Abstract
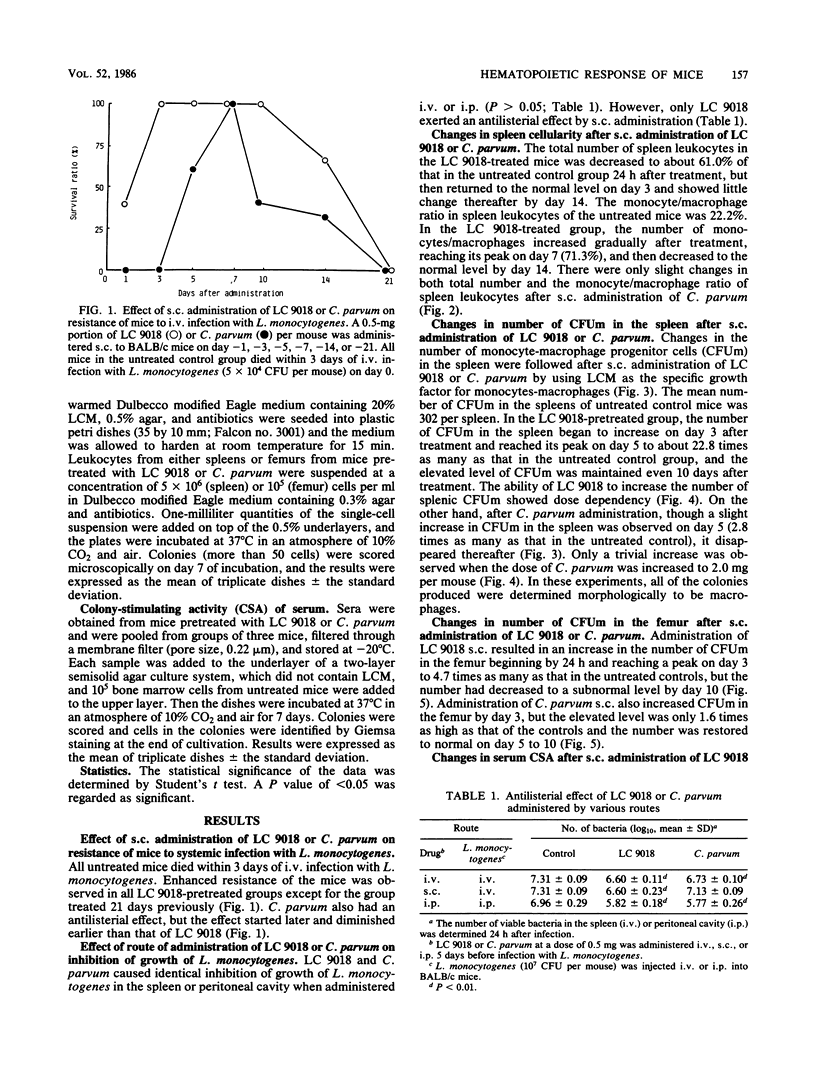
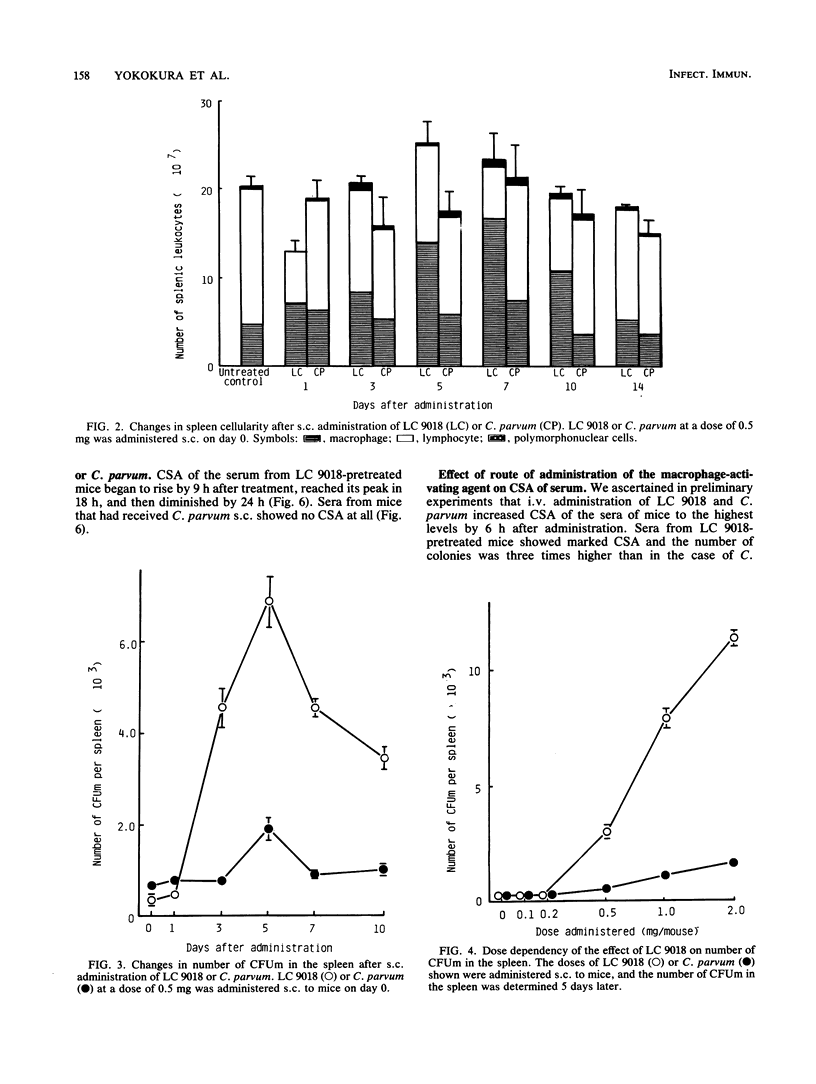
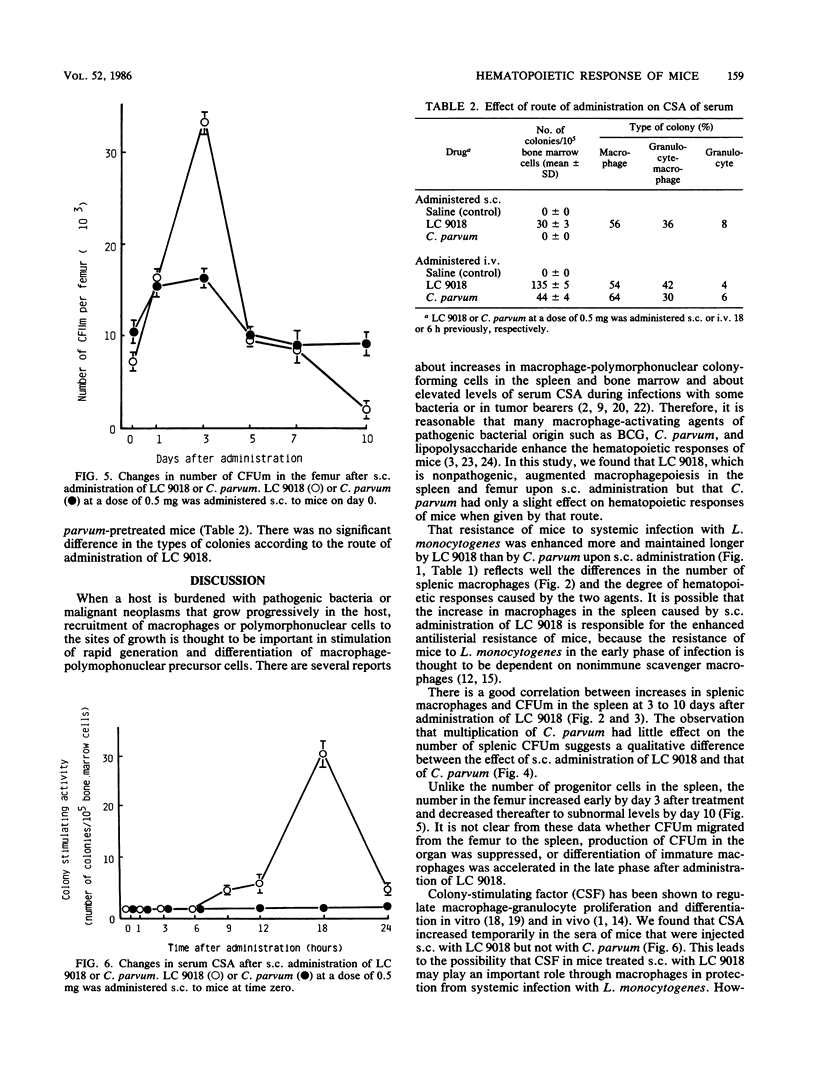
Mice that had received heat-killed Lactobacillus casei (LC 9018) subcutaneously (s.c.) showed enhanced resistance to systemic (i.e., intravenous) infection with Listeria monocytogenes, but the antilisterial resistance of mice was less augmented by s.c. administration of Propionibacterium acnes ("Corynebacterium parvum"). Though there was little change in the total number of splenic leukocytes after s.c. administration of LC 9018, the monocyte-macrophage ratio increased after treatment, reaching its peak on day 5 to 7 after injection. The number of progenitor cells that form macrophage colonies under the stimulus of L-cell-conditioned medium in a semisolid agar culture system increased in the spleens of mice pretreated s.c. with LC 9018, showing a peak response on day 5 after injection. The increase corresponded to the increase in the dose administered, and increased numbers were detected even 10 days after treatment. The number of macrophage colonies in the femurs of mice pretreated s.c. with LC 9018 showed a temporary increase on day 3 after injection but then a decrease until day 10. Colony-stimulating activity was detected in the sera of mice administered LC 9018 s.c. 18 h previously, and the colonies produced were of three types: granulocyte (8%), macrophage (56%), and granulocyte-macrophage (36%). Administration of C. parvum s.c. had little effect on these hematopoietic responses of mice.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessho M., Hirashima K., Ando K., Nara N., Momoi H. Hemopoietic stem cell kinetics in mice bearing CSF-producing fibrosarcoma. Nihon Ketsueki Gakkai Zasshi. 1984 Feb;47(1):21–33. [PubMed] [Google Scholar]
- Foster R. S., Jr, MacPherson B. R., Browdie D. A. Effect of Corynebacterium parvum on colony-stimulating factor and granulocyte-macrophage colony formation. Cancer Res. 1977 May;37(5):1349–1355. [PubMed] [Google Scholar]
- Freedman V. H., Calvelli T. A., Silagi S., Silverstein S. C. Macrophages elicited with heat-killed bacillus Calomette-Guérin protect C57BL/6J mice against a syngeneic melanoma. J Exp Med. 1980 Sep 1;152(3):657–673. doi: 10.1084/jem.152.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galleli A., Le Garrec Y., Chedid L. Increased resistance and depressed delayed-type hypersensitivity to Listeria monocytogenes induced by pretreatment with lipopolysaccharide. Infect Immun. 1981 Jan;31(1):88–94. doi: 10.1128/iai.31.1.88-94.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern B. N., Biozzi G., Stiffel C., Mouton D. Inhibition of tumour growth by administration of killed corynebacterium parvum. Nature. 1966 Nov 19;212(5064):853–854. doi: 10.1038/212853a0. [DOI] [PubMed] [Google Scholar]
- Handman E., Burgess A. W. Stimulation by granulocyte-macrophage colony-stimulating factor of Leishmania tropica killing by macrophages. J Immunol. 1979 Mar;122(3):1134–1137. [PubMed] [Google Scholar]
- Kato I., Yokokura T., Mutai M. Macrophage activation by Lactobacillus casei in mice. Microbiol Immunol. 1983;27(7):611–618. doi: 10.1111/j.1348-0421.1983.tb00622.x. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Sato K., Ohkawa H., Ueyama Y., Okabe T., Sato N., Asano S., Mori M., Ohsawa N., Kosaka K. Association of hypercalcemia with tumors producing colony-stimulating factor(s). Cancer Res. 1983 May;43(5):2368–2374. [PubMed] [Google Scholar]
- Matsuo K., Takeya K., Nomoto K., Shimotori S., Terasaka R. T-cell-independent activation of macrophages by viable BCG in tumor-bearing mice. Cell Immunol. 1981 Jan 15;57(2):293–306. doi: 10.1016/0008-8749(81)90088-5. [DOI] [PubMed] [Google Scholar]
- Miake S., Nomoto K., Yokokura T., Yoshikai Y., Mutai M., Nomoto K. Protective effect of Lactobacillus casei on Pseudomonas aeruginosa infection in mice. Infect Immun. 1985 May;48(2):480–485. doi: 10.1128/iai.48.2.480-485.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Miyata H., Nomoto K., Takeya K. Characteristics of resistance to Listeria monocytogenes enhanced by Corynebacterium parvum in mice. Immunology. 1980 May;40(1):33–39. [PMC free article] [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- Nomoto K., Miake S., Hashimoto S., Yokokura T., Mutai M., Yoshikai Y., Nomoto K. Augmentation of host resistance to Listeria monocytogenes infection by Lactobacillus casei. J Clin Lab Immunol. 1985 Jun;17(2):91–97. [PubMed] [Google Scholar]
- Stanley E. R., Metcalf D. The molecular weight of colony-stimulating factor (CSF). Proc Soc Exp Biol Med. 1971 Jul;137(3):1029–1031. doi: 10.3181/00379727-137-35721. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Robinson W. A., Ada G. L. Properties of the colony stimulating factor in leukaemic and normal mouse serum. Aust J Exp Biol Med Sci. 1968 Dec;46(6):715–726. doi: 10.1038/icb.1968.178. [DOI] [PubMed] [Google Scholar]
- Trudgett A., McNeill T. A., Killen M. Granulocyte-macrophage precursor cell and colony-stimulating factor responses of mice infected with Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):450–455. doi: 10.1128/iai.8.3.450-455.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Waheed A., Shadduck R. K. Changes in serum colony-stimulating factor and monocytic progenitor cells during Listeria monocytogenes infection in mice. Infect Immun. 1984 Jul;45(1):180–184. doi: 10.1128/iai.45.1.180-184.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolmark N., Levine M., Fisher B. The effect of a single and repeated administration of Corynebacterium parvum on bone marrow macrophage colony production in normal mice. J Reticuloendothel Soc. 1974 Oct;16(4):252–257. [PubMed] [Google Scholar]
- Yokochi T., Nakashima I., Kato N., Miyadai T., Yoshida K., Kimura Y. In vivo effects of bacterial lipopolysaccharide on proliferation of macrophage colony-forming cells in bone marrow and peripheral lymphoid tissues. Infect Immun. 1985 Feb;47(2):496–501. doi: 10.1128/iai.47.2.496-501.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokokura T., Kato I., Matsuzaki T., Mutai M., Satoh H. [Antitumor activity of Lactobacillus casei YIT 9018 (LC 9018)--effect of administration route]. Gan To Kagaku Ryoho. 1984 Nov;11(11):2427–2433. [PubMed] [Google Scholar]


