Abstract
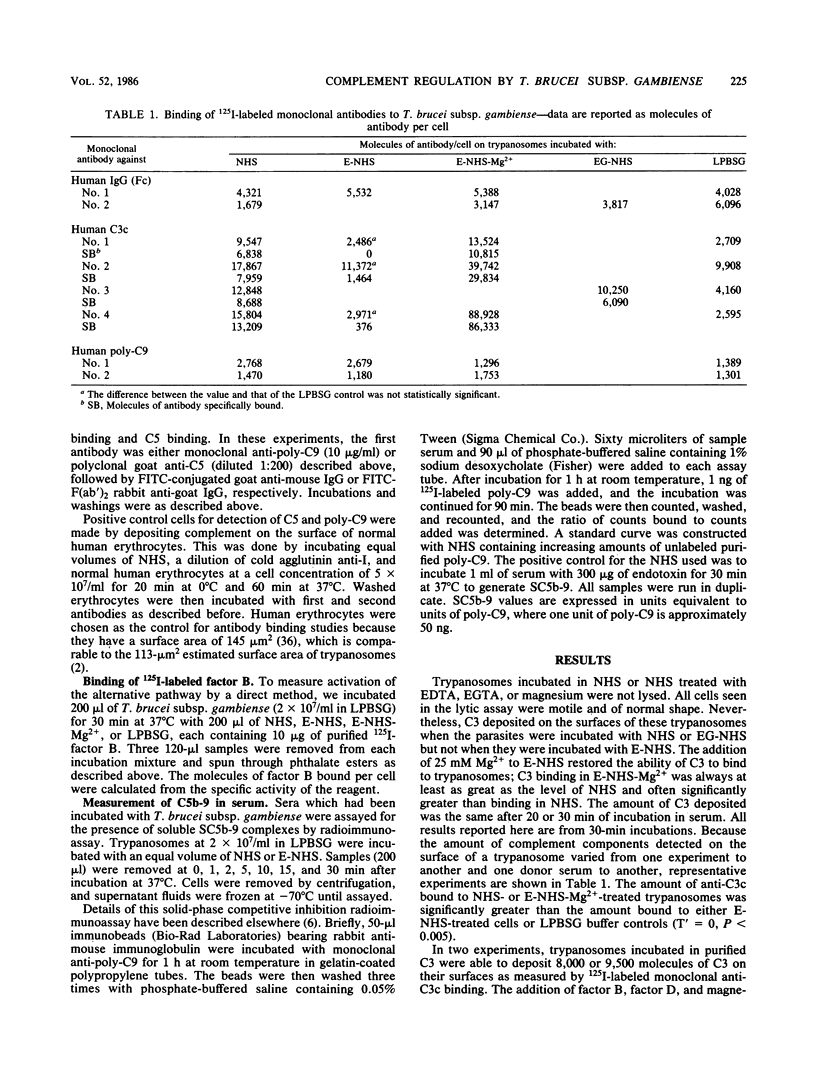
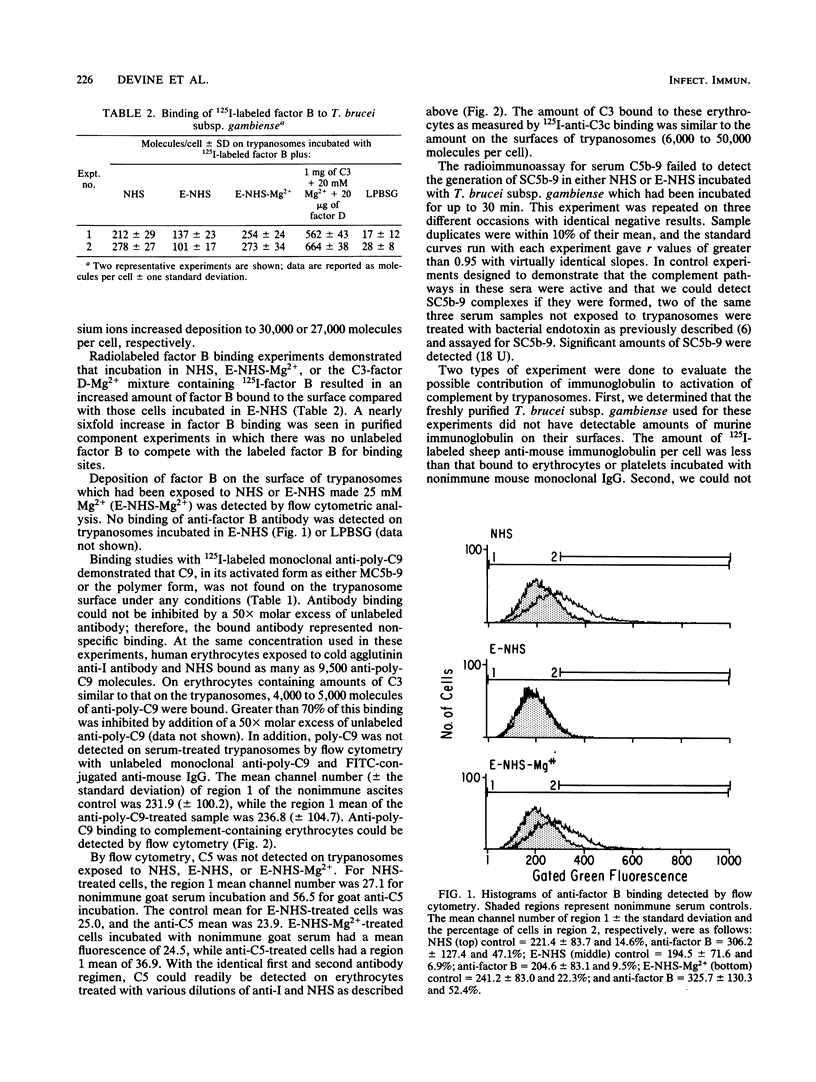
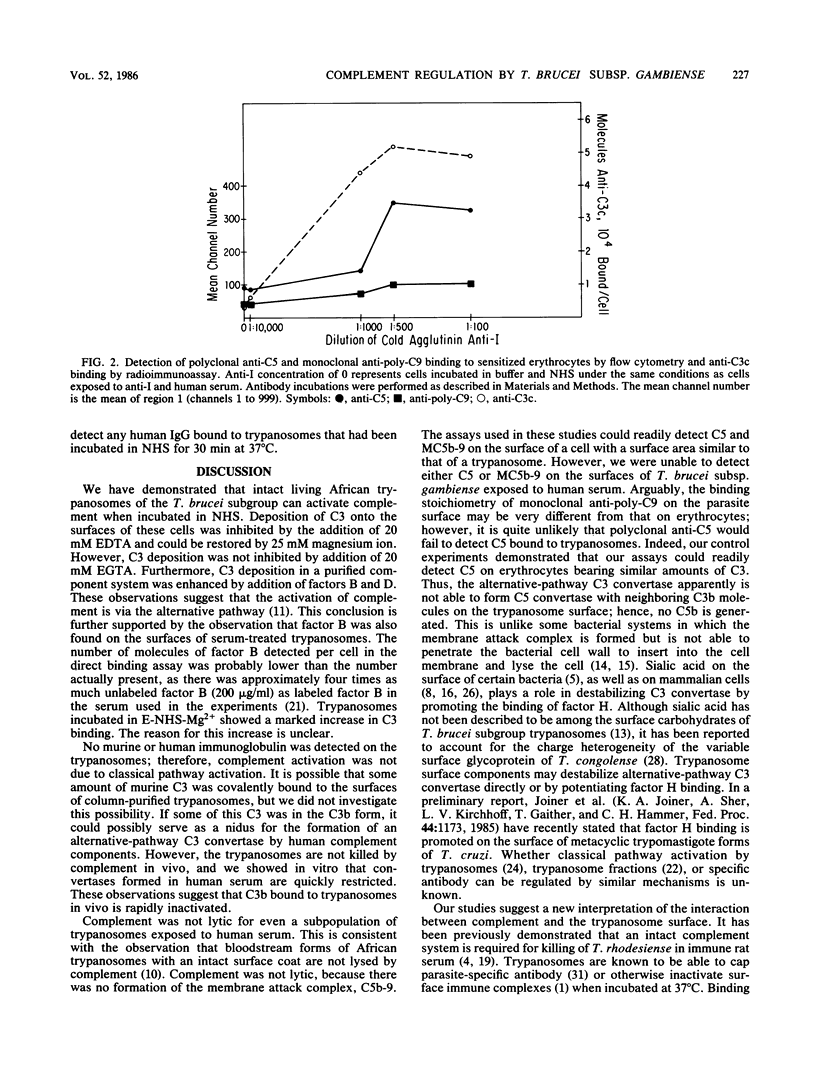
We studied the interaction of African trypanosomes with human complement. Bloodstream forms of Trypanosoma brucei subsp. gambiense isolated from mice activated the alternative pathway of complement during a 30-min incubation in vitro. In human serum, all cells remained intact and motile during this period. C3 was detected on the surface by a direct binding assay with a monoclonal antibody which recognizes C3b and iC3b. C3 deposition could also be detected by this radioimmunoassay when parasites were incubated with purified C3. Such C3 binding was enhanced by factor B, factor D, and magnesium. Surface deposition of factor B was demonstrated both by flow immunofluorescence analysis and binding of radiolabeled factor B. C3 binding and factor B binding were inhibitable by EDTA but not by ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N' -tetraacetic acid (EGTA). The inhibited binding could be restored by addition of magnesium. No human immunoglobulin G or mouse immunoglobulin was detected on the trypanosome surface. By flow cytometry, neither human C5 nor polymerized C9 was detected on trypanosomes incubated in serum, although this assay was able to detect C5 and C9 on the surface of complement-treated human erythrocytes. Using a radioimmunoassay which measures C5b-9 in serum, we found that there was no generation of SC5b-9 in serum which had been incubated with trypanosomes. We concluded that, although trypanosomes activate the alternative pathway of complement, they are not lysed, because the cascade does not continue beyond the establishment of C3 convertase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balber A. E., Bangs J. D., Jones S. M., Proia R. L. Inactivation or elimination of potentially trypanolytic, complement-activating immune complexes by pathogenic trypanosomes. Infect Immun. 1979 Jun;24(3):617–627. doi: 10.1128/iai.24.3.617-627.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975 Dec;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- Dempsey W. L., Mansfield J. M. Lymphocyte function in experimental African trypanosomiasis. V. Role of antibody and the mononuclear phagocyte system in variant-specific immunity. J Immunol. 1983 Jan;130(1):405–411. [PubMed] [Google Scholar]
- Diggs C., Flemmings B., Dillon J., Snodgrass R., Campbell G., Esser K. Immune serum-mediated cytotoxicity against Trypanosoma rhodesiense. J Immunol. 1976 Apr;116(4):1005–1009. [PubMed] [Google Scholar]
- Edwards M. S., Kasper D. L., Jennings H. J., Baker C. J., Nicholson-Weller A. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol. 1982 Mar;128(3):1278–1283. [PubMed] [Google Scholar]
- Falk R. J., Dalmasso A. P., Kim Y., Lam S., Michael A. Radioimmunoassay of the attack complex of complement in serum from patients with systemic lupus erythematosus. N Engl J Med. 1985 Jun 20;312(25):1594–1599. doi: 10.1056/NEJM198506203122502. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Dalmasso A. P., Kim Y., Tsai C. H., Scheinman J. I., Gewurz H., Michael A. F. Neoantigen of the polymerized ninth component of complement. Characterization of a monoclonal antibody and immunohistochemical localization in renal disease. J Clin Invest. 1983 Aug;72(2):560–573. doi: 10.1172/JCI111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Allison A. C. Alternative pathway activation of complement by African trypanosomes lacking a glycoprotein coat. Parasite Immunol. 1983 Sep;5(5):491–498. doi: 10.1111/j.1365-3024.1983.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Ferrante A. The role of natural agglutinins and trypanolytic activity in host specificity of Trypanosoma musculi. Parasite Immunol. 1984 Nov;6(6):509–517. doi: 10.1111/j.1365-3024.1984.tb00821.x. [DOI] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Greenwood B. M., Whittle H. C. Complement activation in patients with Gambian sleeping sickness. Clin Exp Immunol. 1976 Apr;24(1):133–138. [PMC free article] [PubMed] [Google Scholar]
- Johnson J. G., Cross G. A. Carbohydrate composition of variant-specific surface antigen glycoproteins from Trypanosoma brucei. J Protozool. 1977 Nov;24(4):587–591. doi: 10.1111/j.1550-7408.1977.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K., Brown E., Hammer C., Warren K., Frank M. Studies on the mechanism of bacterial resistance to complement-mediated killing. III. C5b-9 deposits stably on rough and type 7 S. pneumoniae without causing bacterial killing. J Immunol. 1983 Feb;130(2):845–849. [PubMed] [Google Scholar]
- Kazatchkine M. D., Fearon D. T., Austen K. F. Human alternative complement pathway: membrane-associated sialic acid regulates the competition between B and beta1 H for cell-bound C3b. J Immunol. 1979 Jan;122(1):75–81. [PubMed] [Google Scholar]
- Lambris J. D., Dobson N. J., Ross G. D. Release of endogenous C3b inactivator from lymphocytes in response to triggering membrane receptors for beta 1H globulin. J Exp Med. 1980 Dec 1;152(6):1625–1644. doi: 10.1084/jem.152.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Mosser D. M., Roberts J. F. Trypanosoma brucei: recognition in vitro of two developmental forms by murine macrophages. Exp Parasitol. 1982 Dec;54(3):310–316. doi: 10.1016/0014-4894(82)90040-6. [DOI] [PubMed] [Google Scholar]
- Musoke A. J., Barbet A. F. Activation of complement by variant-specific surface antigen of trypanosoma brucei. Nature. 1977 Dec 1;270(5636):438–440. doi: 10.1038/270438a0. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Schreiber R. D. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- Nagle R. B., Ward P. A., Lindsley H. B., Sadun E. H., Johnson A. J., Berkaw R. E., Hildebrandt P. K. Experimental infections with African Trypanosomes. VI. Glomerulonephritis involving the alternate pathway of complement activation. Am J Trop Med Hyg. 1974 Jan;23(1):15–26. [PubMed] [Google Scholar]
- Nielsen K., Sheppard J. Activation of complement by trypanosomes. Experientia. 1977 Jun 15;33(6):769–771. doi: 10.1007/BF01944182. [DOI] [PubMed] [Google Scholar]
- Nielsen K., Sheppard J., Holmes W., Tizard I. Experimental bovine trypanosomiasis. Changes in serum immunoglobulins, complement and complement components in infected animals. Immunology. 1978 Nov;35(5):817–826. [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. J., Baker P. J., Rosse W. F. Increased enzymatic activity of the alternative pathway convertase when bound to the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1982 Feb;69(2):337–346. doi: 10.1172/JCI110457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautenberg P., Reinwald E., Risse H. J. Sialic acids are responsible for charge heterogeneity of the variant surface glycoprotein of Trypanosoma congolense. Mol Biochem Parasitol. 1981 Dec;4(3-4):129–138. doi: 10.1016/0166-6851(81)90012-8. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Newman S. L., Lambris J. D., Devery-Pocius J. E., Cain J. A., Lachmann P. J. Generation of three different fragments of bound C3 with purified factor I or serum. II. Location of binding sites in the C3 fragments for factors B and H, complement receptors, and bovine conglutinin. J Exp Med. 1983 Aug 1;158(2):334–352. doi: 10.1084/jem.158.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F., Devine D. V., Ware R. Reactions of immunoglobulin G-binding ligands with platelets and platelet-associated immunoglobulin G. J Clin Invest. 1984 Feb;73(2):489–496. doi: 10.1172/JCI111235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabel H. Activation of the alternative pathway of bovine complement by Trypanosoma congolense. Parasite Immunol. 1982 Sep;4(5):329–335. doi: 10.1111/j.1365-3024.1982.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Tetley L., Vickerman K., Moloo S. K. Absence of a surface coat from metacyclic Trypanosoma vivax: possible implications for vaccination against vivax trypanosomiasis. Trans R Soc Trop Med Hyg. 1981;75(3):409–414. doi: 10.1016/0035-9203(81)90106-1. [DOI] [PubMed] [Google Scholar]
- Turner M. J. Antigenic variation in its biological context. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):27–40. doi: 10.1098/rstb.1984.0106. [DOI] [PubMed] [Google Scholar]


