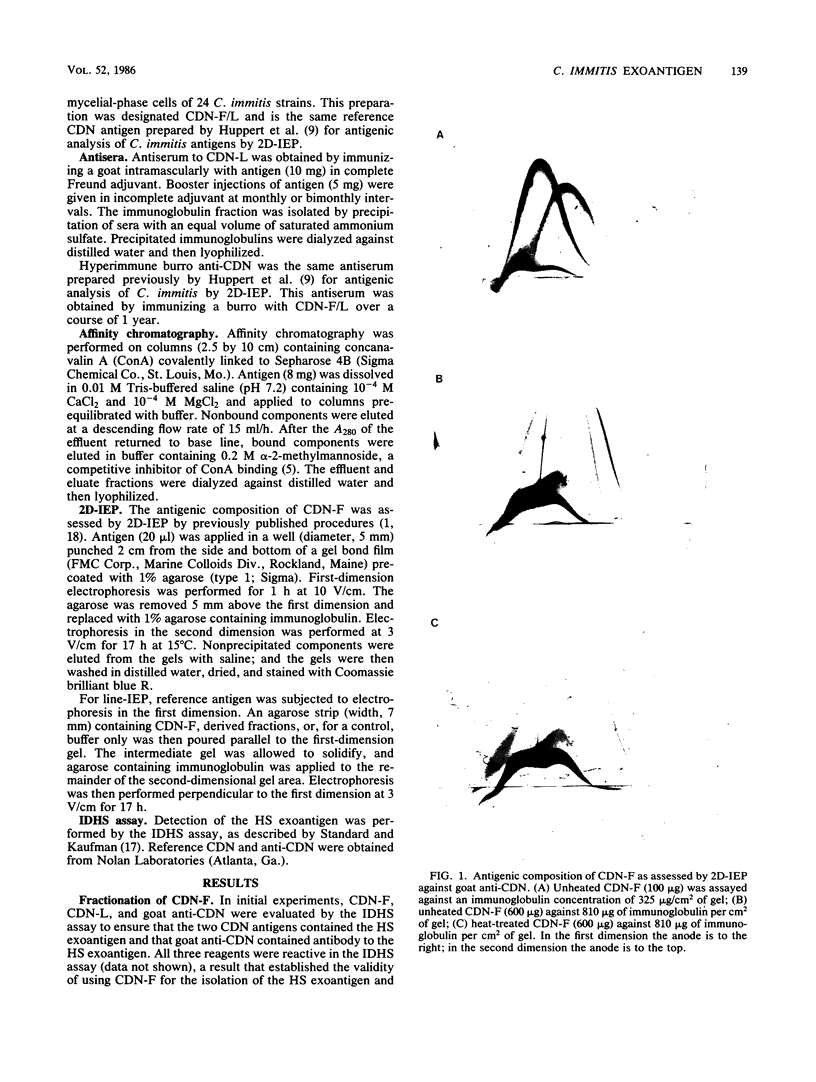
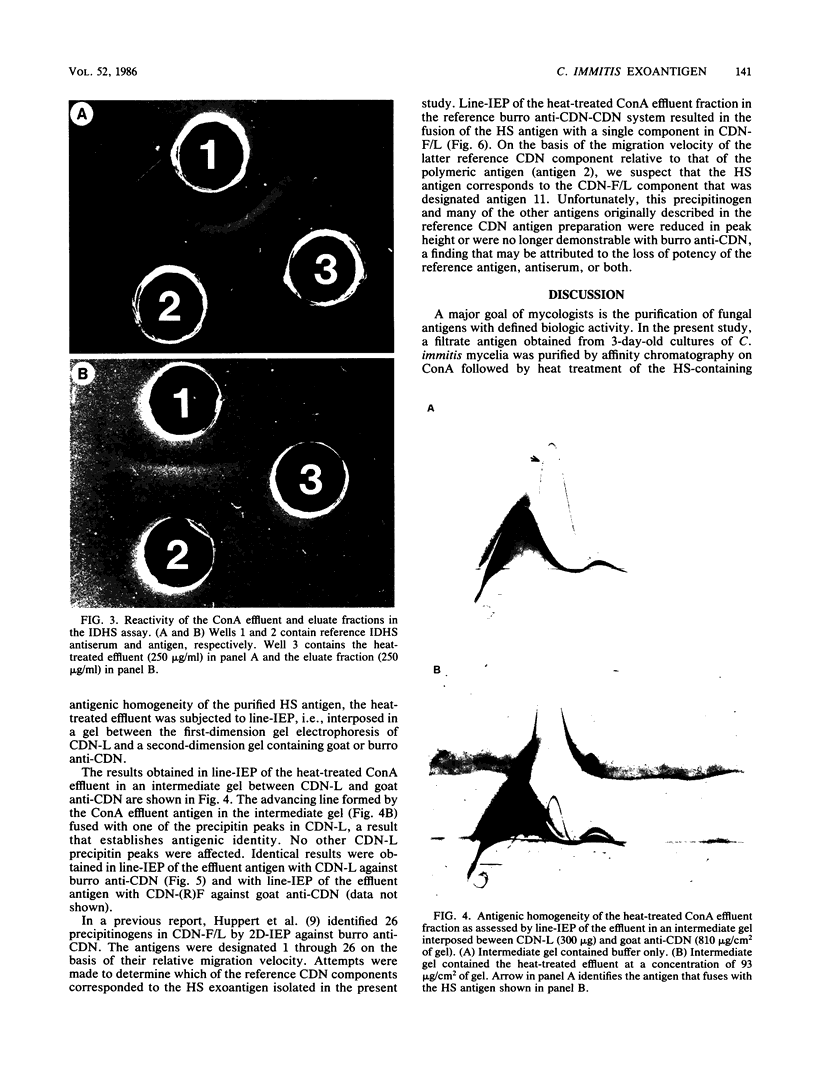
Results of previous studies have established that mycelial-phase cells of Coccidioides immitis produce a heat-stable (HS) exoantigen that is specific for this fungus. In the present study, the HS exoantigen was isolated from a heterogeneous culture filtrate of C. immitis mycelia by using a combination of physicochemical procedures. Affinity chromatography of the culture filtrate on concanavalin A yielded two fractions: an effluent fraction that did not bind to the lectin and an eluate fraction that eluted in alpha-2-methylmannoside. Antigenic analyses by two-dimensional immunoelectrophoresis established that of the seven antigens detected in the unfractionated culture filtrate preparation, only two were present in the column effluent, and of these, only one was stable to heating at 56 degrees C for 30 min. Reactivity in the immunodiffusion assay for the HS exoantigen was demonstrable with the column effluent fraction and not the column eluate. The detection of only one precipitinogen in two-dimensional immunoelectrophoresis of the heat-treated concanavalin A effluent fraction, coupled with the reactivity of this fraction in the immunodiffusion assay for the HS antigen, provides strong, if not definitive, evidence that this antigen is the HS exoantigen. Purification of the HS antigen and the production of monospecific antiserum will provide the necessary reagents for the development of a sensitive and specific immunoassay for detecting the HS antigen in C. immitis cultures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsen N. H. Intermediate gel in crossed and in fused rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:71–77. doi: 10.1111/j.1365-3083.1973.tb03782.x. [DOI] [PubMed] [Google Scholar]
- DiSalvo A. F., Sekhon A. S., Land G. A., Fleming W. H. Evaluation of the exoantigen test for identification of Histoplasma species and Coccidioides immitis cultures. J Clin Microbiol. 1980 Mar;11(3):238–241. doi: 10.1128/jcm.11.3.238-241.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSalvo A. F., Terreni A. A., Wooten A. K. Use of the exoantigen test to identify blastomyces dermatitidis, coccidioides immitis, and Histoplasma capsulatum in mixed cultures. Am J Clin Pathol. 1981 Jun;75(6):825–826. doi: 10.1093/ajcp/75.6.825. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Huppert M., Spratt N. S., Vukovich K. R., Sun S. H., Rice E. H. Antigenic analysis of coccidioidin and spherulin determined by two-dimensional immunoelectrophoresis. Infect Immun. 1978 May;20(2):541–551. doi: 10.1128/iai.20.2.541-551.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Sun S. H., Rice E. H. Specificity of exoantigens for identifying cultures of Coccidioides immitis. J Clin Microbiol. 1978 Sep;8(3):346–348. doi: 10.1128/jcm.8.3.346-348.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L., Standard P. G., Huppert M., Pappagianis D. Comparison and diagnostic value of the coccidioidin heat-stable (HS and tube precipitin) antigens in immunodiffusion. J Clin Microbiol. 1985 Oct;22(4):515–518. doi: 10.1128/jcm.22.4.515-518.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L., Standard P. Improved version of the exoantigen test for identification of Coccidioides immitis and Histoplasma capsulatum cultures. J Clin Microbiol. 1978 Jul;8(1):42–45. doi: 10.1128/jcm.8.1.42-45.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPPAGIANIS D., SMITH C. E., KOBAYASHI G. S., SAITO M. T. Studies of antigens from young mycelia of Coccidioides immitis. J Infect Dis. 1961 Jan-Feb;108:35–44. doi: 10.1093/infdis/108.1.35. [DOI] [PubMed] [Google Scholar]
- Standard P. G., Kaufman L. Immunological procedure for the rapid and specific identification of Coccidioides immitis cultures. J Clin Microbiol. 1977 Feb;5(2):149–153. doi: 10.1128/jcm.5.2.149-153.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke B. Crossed immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:47–56. doi: 10.1111/j.1365-3083.1973.tb03778.x. [DOI] [PubMed] [Google Scholar]