Abstract
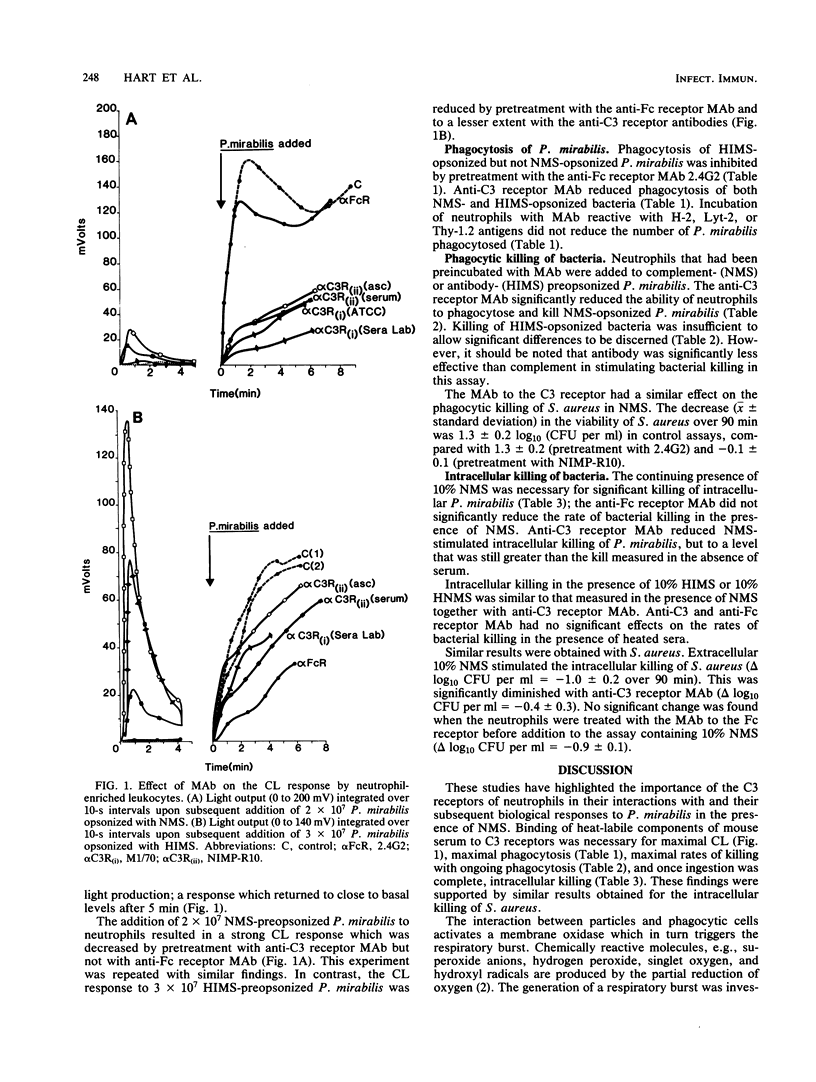
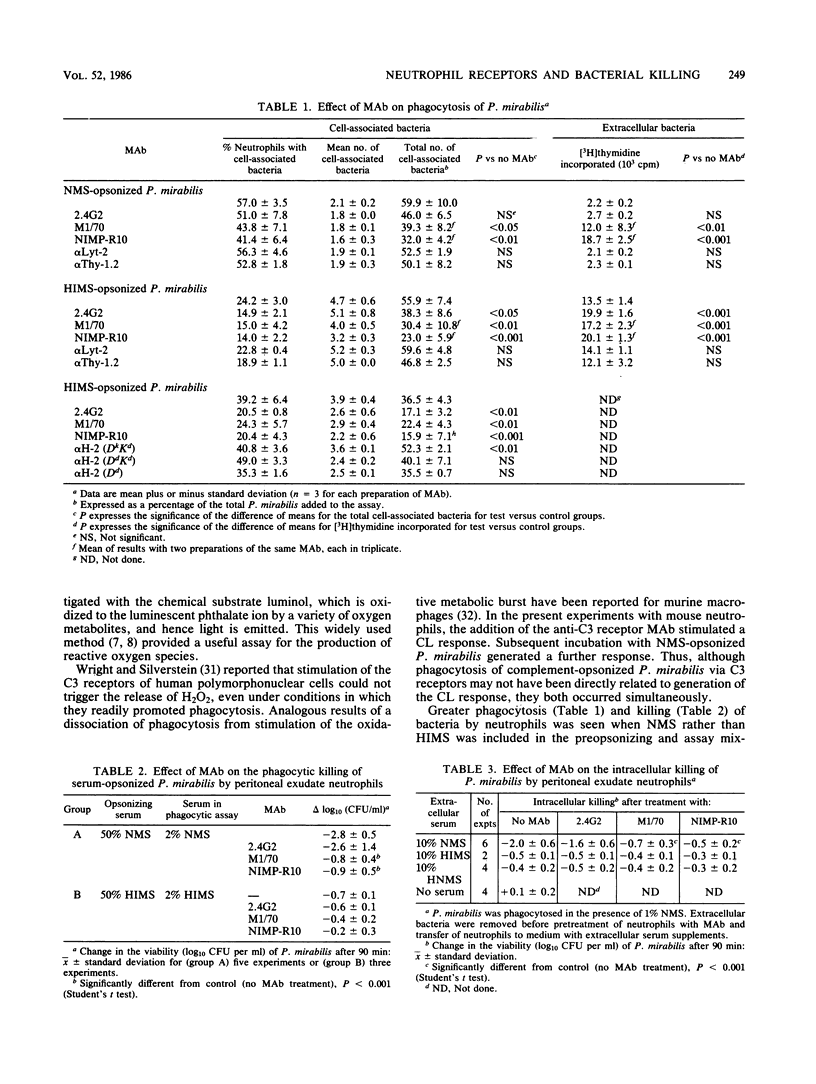
The role of the Fc and third component of complement (C3) receptors on mouse neutrophils in the control of killing of Proteus mirabilis, opsonized in normal mouse serum (NMS) or heated immune mouse serum (HIMS), was studied. The events following incubation of neutrophils with P. mirabilis and the events associated with bacterial killing were assayed. The respiratory burst was quantified by chemiluminescence (CL). Levels of leukocyte-associated bacteria were determined after a 20-min ingestion period as a measure of phagocytosis. Bacterial killing was measured while ingestion was allowed to continue or as a discrete process when extracellular, noningested bacteria had been removed and neutrophils with intracellular bacteria were incubated in the presence of serum. Modification of these responses in the presence of three monoclonal antibodies (MAb), NIMP-R10 and M1/70, which bind to different epitopes of the mouse C3 receptor, and 2.4G2, which binds to the mouse Fc receptor, was investigated. MAb to the C3, but not to the Fc, receptors reduced CL, ingestion, and intracellular killing of NMS-opsonized P. mirabilis. MAb to the Fc receptor diminished CL to and reduced the rate of ingestion of HIMS-opsonized bacteria. The two MAb to the C3 receptor each produced a similar inhibition of ingestion and intracellular killing of HIMS-opsonized bacteria, but they only partially blocked CL. A range of MAb preparations reactive with other murine antigens did not inhibit these events, either with NMS- or HIMS-opsonized P. mirabilis. The results suggest that C3 receptors on mouse neutrophils played a predominant role in regulation of the killing of P. mirabilis. Similar results were found for Staphylococcus aureus. C3 receptors were necessary for maximal expression of all functions culminating in bacterial kill. That MAb to the C3 receptor inhibited phagocytosis of HIMS-opsonized bacteria in similar fashion to the effect of MAb to the Fc receptor and in contrast to the lack of effect of control MAb may reflect steric hindrance of the Fc receptor by MAb binding to the C3 receptor, or it may reflect that the receptors are linked in murine neutrophils as they are in human neutrophils.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Todd R. F., 3rd, Dana N., Melamed J., Schlossman S. F., Colten H. R. Inhibition of phagocytosis of complement C3- or immunoglobulin G-coated particles and of C3bi binding by monoclonal antibodies to a monocyte-granulocyte membrane glycoprotein (Mol). J Clin Invest. 1983 Jul;72(1):171–179. doi: 10.1172/JCI110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Collins L. A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983 Jan;130(1):370–375. [PubMed] [Google Scholar]
- Finlay-Jones J. J., Hill N. L., Nulsen M. F., Pruul H., McDonald P. J. Opsonins in normal mouse serum for the phagocytic killing of Proteus mirabilis by murine neutrophils. Br J Exp Pathol. 1984 Dec;65(6):711–718. [PMC free article] [PubMed] [Google Scholar]
- Gale R., Bertouch J. V., Bradley J., Roberts-Thomson P. J. Direct activation of neutrophil chemiluminescence by rheumatoid sera and synovial fluid. Ann Rheum Dis. 1983 Apr;42(2):158–162. doi: 10.1136/ard.42.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale R., Bertouch J. V., Gordon T. P., Bradley J., Roberts-Thomson P. J. Neutrophil activation by immune complexes and the role of rheumatoid factor. Ann Rheum Dis. 1984 Feb;43(1):34–39. doi: 10.1136/ard.43.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Mullinax P. J. Augmentation of macrophage complement receptor function in vitro. III. C3b receptors that promote phagocytosis migrate within the plane of the macrophage plasma membrane. J Exp Med. 1981 Aug 1;154(2):291–305. doi: 10.1084/jem.154.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. H., Spencer L. K., McDonald P. J., Finlay-Jones J. J. Evaluation of intracellular killing of bacteria by enriched populations of mouse peritoneal exudate neutrophils. Aust J Exp Biol Med Sci. 1985 Aug;63(Pt 4):361–370. doi: 10.1038/icb.1985.42. [DOI] [PubMed] [Google Scholar]
- Jack R. M., Fearon D. T. Altered surface distribution of both C3b receptors and Fc receptors on neutrophils induced by anti-C3b receptor or aggregated IgG. J Immunol. 1984 Jun;132(6):3028–3033. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., Daha M. R., van Furth R. Participation of immunoglobulins and complement components in the intracellular killing of Staphylococcus aureus and Escherichia coli by human granulocytes. Infect Immun. 1981 Sep;33(3):714–724. doi: 10.1128/iai.33.3.714-724.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Strath M., Sanderson C. J. Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. Br J Haematol. 1984 Jul;57(3):489–494. doi: 10.1111/j.1365-2141.1984.tb02923.x. [DOI] [PubMed] [Google Scholar]
- López A. F., Battye F. L., Vadas M. A. Fc receptors on mouse neutrophils and eosinophils: antigenic characteristics, isotype specificity and relative cell membrane density measured by flow cytometry. Immunology. 1985 May;55(1):125–133. [PMC free article] [PubMed] [Google Scholar]
- López A. F., Burns G. F., Stanley I. J. Epitope diversity of monoclonal antibodies revealed by cross-species reactivity. Mol Immunol. 1984 May;21(5):371–374. doi: 10.1016/0161-5890(84)90033-6. [DOI] [PubMed] [Google Scholar]
- López A. F., Strath M., Sanderson C. J. IgG and complement receptors on purified mouse eosinophils and neutrophils. Immunology. 1981 Aug;43(4):779–786. [PMC free article] [PubMed] [Google Scholar]
- López A. F., Strath M., Sanderson C. J. Mouse immunoglobulin isotypes mediating cytotoxicity of target cells by eosinophils and neutrophils. Immunology. 1983 Mar;48(3):503–509. [PMC free article] [PubMed] [Google Scholar]
- Mantovani B. Different roles of IgG and complement receptors in phagocytosis by polymorphonuclear leukocytes. J Immunol. 1975 Jul;115(1):15–17. [PubMed] [Google Scholar]
- Nulsen M. F., Finlay-Jones J. J., Skinner J. M., McDonald P. J. Intra-abdominal abscess formation in mice: quantitative studies on bacteria and abscess-potentiating agents. Br J Exp Pathol. 1983 Aug;64(4):345–353. [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Mayer N. M., Sachs D. H. Monoclonal antibodies to mouse major histocompatibility complex antigens. Transplantation. 1982 Sep;34(3):113–120. doi: 10.1097/00007890-198209000-00001. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Pommier C. G., Inada S., Fries L. F., Takahashi T., Frank M. M., Brown E. J. Plasma fibronectin enhances phagocytosis of opsonized particles by human peripheral blood monocytes. J Exp Med. 1983 Jun 1;157(6):1844–1854. doi: 10.1084/jem.157.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Scribner D. J., Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976 Apr;116(4):892–897. [PubMed] [Google Scholar]
- Sheridan J. W., Finlay-Jones J. J. Studies on a fractionated murine fibrosarcoma: a reproducible method for the cautious and a caution for the unwary. J Cell Physiol. 1977 Mar;90(3):535–552. doi: 10.1002/jcp.1040900316. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfrè G., Secher D. S., Milstein C. Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol. 1978 Aug;8(8):539–551. doi: 10.1002/eji.1830080802. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Tumor-promoting phorbol esters stimulate C3b and C3b' receptor-mediated phagocytosis in cultured human monocytes. J Exp Med. 1982 Oct 1;156(4):1149–1164. doi: 10.1084/jem.156.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Johnston R. B., Jr Dissociation of phagocytosis from stimulation of the oxidative metabolic burst in macrophages. J Exp Med. 1984 Feb 1;159(2):405–416. doi: 10.1084/jem.159.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]


