Abstract
Denaturing gradient gel electrophoresis (DGGE) of PCR-amplified ribosomal DNA (rDNA) is routinely used to compare levels of diversity of microbial communities and to monitor population dynamics. While using PCR-DGGE to examine the bacteria in wine fermentations, we noted that several commonly used PCR primers for amplifying bacterial 16S rDNA also coamplified yeast, fungal, or plant DNA present in samples. Unfortunately, amplification of nonbacterial DNA can result in a masking of bacterial populations in DGGE profiles. To surmount this problem, we developed two new primer sets for specific amplification of bacterial 16S rDNA in wine fermentation samples without amplification of eukaryotic DNA. One primer set, termed WLAB1 and WLAB2, amplified lactic acid bacteria, while another, termed WBAC1 and WBAC2, amplified both lactic acid bacterial and acetic acid bacterial populations found in wine. Primer specificity and efficacy were examined with DNA isolated from numerous bacterial, yeast, and fungal species commonly found in wine and must samples. Importantly, both primer sets effectively distinguished bacterial species in wine containing mixtures of yeast and bacteria.
Winemaking involves a mixed culture of numerous microorganisms including fungal, yeast, and bacteria species (6). The principal bacteria present in wine are members of the lactic acid bacteria (LAB), acetic acid bacteria (AAB), and to a lesser extent species of bacilli (13). LAB and AAB are often present on the surface of the grape and can represent significant populations in musts (17). LAB play dual roles in wine fermentations: as agents of wine spoilage and as the main effector of secondary, or malolactic, fermentation. Most LAB found in wine, including members of Oenococcus, Lactobacillus, Pediococcus, and Leuconostoc, are microaerophilic and able to grow in the anaerobic environment of fermenting wine (24). In contrast, wine-related AAB, such as members of Gluconobacter or Gluconacetobacter, are obligately aerobic and loosely categorized as vinegar bacteria (7, 24). Both AAB and LAB can produce acetic acid, a potential inhibitor of growth and alcohol production by Saccharomyces cerevisiae (18).
Most bacterial species present in wine fermentations have been identified by traditional microbiological techniques involving cultivation. However, as observed with microbial ecological studies of other environments, cultivation-dependent methods often exhibit biases resulting in an incomplete representation of the true bacterial diversity present (1, 16). Applications of culture-independent molecular techniques to monitor the microbial successions of various food and beverage fermentations have revealed microbial constituents and microbial interactions not witnessed by previous plating analyses (14). One example of this is the recent use of epifluorescence microscopy to identify populations of viable but not culturable bacteria (both LAB and AAB) in aging wine (20).
Denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis for separation of bacterial 16S ribosomal DNA (rDNA) amplicons are common methods employed to characterize microbial communities from specific environmental niches (22). These approaches are attractive since they enable detection of individual species as well as overall profiling of community structure changes with time. We have recently developed DGGE methods to characterize the yeast diversity present in commercial wine fermentations (9). In the process of applying DGGE methods to monitor the bacterial populations in wine, we noted that most commonly used 16S rDNA primers readily amplified yeast, fungal, or plant DNAs that copurify with bacterial DNA isolated from wine. Such nontarget amplification from purportedly bacterium-specific primers can create misleading DGGE profiles and result in overestimation of the bacterial diversity present in environmental niches that harbor mixtures of fungi, plants, and bacteria. In this work, we document the amplification of fungal and plant DNA from many 16S rDNA-based primers used for profiling bacterial populations by DGGE and characterize two new primer sets (WBAC1-WBAC2GC and WLAB1-WLAB2GC) for specific amplification of the LAB and AAB populations in wine.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The following bacteria were used in this study: Lactobacillus plantarum UCD1, Pediococcus sp. strain PC8000, Pediococcus parvulus UCD13, Pediococcus pentosaceus UCD12, Leuconostoc mesenteroides ATCC 8293, Lactococcus lactis LM0230, Lactobacillus casei UCD4, Lactobacillus sp. strain UCD5, Lactobacillus brevis UCD24, Lactobacillus hilgardii UCD125, Lactobacillus buchneri UCD124, Lactobacillus fermentum UCD25, Lactobacillus sp. strain UCD9, Gluconacetobacter hansenii ATCC 35959, Gluconacetobacter liquefaciens ATCC 23749, Acetobacter pasteurianus ATCC 9432, Gluconacetobacter sp. strain UCD132 (wine isolate), Gluconobacter oxydans ATCC 23651, Gluconobacter oxydans UCD131 (wine isolate), Gluconobacter oxydans UCD133, Oenococcus oeni UCD20 (ML34), O. oeni UCD158, O. oeni UCD139, O. oeni UCD144, O. oeni UCD141, O. oeni UCD142, O. oeni UCD143, O. oeni UCD161, O. oeni UCD162, O. oeni UCD21 (PSU-1), O. oeni UCD159 (MCW), and O. oeni UCD160. Lactobacillus, Pediococcus, and Leuconostoc strains were grown in MRS media (Becton Dickinson, Sparks, Md.). O. oeni, Gluconobacter, and Gluconacetobacter strains were grown in Apple Rogosa media (7). L. lactis was grown in M17 media supplemented with 0.5% glucose (26). All bacterial strains were grown at 30°C.
Yeast strains and growth conditions.
The yeast strains used in this study were as follows: Saccharomyces cerevisiae S9, Kloeckera apiculata Y1, Metschnikowia pulcherrima UCD125, Pichia farinosa UCD-FST 67-22, Pichia fermentans UCD7, Pichia membranifaciens UCD22, Candida sp. strain EJ1, Candida vini UCD36, Schizosaccharomyces pombe UCD-FST-40-277, Saccharomycodes ludwigii UCD114, Williopsis saturnus UCD20, Brettanomyces sp. strain UCD615, Torulaspora delbrueckii flor 519 UCD817, Zygosaccharomyces bailii UCD795, and Kluveromyces thermotolerans EJ2. All strains were grown in yeast extract-peptone-dextrose medium (Becton Dickinson) at 30°C. All yeast and bacterial strains were provided by the Wine Microbiology Culture Collection in the Department of Viticulture and Enology at the University of California, Davis.
Fungal strains and growth conditions.
Cultures of a Trichoderma sp., a Cladosporium sp., Pythium ultimum, a Penicillium sp., Stemphylium botryosum, Alternaria alternata, and Humicola spp. were obtained from R. Michael Davis, and Eutypa lata and Fusarium lateritium were obtained from W. Douglas Gubler, both at the Plant Pathology Department, University of California, Davis. A culture of Botrytis cinerea was obtained from Far Niente Winery in Oakville, Calif. All fungal cultures were maintained on potato dextrose agar (Becton Dickinson) plates at 26°C.
DNA extractions.
For the isolation of DNA from yeast and fungal species, DNA was purified by procedures described previously (21). For isolation of bacterial DNA, the cell pellet from a 1.5-ml culture sample was resuspended in 200 μl of breaking buffer (2% Triton X-100, 1% sodium dodecyl sulfate, 100 mM NaCl, 10 mM Tris [pH 8], 1 mM EDTA [pH 8])-20 μl of lysozyme (0.1 g/liter) and incubated for 15 min at 37°C. The mixture was transferred to a microcentrifuge tube containing 0.3 g of 0.5-mm-diameter glass beads (BioSpec Products Inc., Bartlesville, Okla.). The cells were homogenized twice for 45 s at a speed setting of 4.5 in a bead beater instrument (Fast Prep; Q-BIOgene, Carlsbad, Calif.) in the presence of 200 μl of phenol-chloroform-isoamyl alcohol (25:24:1). Two hundred microliters of TE (10 mM Tris, 1 mM EDTA, pH 8) was added, and the bead-cell mixture was centrifuged at 11,000 × g for 5 min at 4°C. The aqueous phase was removed to another microcentrifuge tube, and the DNA was precipitated with 1 ml of 100% ethanol and centrifuged at 12,000 rpm for 10 min at 4°C. The pellet was washed with 70% ethanol, dried, and resuspended in 50 μl of sterile distilled water containing 1 μl of RNase (500 μg per ml; Sigma Chemical Co., St. Louis, Mo.). The sample was then incubated at 37°C for 30 min before storage at −20°C.
Plant DNA was isolated from sterilely propagated Vitis vinifera L. cv. Chardonnay with the Plant DNeasy kit as described by the manufacturer (Qiagen, Valencia, Calif.).
DNA amplification and primers.
Primers used in this study and their approximate positions on the Lactobacillus plantarum 16S rDNA partial gene sequence (accession number AJ271852; see Fig. 1) are as follows: HDA1 (5′-ACTCCTACGGGAGGCAGCAGT-3′; nucleotides [nt] 349 to 369) and HDA2 (5′-GTATTACCGCGGCTGCTGGCAC-3′; nt 526 to 547) (25), Ec338f (5′-ACTCCTACGGGAGGCAGCAG-3′; nt 349 to 368) and Ec518r (5′-ATTACCGCGGCTGCTGG-3′; nt 529 to 545) (2, 4), U968 (5′-ACGCGAAGAACCTTAC-3′; nt 977 to 992) and L1401 (5′-GCGTGTGTACAAGACCC-3′; nt 1392 to 1409) (29), Ec1055 (5′-ATGGCTGTCCGTCAGCT-3′; nt 1063 to 1078) and Ec1406 (5′-ACGGGCGGTGTGTAC-3′; nt 1399 to 1413) (12), GM5f (5′-CCTACGGGAGGCAGCAG-3′; nt 352 to 368) and 907R (5′-CCGTCAATTCCTTTRAGTTT-3′; nt 916 to 935) (23), Lac1 (5′-AGCAGTAGGGAATCTTCCA-3′; nt 364 to 382) and Lac2 (5′-ATTTCACCGCTACACATG-3′; nt 690 to 707) (28), 63f (5′-CAGGCCTAACACATGCAAGTC-3′; nt 27 to 48) (11, 19), WBAC1 (5′-GTCGTCAGCTCGTGTCGTGAGA-3′; nt 1069 to 1090), WBAC2 (5′-CCCGGGAACGTATTCACCGCG-3′; nt 1374 to 1394), WLAB1 (5′-TCCGGATTTATTGGGCGTAAAGCGA-3′; nt 565 to 589), WLAB2 (5′-TCGAATTAAACCACATGCTCCA-3′; nt 951 to 972). To facilitate DGGE separation, a GC-rich sequence (5′-CGCCCGCCGCGCCCCGCGCCCGGCCCGCCGCCCCCGCCCC-3′) was attached to one of the primers in each primer pair. PCR was performed using an MJ Research PTC-200 Peltier thermal cycler at a final volume of 50 μl containing 10 mM Tris-HCl; 50 mM KCl; 1.5 mM MgCl2; 0.2 mM (each) dATP, dCTP, dGTP, and dTTP; 0.2 μM primers; 1.25 IU of Taq DNA polymerase (Promega Corp, Madison, Wis.); and 3 μl of the extracted DNA (approximately 150 ng). The amplification programs, different for each primer set, are shown in Table 1. Amplicons were run on 2% agarose gels, stained with ethidium bromide, visualized under UV light, and photographed with a Multimage light cabinet (Alpha Innotech Corporation, San Leandro, Calif.).
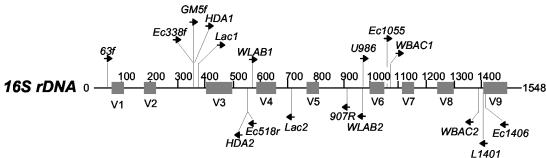
FIG. 1.
Relative positions on the Lactobacillus plantarum 16S rDNA gene (Gene Bank accession number AJ271852) for the various primers employed in this study.
TABLE 1.
PCR amplification programs
| Primer pair | Initial denaturation temp (°C), time (min) | Main cycling conditions
|
Final extension temp (°C), time (min) | Source or reference(s) | |||
|---|---|---|---|---|---|---|---|
| No. of cycles | Denaturing temp (°C), time | Annealing temp (°C), time | Extension temp (°C), time (min) | ||||
| HDA1GC-HDA2 | 94, 4 | 30 | 94, 30 s | 56, 30 s | 68, 1 | 68, 7 | 25 |
| Ec338fGC-Ec518r | 94, 5 | 25 | 94, 1 min | 52, 1 min | 72, 1 | 72, 10 | 2, 4 |
| U968GC-L1401 | 94, 3 | 30 | 94, 30 s | 56, 30 s | 68, 1 | 68, 7 | 29 |
| Ec1055-Ec1406GC | 94, 5 | 10 | 94, 1 min | TDa 53-43, 1 min | 72, 3 | 72, 10 | 12 |
| 20 | 94, 1 min | 43, 1 min | 72, 3 | ||||
| GM5FGC-907R | 94, 5 | 20 | 94, 1 min | TD 70-60, 1 min | 72, 3 | 72, 10 | 23 |
| 10 | 94, 1 min | 60, 1 min | 72, 3 | ||||
| Lac1-Lac2GC | 94, 2 | 35 | 94, 30 s | 61, 1 min | 68, 1 | 68, 7 | 28 |
| 63fGC-HDA2 | 94, 5 | 30 | 92, 1 min | 55, 1 min | 72, 1 | 72, 10 | 11, 19 |
| WBAC1-WBAC2GC | 95, 5 | 30 | 95, 1 min | 67, 30 s | 72, 1 | 72, 5 | This work |
| WLAB1-WLAB2GC | 95, 5 | 30 | 95, 1 min | 60, 30 s | 72, 1 | 72, 5 | This work |
TD, touchdown.
DGGE analysis.
The DCode universal mutation detection system (Bio-Rad, Hercules, Calif.) was used for sequence-specific separation of PCR products. PCR products obtained from WLAB1-WLAB2GC and WBAC1-WBAC2GC primers were run on 8% (wt/vol) polyacrylamide gels in a running buffer containing 40 mM Tris-acetate and 2 mM Na2-EDTA · H2O, pH 8.5 (TAE), and a denaturing gradient from 30 to 60% of urea and formamide. The electrophoresis was performed at 20 V for 10 min, 200 V for 2 h, and 120 V for 2 h at a constant temperature of 60°C. PCR products generated with the Ec338fGC-Ec518r primer pair were analyzed by DGGE as described previously (2). Ec1055-Ec1406GC amplicons were separated with 8% polyacrylamide gels containing a 40 to 60% urea-formamide gradient. Electrophoresis was performed for 10 h at 120 V at a constant temperature of 60°C. After electrophoresis, the DGGE gels were stained in 1.25× TAE solution containing ethidium bromide and photographed under UV transillumination. Bands of interest were excised directly from the gels with a sterile blade, mixed with 40 μl of water, and incubated overnight at 4°C. Two microliters of this solution was used to reamplify the PCR product. The PCR products were purified with a Wizard PCR purification kit (Promega) and sent to a commercial sequencing facility for sequencing (Davis Sequencing, Davis, Calif.).
Sequence analysis.
Sequence compilation and comparison were performed with Genetics Computer Group sequence analysis software using the BLAST program. Primer sets WLAB1-WLAB2GC and WBAC1-WBAC2GC were tested for specificity against the 16S rRNA Ribosomal Database (10) with the program OligoCheck, version 0.93. All analyses allowed for two mismatches per primer. Individual primers were checked for specificity against eukaryotic targets by BLAST analysis.
RESULTS
Amplification of eukaryotic DNA using common 16S rDNA primers.
Most 16S rDNA-based primer sets used for DGGE studies amplify either the V3 or the V6 to V8 variable regions (variable regions as described in reference 27) (Fig. 1). Initial PCR-DGGE and sequence analyses of DNA samples purified directly from a botrytized white wine fermentation revealed that bacterium-specific 16S primers Ec338fGC and Ec518r (2) readily amplified yeast 18S rDNA (Fig. 2). Given that DNA isolated from wine comes from eukaryotic as well as bacterial sources, we tested these primer sets for amplification of various yeast, fungal, and plant DNAs known to be present in the grape, wine, or winery environment. As shown in Table 2, the V3 region primers HDA1-HDA2GC (25) and Ec338fGC-Ec518r produced amplicons from most of the yeasts found in the wine environment. In addition, the Ec338fGC-Ec518r primer pair generated an amplicon from V. vinifera L. cv. Chardonnay DNA. Note that HDA1-HDA2GC and Ec338fGC-Ec518r are nearly identical primer sets developed independently (2, 25). V6 to V8 region PCR primers U968GC-L1402 (29) and Ec1055-Ec1406GC (12) did not produce amplicons from yeast DNA although the Ec1055-Ec1406GC pair generated amplicons from the fungal strains Fusarium lateritium, Botrytis cinerea, and Stemphylium botryosum (Table 2). Both V6 to V8 region primer pairs produced amplicons from V. vinifera DNA. To ensure that the amplicons were truly generated from the target DNAs and not emanating from bacterial contamination of these DNAs, select fungus-, yeast-, and plant-generated amplicons were sequenced. Results indicated that, in general, the V3 region primers amplified fungal or yeast 18S rDNA while the V6 to V8 region primers amplified plant chloroplast rDNA.
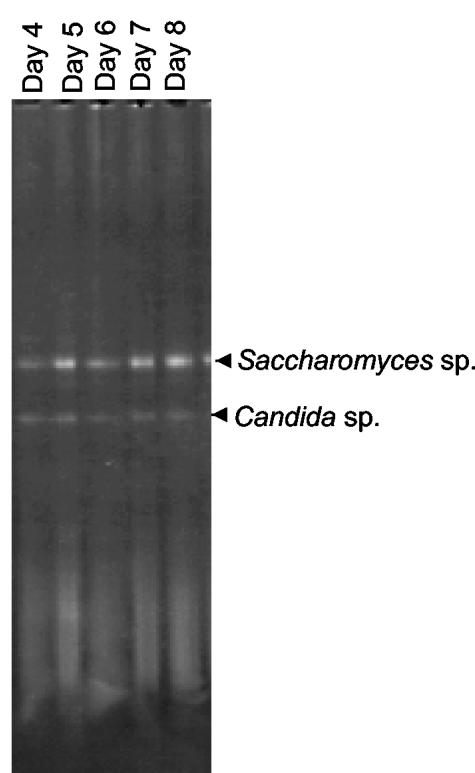
FIG. 2.
“Bacterial” DGGE pattern obtained with the Ec338fGC-Ec518r2 primer set on samples collected from a botrytis-affected wine fermentation (21). The two observable bands were excised, reamplified, sequenced, and compared to GenBank sequences for identification. Both bands were determined to be 18S rDNA from Saccharomyces or Candida yeast species.
TABLE 2.
Specificity of primer pairs
| Target DNA | Specificitya of primer pair:
|
|||
|---|---|---|---|---|
| HDA1- HDA2GC | Ec338fGC- Ec518r | Ec1055- Ec1406GC | U968GC- L1401 | |
| Bacteria | ||||
| L. plantarum | + | + | + | + |
| O. oeni | + | + | + | + |
| Pediococcus sp. strain PC8000 | ND | + | + | + |
| G. oxydans | ND | + | + | + |
| Plant | ||||
| V. vinifera | ND | + | + | + |
| Yeasts | ||||
| M. pulcherrima | + | + | − | − |
| S. cerevisiae | + | + | − | − |
| K. apiculata | + | + | − | − |
| P. farinosa | + | + | − | − |
| P. membranifaciens | + | + | − | − |
| P. fermentans | + | + | − | − |
| Candida sp. strain EJ1 | + | + | − | − |
| C. vini | + | + | − | − |
| S. pombe | + | + | − | − |
| S. ludwigii | + | + | − | − |
| W. saturnus | + | + | − | − |
| Brettanomyces sp. | + | + | − | − |
| T. delbrueckii | + | FB | − | − |
| Z. bailii | + | + | − | − |
| K. thermotolerans | FB | FB | − | − |
| Fungi | ||||
| F. lateritium | + | FB | + | − |
| B. cinerea | + | + | + | − |
| Trichoderma sp. | − | − | − | − |
| Cladosporium sp. | − | + | − | − |
| Penicillium sp. | FB | + | − | − |
| E. lata | − | − | − | − |
| S. botryosum | + | + | + | − |
| A. alternata | − | − | − | − |
| Humicola sp. | − | − | − | − |
| P. ultimum | + | + | − | FB |
ND, not determined; FB, faint band.
We then examined several other published 16S rDNA-based primers on select bacterial, yeast, fungal, and plant DNAs (Table 3); however, all were found to be unsuitable for wine analysis. Primer pair Lac1-Lac2GC was specific for LAB but did not amplify the common wine LAB O. oeni. Primer set GM5FGC-907R did not amplify O. oeni and Pediococcus sp. strain PC8000 and produced an amplicon on plant DNA. Primer set 63fGC-HDA2 amplified LAB and AAB, but it also produced a product with grape DNA.
TABLE 3.
Specificity of additional primer pairs
| Target DNA | Specificity of primer pair:
|
||
|---|---|---|---|
| GM5FGC-907R | Lac1-Lac2GC | 63fGC-HDA2 | |
| Bacteria | |||
| L. plantarum | + | + | + |
| O. oenia | − | − | + |
| Pediococcus spp.b | − | vc | + |
| G. oxydans | NDd | − | + |
| Plant | |||
| V. vinifera | + | − | + |
| Yeast | |||
| S. cerevisiae | − | − | − |
| Fungus | |||
| F. lateritium | − | − | − |
Primers were tested on 11 strains of O. oeni.
P. pentosaceus and Pediococcus sp. strain PC8000 were examined.
v, variable.
ND, not determined.
Competition between bacterial and eukaryotic DNA in mixed-template PCRs.
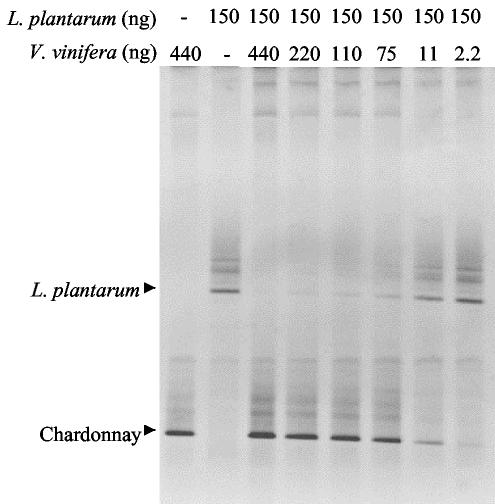
In addition to causing an overestimation of the bacterial diversity inherent in an environmental sample, amplification of nontarget organisms can limit the detection of true bacterial species because DNA from the nontarget organisms competes with the bacterial DNA for primers and deoxynucleoside triphosphates during PCR amplification. It has been suggested that competition between bacterial templates that represent less than 1% of the total microbial content results in omission of populations on DGGE gels (22). Using yeast-specific primers, we observed that wine yeast populations as low as 0.1% of the total yeast population could be revealed by PCR-DGGE (8). Since the V6 to V8 region primers Ec1055-Ec1406GC and U968GC-L1402 did not produce amplicons from yeast DNAs but did with plant DNAs, we examined the impact of the amplification of grape DNA on the ability to detect a target bacterial species by DGGE. Different amounts of V. vinifera grape DNA were mixed with a constant level of DNA from Lactobacillus plantarum (150 ng), and the mixture was amplified with the Ec1055-Ec1406GC primer set. The resultant amplicons were then run on DGGE. As shown in Fig. 3, the clarity of the Lactobacillus plantarum band was impacted when the Lactobacillus plantarum-to-V. vinifera DNA template ratio fell between 13:1 and 2:1 (Fig. 3). When equal or larger amounts of V. vinifera DNA relative to Lactobacillus plantarum DNA were included in the PCR, little or no evidence of the corresponding Lactobacillus plantarum band was observed in the resultant DGGE gel. These results demonstrate the potential masking effect of nontarget DNA on DGGE gel profiling and signify the importance of considering potential nontarget amplification when using DGGE to characterize bacterial communities.
FIG. 3.
Masking effect of V. vinifera plant (Chardonnay) DNA on bacterial populations in DGGE profiles with the Ec1055-Ec1406GC primer set. PCR was carried out on mixtures containing differing amounts of bacterial and plant DNA as indicated in Table 1 for the Ec1055-Ec1406GC primer set.
Wine bacterium-specific primers.
Unlike that in many ecological niches, the diversity of bacterial genera found in wine is relatively limited, consisting mostly of LAB and AAB species (13). Given the prevalence of nontarget yeast, fungal, or plant amplifications using previously published primers, we designed new primer sets that described the majority of LAB and AAB species while avoiding nontarget amplifications. The first primer set, WBAC1-WBAC2GC, targets the V7 to V8 16S rDNA region and produced a ∼320-bp amplicon on 14 LAB species and 6 AAB species (Table 4). Database analysis indicated that the WBAC1-WBAC2GC primers cover 70% of the eubacteria (allowing for two mismatches per primer). In particular, the primer set described 70% of the Proteobacteria (90% of the Acetobacter aceti subgroup) and 75% of the gram-positive bacteria (80% of the Bacillus-Lactobacillus-Streptococcus grouping; 90% of the Lactobacillus subgroup). Additional analysis indicated the WBAC1-WBAC2GC primers possess homology to a variety of eukaryotic targets (mostly plastid rDNAs); however, when tested empirically, the primer pair failed to produce amplicons from DNA from 15 yeast species, 10 fungal species, and a grape plant (Table 4).
TABLE 4.
Specificity of WBAC and WLAB primer pairs
| Target DNA | Specificity of primer pairs:
|
|
|---|---|---|
| WBAC1- WBAC2GC | WLAB1- WLAB2GC | |
| Bacteria | ||
| L. plantarum | + | + |
| O. oeni | + | + |
| Pediococcus sp. strain PC8000 | + | + |
| G. oxydans | + | − |
| L. lactis | + | + |
| P. pentosaceus | + | + |
| P. parvulus | + | + |
| L. mesenteroides | + | + |
| L. brevis | + | + |
| L. casei | + | + |
| L. fermentans | + | + |
| L. hilgardii | + | + |
| L. buchneri | + | + |
| Lactobacillus sp. strain UCD5 | + | + |
| Plant | ||
| V. vinifera | − | − |
| Yeasts | ||
| M. pulcherrima | − | − |
| S. cerevisiae | − | − |
| K. apiculata | − | − |
| P. farinosa | − | − |
| P. membranifaciens | − | − |
| P. fermentans | − | − |
| Candida sp. strain EJ1 | − | − |
| C. vini | − | − |
| S. pombe | − | − |
| S. ludwigii | − | − |
| W. saturnus | − | − |
| Brettanomyces sp. | − | − |
| T. delbrueckii | − | − |
| Z. bailii | − | − |
| K. thermotolerans | − | − |
| Fungi | ||
| F. lateritium | − | − |
| B. cinerea | − | − |
| Trichoderma sp. | − | − |
| Cladosporium sp. | − | − |
| Penicillium sp. | − | − |
| E. lata | − | − |
| S. botryosum | − | − |
| A. alternata | − | − |
| Humicola sp. | − | − |
| P. ultimum | − | − |
We then designed an additional primer set to identify the LAB commonly found in wine. Primer pair WLAB1-WLAB2GC targets the V4 and V5 16S rDNA regions and produced a ∼400-bp product from 14 LAB species. Database analysis indicates that this primer set describes 75% of the Bacillus-Lactobacillus-Streptococcus subgroup (including 90% of the Lactobacillus subgroup, 90% of the Enterococcus subgroup, and 80% of the Streptococcus subgroup). WLAB1-WLAB2GC is not unique to LAB, as it also describes 80% of the Fusobacteria and relatives. Importantly this set does not describe the Proteobacteria. In particular, the Acetobacter aceti subgroup is not covered, and, when tested empirically, the primer set did not produce amplicons from six AAB species (Table 4). Like WBAC1-WBAC2GC, the WLAB1-WLAB2GC primers possessed homology to eukaryotic targets but failed to produce amplicons on DNA from 15 yeast species, 10 fungal species, and a grape plant (Table 4).
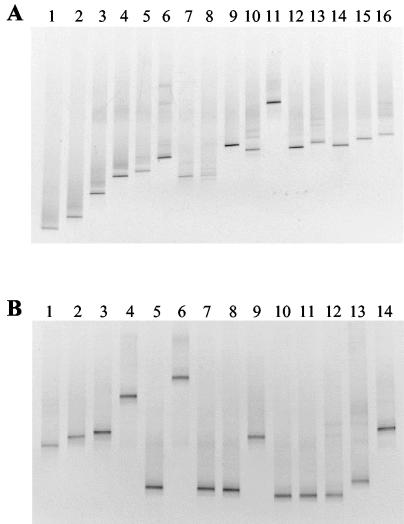
DGGE separation of WBAC1-WBAC2GC and WLAB1-WLAB2GC amplicons was sufficient to be useful for ecological studies of wines and musts (Fig. 4). As shown in Fig. 4A, the WBAC1-WBAC2GC primer pair worked particularly well to resolve AAB strains by DGGE. Amplicons generated by WLAB1-WLAB2GC resulted in less-diffuse DGGE bands than the WBAC1-WBAC2GC pair; however, several LAB species tested (Lactobacillus casei, Lactobacillus sp. strain UCD9, Lactobacillus fermentans, Lactobacillus sp. strain UCD5, Lactobacillus hilgardii, and Leuconostoc mesenteroides) exhibited similar electrophoretic mobilities (Fig. 4B).
FIG. 4.
DGGE profiles of amplified 16S rDNA regions obtained from different species of LAB and AAB. (A) WBAC1-WBAC2GC primer set. Lanes: 1, G. hansenii; 2, G. liquefaciens; 3, A. pasteurianus; 4, G. oxydans ATCC 23651; 5, G. oxydans UCD133; 6, Lactobacillus buchneri; 7, Lactobacillus hilgardii; 8, Lactobacillus fermentans; 9, Lactobacillus brevis; 10, Lactobacillus casei; 11, Lactococcus lactis; 12, Leuconostoc mesenteroides; 13, P. parvulus; 14, P. pentosaceus; 15, O. oeni; 16, Lactobacillus plantarum. (B) WLAB1-WLAB2GC primer set. Lanes: 1, Lactobacillus plantarum; 2, O. oeni; 3, P. pentosaceus; 4, P. parvulus, 5, Leuconostoc mesenteroides; 6, Lactococcus lactis; 7, Lactobacillus casei; 8, Lactobacillus sp. strain UCD9; 9, Lactobacillus brevis; 10, Lactobacillus fermentans; 11, Lactobacillus sp. strain UCD5; 12, Lactobacillus hilgardii; 13, Lactobacillus buchneri; 14, Pediococcus sp. strain PC8000.
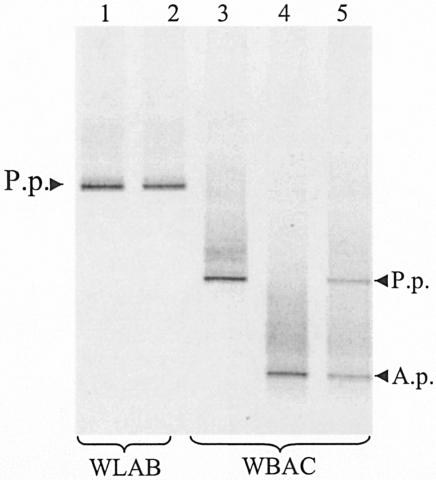
To see if WBAC1-WBAC2GC and WLAB1-WLAB2GC would selectively amplify LAB and AAB from grape must containing high yeast populations, a mixed culture of S. cerevisiae, Kloeckera apiculata, P. pentosaceus, and A. pasteurianus was prepared in sterile Chardonnay must (approximately 5 × 108 CFU per ml of each microbe). DNA was then extracted from this mixture, and PCR-DGGE was carried out using the WBAC1-WBAC2GC and WLAB1-WLAB2GC primers. As shown in Fig. 5, the WBAC1-WBAC2GC primers effectively distinguished the two bacterial species present in the must while WLAB1-WLAB2GC selectively amplified P. pentosaceus. Importantly, neither primer pair produced additional DGGE bands from the large population of yeasts (S. cerevisiae and Kloeckera apiculata) present in the mixture.
FIG. 5.
DGGE profiles with the WBAC1-WBAC2GC and WLAB1-WLAB2GC primer sets for grape must containing either a single bacterial species (P. pentosaceus or A. pasteurianus) or mixtures of bacteria and yeast (S. cerevisiae and Kloeckera apiculata). Lane 1, P. pentosaceus; lane 2, mixture of P. pentosaceus, A. pasteurianus, S. cerevisiae, and K. apiculata; lane 3, P. pentosaceus; lane 4, A. pasteurianus; lane 5, mixture of P. pentosaceus, A. pasteurianus, S. cerevisiae, and K. apiculata. P.p., P. pentosaceus; A.p., A. pasteurianus.
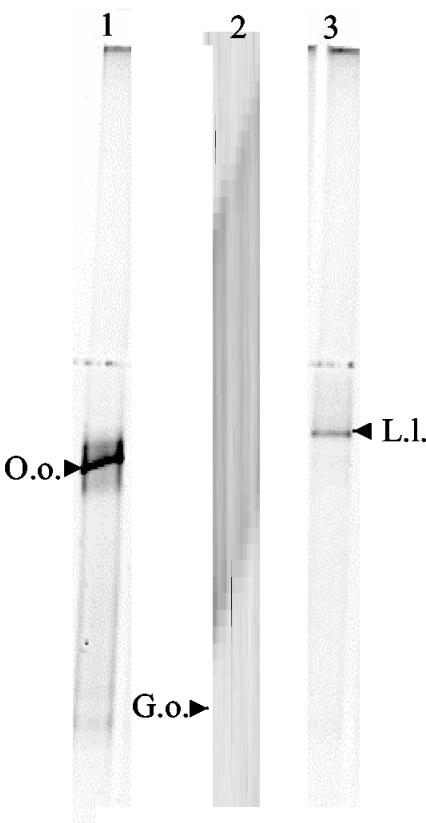
To ensure that the WBAC primer set is applicable with actual wine samples, several commercial wine samples were examined. Two spoiled red wines which appeared to possess bacterial growth (as determined microscopically) were obtained from commercial wineries. PCR-DGGE analysis of these two wines using the WBAC primers revealed this spoilage to be individual populations of Oenococcus and Lactococcus, respectively (Fig. 6, lanes 1 and 3). In addition, a prefermentation sample obtained from a commercial botrytized white wine fermentation was examined. Botrytized wine fermentations are known to contain large populations of Gluconobacter species (3), and previous analysis of this particular commercial fermentation revealed substantial populations of yeasts (∼107 CFU per ml) at the prefermentation stage (21). As seen in Fig. 6, a single dominant population of G. oxydans was identified in the botrytized white wine prefermentation sample. The fact that no yeast-derived DGGE bands were witnessed in this sample, in contrast to the Saccharomyces and Candida populations observed with the Ec338fGC-Ec518r primer pair (Fig. 1), confirms the bacterial specificity of the WBAC primers in the presence of a large yeast population.
FIG. 6.
DGGE profiles using the WBAC1-WBAC2GC primer pair on two wine spoilage samples (lanes 1 and 3) and a prefermentation sample taken from a botrytized white wine fermentation (lane 2). Designated bands of interest were excised, reamplified, sequenced, and compared to the GenBank database for species identification. Abbreviations: O.o., O. oeni; G.o., G. oxydans; L.l., Lactococcus lactis.
DISCUSSION
Bacteria are a ubiquitous presence in the production of wine. Various genera of LAB and AAB are found in fresh must, and different species are capable of persisting and growing throughout the fermentation, even after the production of significant levels of ethanol by the wine yeasts (13). Bacterial growth in wine can have a positive or negative impact on wine quality. Growth of AAB and some LAB can result in unacceptable levels of acetic acid, potentially influencing proper growth and sugar utilization by wine yeast as well as altering the sensory attributes of the finished wine (5). Conversely, other LAB species perform the malolactic conversion in wine, thereby enhancing stability and sensory aspects (15).
We have previously employed DGGE to directly characterize the yeast populations in wine (9). In the process of evaluating commonly used PCR primers for bacterial DGGE analysis, we noted that common 16S rDNA primer sets readily amplified yeast, fungal, or plant DNAs, three common constituents in any DNA sample purified directly from wine. PCR primers targeting the bacterial 16S rDNA V3 variable region (HDA1GC-HDA2 and Ec338fGC-Ec518r) were shown to readily amplify select yeast and fungal strains. Other primers that target the V6 to V8 regions (U968GC-L1401 and Ec1055-Ec1406GC) did not amplify select fungal and yeast populations; however, they did amplify plant DNA. Coamplification of nonbacterial DNA is problematic since it can result in an overestimation of the bacterial content of any particular niche. Moreover, competition between bacterial and nontarget templates during PCR may mask lower bacterial populations. This work demonstrates the importance of testing purportedly bacterium-specific PCR primers on potential eukaryotic DNAs that might copurify with bacterial DNA in environmental samples prior to embarking on a detailed analysis.
To overcome this problem, we developed new primers sets WBAC1-WBAC2GC and WLAB1-WLAB2GC, which readily amplify the LAB and AAB species in wine but which avoid amplification of eukaryotic DNAs. Each primer set produced a similar PCR product yield from the bacterial species analyzed (14 LAB and 6 AAB species tested with WBAC1-WBAC2GC and 14 LAB species tested with WLAB1-WLAB2GC). While database analysis indicated that the WBAC1-WBAC2GC primer set does not comprehensively cover all eubacteria, it well describes the Proteobacteria and gram-positive taxa, particularly the Acetobacter aceti and Lactobacillus subgroupings. In a similar vein the WLAB1-WLAB2GC primer set is not exclusively specific for LAB, as it describes another bacterial taxon, Fusobacteria, albeit a group not generally found in the wine environment. Importantly, the WLAB primer set does not amplify members of the AAB (Acetobacteraceae), the other major bacterial taxon found in wines and musts. Given that the WLAB1-WLAB2GC primer set covers the V4 and V5 16S rDNA regions, the resultant sequence analysis may not be as discriminatory as that obtained with WBAC1-WBAC2GC (V6 to V8 region). Regardless, the WLAB1-WLAB2GC primer set should be useful for differentiating LAB species from wine and grape environments.
Acknowledgments
This work was funded in part by the American Vineyard Foundation and the California Competitive Grants Program for Research in Enology and Viticulture (D.A.M.). I.L. was supported by a scholarship from the Spanish Ministry of Education and Culture (grant AP99 16580683). E.O. was supported in part by a scholarship from the American Society for Enology and Viticulture.
D.A.M. thanks the Ribosome Database Project staff and Kevin Ashford at the Cardiff School of BioSciences for assistance with the bioinformatics analyses.
REFERENCES
- 1.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ampe, F., N. B. Omar, C. Moizan, C. Wacher, and J.-P. Guyot. 1999. Polyphasic study of the spatial distribution of microorganisms in Mexican pozol, a fermented maize dough, demonstrates the need for cultivation-independent methods to investigate traditional fermentations. Appl. Environ. Microbiol. 65:5464-5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbe, J. C., G. de Revel, A. Joyeux, A. Bertrand, and A. Lonvaud-Funel. 2001. Role of botrytized grape microorganisms in SO2 binding phenomena. J. Appl. Microbiol. 90:34-42. [DOI] [PubMed] [Google Scholar]
- 4.ben Omar, N., and F. Ampe. 2000. Microbial community dynamics during production of the Mexican fermented maize dough pozol. Appl. Environ. Microbiol. 66:3664-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisson, L. F. 1999. Stuck and sluggish fermentations. Am. J. Enol. Vitic. 50:107-119. [Google Scholar]
- 6.Bisson, L. F., and R. E. Kunkee. 1993. Microbial interactions during wine production, p. 37-68. In J. G. Zeikus and E. A. Johnson (ed.), Mixed cultures in biotechnology. McGraw-Hill, New York, N.Y.
- 7.Boulton, R. B., V. L. Singleton, L. F. Bisson, and R. E. Kunkee. 1996. Principles and practices of winemaking. Chapman & Hall, New York, N.Y.
- 8.Cocolin, L., L. F. Bisson, and D. A. Mills. 2000. Direct profiling of the yeast dynamics in wine fermentations. FEMS Microbiol. Lett. 189:81-87. [DOI] [PubMed] [Google Scholar]
- 9.Cocolin, L., A. Heisey, and D. A. Mills. 2001. Direct identification of the indigenous yeasts in commercial wine fermentations. Am. J. Enol. Vitic. 52:49-53. [Google Scholar]
- 10.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.el Fantroussi, S., L. Verschuere, W. Verstraete, and E. M. Top. 1999. Effect of phenylurea herbicides on soil microbial communities estimated by analysis of 16S rRNA gene fingerprints and community-level physiological profiles. Appl. Environ. Microbiol. 65:982-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferris, M. J., G. Muyzer, and D. M. Ward. 1996. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community. Appl. Environ. Microbiol. 62:340-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleet, G. H. 1997. The microbiology of alcoholic beverages, p. 217-262. In B. J. B. Wood (ed.), Microbiology of fermented foods, 2nd ed., vol. 1. Blackie Academic & Professional, London, United Kingdom.
- 14.Giraffa, G., and E. Neviani. 2001. DNA-based, culture-independent strategies for evaluating microbial communities in food-associated ecosystems. Int. J. Food Microbiol. 67:19-34. [DOI] [PubMed] [Google Scholar]
- 15.Henick-Kling, T. 1993. Malolactic fermentation, p. 289-326. In G. Fleet (ed.), Wine microbiology and bio/technology. Harwood Academic Publishers, Philadelphia, Pa.
- 16.Hugenholtz, P., B. M. Goebel, and N. R. Pace. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180:4765-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lonvaud-Funel, A. 1999. Lactic acid bacteria in the quality improvement and depreciation of wine. Antonie Leeuwenhoek 76:317-331. [PubMed] [Google Scholar]
- 18.Ludovico, P., M. J. Sousa, M. T. Silva, C. Leao, and M. Corte-Real. 2001. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology 147:2409-2415. [DOI] [PubMed] [Google Scholar]
- 19.Marchesi, J. R., T. Sato, A. J. Weightman, T. A. Martin, J. C. Fry, S. J. Hiom, D. Dymock, and W. G. Wade. 1998. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 64:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millet, V., and A. Lonvaud-Funel. 2000. The viable but non-culturable state of wine microorganisms during storage. Lett. Appl. Microbiol. 30:136-141. [DOI] [PubMed] [Google Scholar]
- 21.Mills, D. A., E. A. Johannsen, and L. Cocolin. 2002. Yeast diversity and persistence in botrytis-affected wine fermentations. Appl. Environ. Microbiol. 68:4884-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muyzer, G., and K. Smalla. 1998. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Leeuwenhoek 73:127-141. [DOI] [PubMed] [Google Scholar]
- 23.Muyzer, G., A. Teske, C. O. Wirsen, and H. W. Jannasch. 1995. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch. Microbiol. 164:165-172. [DOI] [PubMed] [Google Scholar]
- 24.Ribéreau-Gayon, P. 2000. Handbook of enology. Wiley, Chichester, United Kingdom.
- 25.Tannock, G. W., A. Tilsala-Timisjarvi, S. Rodtong, J. Ng, K. Munro, and T. Alatossava. 1999. Identification of Lactobacillus isolates from the gastrointestinal tract, silage, and yogurt by 16S-23S rRNA gene intergenic spacer region sequence comparisons. Appl. Environ. Microbiol. 65:4264-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van de Peer, Y., P. De Rijk, J. Wuyts, T. Winkelmans, and R. De Wachter. 2000. The European small subunit ribosomal RNA database. Nucleic Acids Res. 28:175-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter, J., C. Hertel, G. W. Tannock, C. M. Lis, K. Munro, and W. P. Hammes. 2000. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces group-specific PCR primers and by using denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67:2578-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zoetendal, E. G., A. D. L. Akkermans, and W. M. De Vos. 1998. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 64:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]