Abstract
Bovine β-lactoglobulin (Blg) is one of the major cow's milk allergens. Peptide 41-60 of Blg (Blg41-60) was described as a murine T-cell determinant and a murine, rat, and human immunoglobulin E (IgE) epitope. The aim of this study was the expression of Blg41-60 as a fusion protein in the food-grade bacterium Lactococcus lactis and the characterization of its immunogenicity in mice. We constructed a recombinant strain of L. lactis capable of inducible production and secretion of Blg41-60::Nuc, a fusion protein between Blg41-60 and the mature part of the staphylococcal nuclease (Nuc). The highest production yield of Blg41-60::Nuc (32.5 mg/liter) was reached 4 h after induction. At this time, up to 75% of Blg41-60::Nuc was secreted. When monoclonal antibodies specific for Blg41-60 were used, purified Blg41-60::Nuc and synthetic Blg41-60 exhibited very similar immunoreactivities. Subcutaneous coadministration of purified Blg41-60::Nuc and killed nonrecombinant L. lactis resulted in the induction of specific anti-Blg41-60 IgG2a and IgG1. The IgG1/IgG2a ratio and the lack of specific IgE suggest a Th1-type immune response, i.e., a nonallergic response. Similar administrations of the killed Blg41-60::Nuc-producing L. lactis strain did not elicit a specific immune response, whereas a transitory mucosal IgA-specific immune response was induced in mice after oral administration of the live Blg41-60::Nuc-producing L. lactis strain.
Food allergy generally corresponds to an immediate immunoglobulin E (IgE)-mediated immune response (13, 17). This humoral response is considered to result from the activation of the Th2 type of T-helper lymphocytes. In mice, the Th2 response results in IgE and IgG1 production, while the Th1 response leads to IgG2a production (16, 31). Treatment of allergic disease can be achieved by immunotherapy with T-cell epitope peptides, which will avoid the potential risk of anaphylaxis incurred with use of the entire native allergen (21, 38). Intranasal administration of a single peptide containing the immunodominant T-cell epitope of Der p 1, one of the major house dust mite allergens, to H-2b mice was previously shown to inhibit T-cell responses to the whole protein (22). Feeding large amounts of recombinant fusion protein containing this epitope to mice can likewise eliminate T-cell responses to the whole protein (20).
Cow's milk allergy is an important problem in infants, affecting 1.9 to 2.8% of infants in the first 2 years of life in various countries of northern Europe (18, 19). β-Lactoglobulin (Blg; 18 kDa) is the most abundant protein of the soluble fraction of cow's milk and is regarded as a dominant allergen. Major human IgE epitopes were shown to be fragments 41 to 60, 102 to 124, and 149 to 162 (41). Peptide 41-60 (Blg41-60) has also been described as a mouse and rat IgE epitope (2, 30) and as a mouse T-cell determinant (47, 48). Moreover, it can be detected easily by Western blot experiments and by a competitive enzyme immunoassay (EIA) developed in our laboratory that uses two monoclonal antibodies (MAbs), Blg-21R and Blg-31R (32), specific for Blg41-60 (8).
Lactococcus lactis, the model gram-positive lactic acid bacterium, is nonpathogenic and noninvasive and possesses both GRAS (generally regarded as safe) and food-grade status. L. lactis has been extensively engineered for the production of heterologous therapeutic proteins (4, 7, 11, 14, 24, 35, 44). L. lactis has already been used as an antigen delivery vehicle for vaccination against tetanus (52) and, more recently, for treatment of murine colitis (43).
L. lactis was already used to produce entire Blg protein, and recombinant strains were shown to be immunogenic after intranasal and oral administration in mice (7). More recently, Bernasconi et al. (5) showed that the Lactobacillus bulgaricus exported protease PrtB could enhance the export of both entire Blg and a poorly antigenic Blg peptide in L. lactis. Here, we constructed an L. lactis strain producing Blg41-60::Nuc, a recombinant fusion protein between Blg41-60 and the mature part of the staphylococcal nuclease (Nuc) (26), by use of the nisin-inducible expression system (9). Binding of anti-Blg41-60 MAbs to purified Blg41-60::Nuc or synthetic Blg41-60 was very similar. Four hours after induction, up to 75% of Blg41-60::Nuc is secreted and the amount of Blg41-60::Nuc produced reaches its maximum (32.5 mg/liter). In vivo studies showed that subcutaneous administration of the killed recombinant strain did not elicit anti-Blg41-60 antibodies, but a Blg41-60-specific response can be obtained after administration of purified Blg41-60::Nuc emulsified in complete Freund's adjuvant (CFA) or after coadministration with nonrecombinant killed L. lactis, mimicking the live recombinant secreting strain. The effect of oral administration of the live L. lactis strain secreting Blg41-60::Nuc was then tested, and the induction of a transitory mucosal IgA-specific immune response was observed.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
L. lactis NZ9000 (23) and derivative strains were grown at 30°C in M17 medium containing 0.5% glucose (GM17) (45). Escherichia coli TG1 (15) was grown in Luria-Bertani (LB) medium at 37°C under vigorous shaking conditions. When required, antibiotics were added at the following concentrations: ampicillin, 50 μg/ml; chloramphenicol, 25 μg/ml for E. coli and 10 μg/ml for L. lactis; and erythromycin, 5 μg/ml for L. lactis. Expression of the Blg41-60::nuc gene placed under the control of the promoter PnisA was induced as follows. An overnight culture of L. lactis NZ9000 was used to inoculate fresh medium at a dilution of 1:250. At an optical density at 600 nm (OD600) of about 0.5, 1 ng of nisin (Sigma) per ml was added to the culture, which was further incubated for various times.
General DNA techniques, PCR, and transformations.
Plasmid DNA was isolated essentially as previously described (6); for L. lactis, cells were incubated in TES buffer (50 mM Tris-HCl, 1 mM EDTA, 25% sucrose, pH 8) containing 10 mg of lysozyme per ml at 37°C for 10 min before alkaline lysis. Restriction enzymes and T4 DNA ligase were obtained from Gibco BRL or New England Biolabs and used according to the instructions of the suppliers. PCR was performed with a Perkin-Elmer (Norwalk, Conn.) Cetus thermocycler. Electroporation of L. lactis was performed as described previously (25), and transformants were plated on GM17 agar plates containing the required antibiotic.
Construction of expression plasmids carrying the Blg41-60::nuc gene.
All plasmid constructions were performed in E. coli TG1 and then transferred into L. lactis NZ9000. To obtain the DNA fragment encoding the Blg41-60 epitope, two 79-base oligonucleotides (5′-ATGCATCAGTTTATGTTGAAGAACTTAAACCAACTCCTGAAGGAGATCTTGAAATTCTTCTTCAAAAAAATGCATGG and its complement) were annealed, blunted, inserted into SmaI-cut pBlueScript (pBS) II SK(+) vector, and cloned in E. coli TG1, resulting in pBS:Blg41-60. At both ends, an NsiI restriction site (underlined) was added. The BglII restriction site (italics), absent from pBS and from our expression vectors, allowed for screening and control of the insertion of the epitope among the candidates first selected by blue-white screening based on β-galactosidase activity (39).
The Blg41-60 fragment was then produced by NsiI digestion to be inserted in an expression cassette composed of the nuc gene encoding the staphylococcal nuclease (Nuc) with its own signal peptide (SP) under the control of the nisin-inducible promoter carried by pSEC:Nuc1 plasmid (27). The presence of a unique NsiI restriction site in this expression cassette between SPNuc and nuc allows for the insertion of DNA encoding the epitope Blg41-60. The Blg41-60 fragment could be inserted in two possible orientations: the correct orientation results in translational fusion between Blg41-60 and nuc, whereas the opposite orientation results in a premature stop codon. By a Nuc activity plate test, clones expressing the hybrid active protein Blg41-60::Nuc were Nuc+, whereas clones with the opposite orientation were Nuc−. Among Nuc+ clones, the insertion of one (or more) Blg41-60 fragment between SPNuc and Nuc was confirmed by digestion by BglII. Selected constructs were then sequenced to determine the number of inserted Blg41-60 oligopeptides. The resulting plasmid (pSEC:Blg41-60::Nuc) carrying the Blg41-60::nuc gene was then introduced into L. lactis NZ9000, which contains the PnisA regulatory genes nisRK.
Preparation of cellular and supernatant protein fractions of L. lactis for Western blot analysis.
An overnight culture of each L. lactis strain was used to inoculate fresh medium at a dilution of 1:250. For induction of the promoter PnisA, strains were grown until an OD600 of 0.5 was reached, and then nisin was added at a final concentration of 1 ng/ml. For fractionation between cell (C) and supernatant (S) fractions, 2 ml of L. lactis culture was centrifuged for 5 min at 6,000 × g at 4°C. Protein extracts were then prepared as previously described (27).
Preparation of cytoplasmic and secreted protein extracts for immunoassays.
The induced cells were pelleted by centrifugation at 5,000 × g for 15 min at 4°C, and the culture supernatant (S) was stored at −20°C until assay. The soluble cytoplasmic (Cs) proteins were extracted by disruption of the bacteria with glass beads and resuspension in 1/10 the initial volume in 50 mM Tris-HCl, pH 7.4. These extracts were then centrifuged at 10,000 × g for 15 min at 4°C. The supernatant containing Cs proteins was stored at −20°C until assay. The pellet was resuspended in 1/10 the initial volume in 50 mM Tris-HCl (pH 7.4)-8 M urea-100 mM dithiothreitol for 1 h at room temperature (RT). After centrifugation at 10,000 × g for 15 min at RT, the supernatant containing resolubilized insoluble cytoplasmic (Ci) proteins was stored at −20°C until assay. Blg41-60::Nuc was assayed in the S, Cs, and Ci fractions by the competitive EIA described below. Before assays, the Ci fraction was dialyzed against 50 mM Tris-HCl, pH 7.4.
Immunodetection of purified Blg41-60::Nuc.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis was performed with a Tricine buffer as previously described (40). Proteins were stained with Gelcode Blue stain reagent (Pierce, Rockford, Ill.). For immunoblot analysis, proteins were separated by SDS-PAGE (12% gel) and electroblotted (46) onto polyvinylidene difluoride membranes (Millipore, Bedford, Mass.). After blotting, treatment of the membranes depended on the antibodies used as follows.
(i) Specific anti-Blg41-60 MAbs.
Nonspecific protein binding sites were blocked with 1% bovine serum albumin in 50 mM Tris-HCl (pH 8)-150 mM NaCl-0.5% Tween 20. Membranes were then incubated overnight with a 1/1,000 dilution of Blg-21R MAb specific for Blg41-60 (8). After washing in 50 mM Tris-HCl (pH 8)-150 mM NaCl-0.5% Tween 20, the membranes were incubated for 1 h with alkaline phosphatase-conjugated anti-mouse antibody (1/5,000) (Jackson ImmunoResearch Laboratories, West Grove, Pa.). Color development was obtained according to the supplier's instructions.
(ii) Polyclonal anti-Nuc antibodies.
After blotting, nonspecific protein binding sites were blocked with 1% bovine serum albumin in 50 mM Tris-HCl (pH 8.0)-150 mM NaCl-0.5% Tween 20. Membranes were incubated for 2 h with a 1/2,000 dilution of anti-Nuc antibodies (Eurogentec). After washing, membranes were incubated for 45 min with protein G-horseradish peroxidase conjugate (Bio-Rad) and signals were detected by use of an enhanced chemiluminescence kit (ECL; Dupont-NEN). After ECL detection, different nonsaturated film exposures were scanned by a Scanjet II (Hewlett Packard). For quantification, signals were compared to those of known amounts of purified Nuc.
Purification of Blg41-60::Nuc.
Blg41-60::Nuc was purified from the supernatant culture on an immunoaffinity column. The column was prepared by immobilizing the Blg-31R MAb (1 mg/ml of gel) on CNBr-activated Sepharose 4B, as described by the supplier (Pharmacia, Uppsala, Sweden). Expression of recombinant peptide was induced for 4 h as described above. Cells were pelleted by centrifugation. The culture supernatant (250 ml) was then added on 5 ml of gel and flowed through the column by gravity. The column was then washed with 50 ml of 50 mM Tris-HCl (pH 8)-150 mM NaCl-0.05% Tween 20. Blg41-60::Nuc was eluted in 5× column volume (elution buffer, 100 mM glycine-HCl, pH 2.5). Blg41-60::Nuc was usually eluted in the first two volumes. The elution was immediately neutralized by 1 M phosphate buffer, pH 7.4 (1/10 the elution volume). The column was regenerated by extensive washing with 50 mM Tris-HCl, pH 8. The solution containing Blg41-60::Nuc was dialyzed against 50 mM Tris-HCl, pH 8. The Blg41-60::Nuc concentration was measured by the BCA protein assay (Pierce) or the competitive EIA described below.
Preparation and purification of a synthetic peptide Blg41-60 and tracer Blg41-60 coupled to AChE.
Synthetic peptide Blg41-60 was synthesized by use of a Milligen 9050 apparatus (Millipore) and standard solid-phase synthesis by the 9-fluorenylmethoxycarbonyl continuous flow method. Synthetic peptide was purified by reversed-phase high-performance liquid chromatography with a Waters system (Milford) and a C18 Vydac column (250 by 10 mm; SFCC, Eragny, France). Tracer Blg41-60 coupled to acetylcholinesterase (Blg41-60-AChE) was obtained by reaction of thiol groups previously introduced in the synthetic peptide with maleimido groups incorporated into AChE as previously described (28).
Competitive EIA for Blg41-60.
Competitive EIAs were performed in 96-well microtiter plates coated with AffiniPure goat anti-mouse IgG plus IgM (heavy plus light chains) (Jackson ImmunoResearch). Blg-21R MAb (capture antibody) (50 μl), 50 μl of a dilution of standard (synthetic Blg41-60) or of samples (containing Blg41-60::Nuc), and 50 μl of tracer Blg41-60-AChE were added to wells. After an 18-h reaction at 4°C, the plates were washed and solid-phase-bound AChE was measured by Ellman et al.'s method (10).
Competitive ELISA.
Competitive enzyme-linked immunosorbent assays (ELISA) were performed in 96-well microtiter plates coated with purified Blg41-60::Nuc (5 μg/ml). Blg-21R MAb (50 μl) and 50 μl of a dilution of Blg41-60 or of purified Blg41-60::Nuc were added. After an 18-h reaction at 4°C, the plates were washed, and 100 μl of goat anti-Ig mouse antibody labeled with AChE was added for 4 h at RT. Plates were then washed, and solid-phase-bound AChE was measured by Ellman et al.'s method.
Immunizations. (i) Subcutaneous administrations.
Recombinant strain NZ9000(pSEC:Blg41-60::Nuc) and control strain NZ9000(pVE3655) (27) were grown as described above and induced for 4 h with nisin. Cells were washed and resuspended in sterile phosphate-buffered saline, divided into aliquots, and frozen at −80°C until subcutaneous administration. Yields of cellular Blg41-60::Nuc were determined by competitive EIA of 1 aliquot as described above. We considered only cellular Blg41-60::Nuc because administered bacteria were killed by freezing. Doses of 20 μg of purified Blg41-60::Nuc emulsified in CFA, the quantity of cells of NZ9000(pSEC:Blg41-60::Nuc) corresponding to 20 μg of Blg41-60, and the same quantity of cells of NZ9000(pVE3655) with or without 20 μg of purified Blg41-60::Nuc were administered subcutaneously in 200 μl of suspension to groups of five BALB/c female mice on days 1 and 21. Serum samples were taken on days 0, 18, and 32.
(ii) Oral administrations.
Control strain NZ9000(pVE3655) and two recombinant strains, NZ9000(pSEC:Blg) (7) and NZ9000(pSEC:Blg41-60::Nuc), were grown as described above and induced for 4 h with nisin to ∼109 bacteria/ml. Ten milliliters of each induced culture was pelleted, concentrated 30-fold in sterile phosphate-buffered saline, and immediately administered to mice. Groups of seven mice were intragastrically immunized for five consecutive days (day 1 to day 5) with induced recombinant and control strains. Serum samples and fecal pellets were collected on days 0, 21, 28, and 35 as previously described (7).
ELISA for detection of Blg41-60-specific antibody.
Ninety-six-well microtiter plates (Maxisorp; Nunc, Roskilde, Denmark) coated with 5 μg of synthetic Blg41-60 per ml were incubated overnight with serial dilutions of sera from immunized mice or with standards when available. Purified and quantified MAbs specific for Blg41-60 were used as standards as follows: a mixture of two IgG1 MAbs (Blg-8R and Blg-83R) or one IgG2a MAb (Blg-92R) was diluted from 2 to 0.001 μg/ml. After washing, IgE, IgG1, or IgG2a binding to Blg41-60 was revealed by incubation with an anti-IgE (Serotec, Oxford, England), anti-mouse IgG1, or anti-mouse IgG2a (Southern Biotechnology Associated, Birmingham, Ala.) antibody labeled with AchE (1). AChE activity was detected by Ellman et al.'s method. Specific IgA activity was assayed as previously described on Blg-coated plates (7) and on Blg41-60-coated plates.
RESULTS
Blg41-60::Nuc is efficiently secreted from L. lactis.
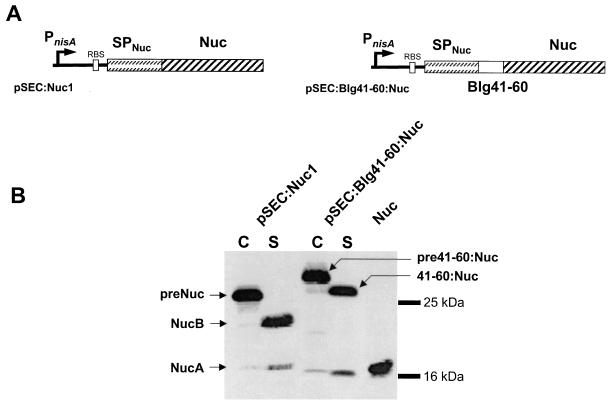
The Blg41-60 epitope was fused at the N-terminal end of the staphylococcal nuclease (Nuc), resulting in the protein fusion Blg41-60::Nuc produced in L. lactis (Fig. 1A). Western blot analysis of cell (C) and supernatant (S) protein extracts using polyclonal anti-Nuc antibodies was performed on nisin-induced and noninduced cultures of three L. lactis strains: the negative control, NZ9000(pVE3655), and the protein-producing strains NZ9000(pSEC:Nuc1) and NZ9000(pSEC:Blg41-60::Nuc). No protein was detected in protein extracts of noninduced cultures of producing strains and of NZ9000(pVE3655) induced culture (data not shown). The band corresponding to the precursor pre-Blg41-60::Nuc in the C fraction of NZ9000(pSEC:Blg41-60::Nuc) has a higher molecular weight (MW) than pre-Nuc in the C fraction of NZ9000(pSEC:Nuc1) due to the presence of the additional 23 amino acids of Blg41-60 fused at the N terminus of NucB (Fig. 1B).
FIG. 1.
Secretion of Blg41-60::Nuc fusion protein in L. lactis. (A) Expression cassettes for production and export of Nuc and fusion protein Blg41-60::Nuc. Schematic structures of Nuc and the fusion protein carried by the indicated plasmids are shown. For details of plasmid construction, see Materials and Methods. PnisA, nisin-inducible promoter; RBS, ribosomal binding site of usp45 gene; SPNuc, SP of staphylococcal Nuc; Nuc, DNA fragment encoding the mature protein. (B) Western blot analysis of L. lactis strains NZ9000(pSEC:Nuc1) and NZ9000(pSEC:Blg41-60:Nuc) using anti-Nuc antibodies. Blg41-60::Nuc production was estimated by Western blot analysis on exponential-phase-induced cultures of lactococcal strains containing pSEC:Nuc1 or pSEC:Blg41-60:Nuc. Protein extracts were prepared 1 h after nisin induction and separated into cellular (C) and supernatant (S) fractions. At an OD600 of 0.5, cultures were induced with 1 ng of nisin per ml of culture. C and S extracts of the induced cultures of NZ9000(pSEC:Nuc1) and NZ9000(pSEC:Blg41-60:Nuc) were hybridized with anti-Nuc antibodies. preNuc, precursor of Nuc; NucB and NucA, mature forms of Nuc; pre41-60:Nuc, precursor of the fusion protein Blg41-60::Nuc; 41-60:Nuc, mature form of Blg41-60::Nuc.
For S fractions, the profile obtained for the NZ9000(pSEC:Nuc1) strain showed one major band and one minor band, corresponding to the MW of mature NucB and NucA, respectively (27) (Fig. 1B). For the Blg41-60::Nuc-producing strain, one major band was detected in the supernatant showing a higher MW than NucB, indicating the presence of one Blg41-60 epitope. Using a Blg41-60-specific MAb, we obtained the following results: (i) a band with the same MW as the band detected with anti-Nuc polyclonal antibodies was detected in the supernatant of the NZ9000(pSEC:Blg41-60::Nuc) strain, confirming the production of Blg41-60::Nuc (data not shown); and (ii) no protein was detected in the Nuc-producing strain (data not shown). The secretion efficiencies (SE; i.e., the proportion of the mature form secreted in the supernatant) of both Nuc and Blg41-60::Nuc were estimated by densitometry to be about 50%. No degradation band was observed.
Purification and antigenic characterization of Blg41-60::Nuc.
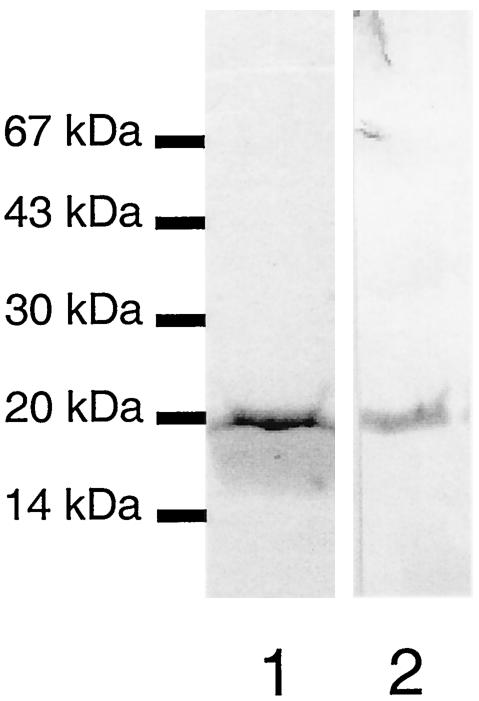
To compare the antibody reactivity of Blg41-60::Nuc with that of synthetic Blg41-60, Blg41-60::Nuc was purified from the culture supernatant of the induced NZ9000(pSEC:Blg41-60::Nuc) recombinant strain by use of an immunoaffinity column, with Blg-31R MAb coupled to Sepharose gel. After Coomassie blue staining of the SDS-PAGE gel, the elution appeared to contain a single band, with a MW corresponding to that of Blg41-60::Nuc fusion protein (Fig. 2, lane 1). In a Western blot using Blg-31R MAb, elution also appeared as a unique band with the same MW after Coomassie blue staining (Fig. 2, lane 2). Under nonreducing conditions, the elution also showed a unique band, indicating that Blg41-60::Nuc is purified as a monomer, and no degradation bands were observed (data not shown).
FIG. 2.
Purification of Blg41-60::Nuc fusion protein. Blg41-60::Nuc was purified from the supernatant of an induced culture of NZ9000(pSEC:Blg41-60:Nuc) by use of an immunoaffinity column. Lane 1, purified Blg41-60::Nuc stained by Coomassie blue; lane 2, purified Blg41-60:Nuc detected by immunoblotting with anti-41-60 Blg-21R MAb.
Recognition of Blg41-60::Nuc by Blg41-60-specific MAbs. (i) Competitive EIA.
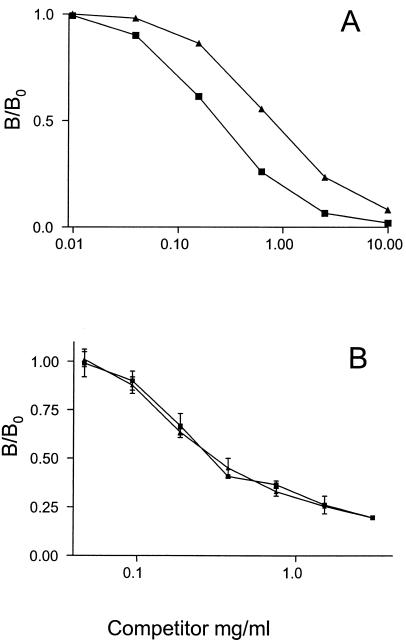
The reactivity of purified Blg41-60::Nuc to Blg-21R and Blg-31R MAbs was tested by competitive EIA. For these experiments, the concentration of purified Blg41-60::Nuc used was measured by BCA colorimetric assay. As seen in Fig. 3A, competition curves obtained by competitive EIA with Blg-21R MAb are strictly parallel in the linear portion. A B/B0 ratio (i.e., the ratio of the absorbance measured in the presence of the competitor to the absorbance measured in the absence of the competitor) of 0.5 is reached for Blg41-60 at 250 pmol/ml and for Blg41-60::Nuc at 750 pmol/ml. The same results were obtained with Blg-31R MAb. These results indicate that Blg41-60::Nuc in solution is well recognized by specific Blg41-60 MAbs. This observation shows that competitive EIA developed for Blg41-60 can be used further to assay Blg41-60::Nuc.
FIG. 3.
Determination of Blg41-60::Nuc antibody reactivity. (A) Competitive EIA. Anti-41-60 Blg-21R MAb, synthetic Blg41-60 labeled with AChE, and increasing concentrations of synthetic Blg41-60 (▪) or recombinant Blg41-60::Nuc (▴) were added to a microtiter plate that was previously coated with anti-mouse antibody. (B) Competitive ELISA. Blg-21R MAb labeled with AChE and increasing concentrations of Blg41-60 or Blg41-60::Nuc were added to microtiter plates that were previously coated with Blg41-60::Nuc. For both experiments, concentrations of both competitors are expressed as picomoles of Blg41-60 per milliliter. Data are means of duplicate determinations. Absorbance was measured at 414 nm.
(ii) Competitive ELISA.
The reactivity of Blg41-60::Nuc was then tested by competitive ELISA. For these experiments, the concentration of purified Blg41-60::Nuc was measured by BCA colorimetric assay. Blg41-60::Nuc was used to coat plates, and the binding rate of Blg-21R was measured after the addition of several dilutions of synthetic Blg41-60 or Blg41-60::Nuc. As seen in Fig. 3B, competition curves are strictly superimposable. The same results were found with Blg-31R MAb. These results indicate that Blg41-60::Nuc-coated plates are specifically recognized by MAbs specific to Blg41-60 (Blg-21R and Blg-31R).
Expression kinetics of Blg41-60::Nuc.
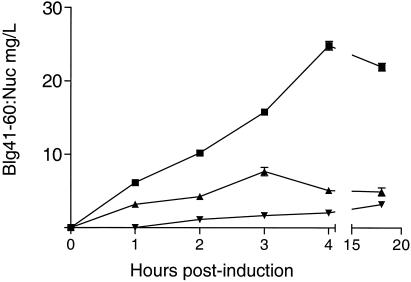
Production of Blg41-60::Nuc was assayed at different times after induction by competitive EIA specific for Blg41-60, as allowed by the previous results. Blg41-60::Nuc was assayed in supernatant (S), soluble cytoplasmic protein (Cs), and insoluble cytoplasmic protein (Ci) fractions. Four hours after induction, the total amount of Blg41-60::Nuc produced reached its maximum, 32.5 ± 0.12 mg/liter, and remained stable until 18 h after induction (Fig. 4). Similar results were obtained by densitometry analysis of Western blot experiments (data not shown). Four hours after induction, 70 to 75% of the total production of Blg41-60::Nuc was secreted, corresponding to 25 mg/liter. The remainder was found preferentially in the Cs fraction.
FIG. 4.
Kinetics of Blg41-60::Nuc expression. L. lactis strain NZ9000(pSEC:Blg41-60:Nuc) was grown to an OD600 of 0.5, and then Blg41-60::Nuc expression was induced with nisin. Blg41-60::Nuc was assayed by competitive EIA at different times postinduction (1, 2, 3, 4, and 18 h) in supernatant (▪), cytoplasmic soluble proteins (▴), and cytoplasmic insoluble proteins (▾). Results are expressed as concentrations of Blg41-60::Nuc. Values given represent the means ± standard deviations for three independent experiments.
Immune response elicited by subcutaneous administration of killed recombinant L. lactis strain in mice.
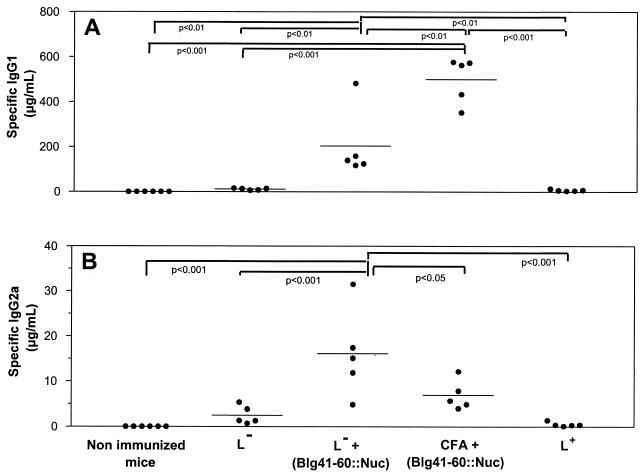
Killed recombinant L. lactis strain NZ9000(pSEC:Blg41-60::Nuc), containing 20 μg of Blg41-60::Nuc, killed control strain NZ9000(pVE3655), killed control strain plus 20 μg of pure Blg41-60::Nuc, or 20 μg of pure Blg41-60::Nuc emulsified in CFA was administered subcutaneously to mice. Injections were performed on days 1 and 21, and serum samples were taken at days 0 (S0), 18 (S1), and 32 (S2). No significant antibody response was detected in S1 samples. After the boost injection, anti-Blg41-60 IgG1 and IgG2a were detected in mice immunized with Blg41-60::Nuc coadministered with the control strain or with CFA (Fig. 5). No specific IgE response was detected. The specific IgG1/IgG2a ratio was higher for the CFA group than for the lactococci group. No specific immune response was induced when synthetic Blg41-60 peptide was used for immunization instead of purified Blg41-60::Nuc (data not shown).
FIG. 5.
Immunogenicity of Blg41-60::Nuc fusion protein. Control strain NZ9000(pVE3655) (L−), control strain plus 20 μg of purified Blg41-60::Nuc [L− + (Blg41-60::Nuc)], 20 μg of purified Blg41-60::Nuc emulsified in CFA [CFA + (Blg41-60::Nuc)], or recombinant strain NZ9000(pSEC:Blg41-60::Nuc) (L+) was administrated subcutaneously to mice on days 1 and 21. Serum samples were taken on day 32. Specific IgG1 (A) and IgG2a (B) levels were quantified as described in Materials and Methods. Values for each mouse were determined in duplicate (•; five mice per group), and group means are shown. Statistical analyses were performed by one-way analysis of variance and Tukey's multiple comparison test. P values on the graph are precise.
Immune response elicited by oral administration of live recombinant L. lactis strain in mice.
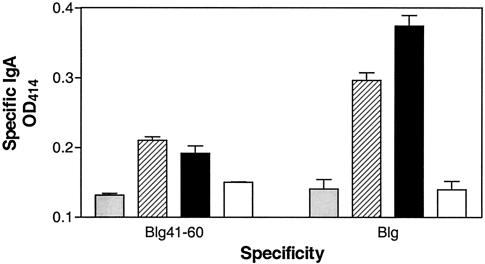
Groups of seven mice were inoculated intragastrically with live recombinant strains NZ9000(pSEC:Blg) or NZ9000(pSEC:Blg41-60::Nuc) or nonrecombinant control strain NZ9000(pVE3655). Three weeks after J1 (day 22), anti-Blg41-60 and anti-Blg IgAs were detected in fecal pellets of mice that had received NZ9000(pSEC:Blg41-60::Nuc) (Fig. 6), demonstrating the induction of a specific mucosal immune response. This immune response was not detected further in fecal pellets collected on days 28 and 35 (data not shown). No anti-Blg41-60 or anti-Blg IgG was detected in fecal pellets (data not shown). Anti-Blg41-60 and anti-Blg IgAs were also detected in fecal pellets of mice that had received NZ9000(pSEC:Blg) (Fig. 6), confirming previous results (7). This immune response was still detected in fecal pellets collected on days 28 and 35 (data not shown). No specific antibodies (IgG1, IgG2a, or IgG) were detected in sera from immunized mice, suggesting that the immune response is restricted to the mucosal level.
FIG. 6.
Specific IgA response elicited after oral administration of live recombinant L. lactis strain to mice. Groups of seven mice were intragastrically administered live recombinant strain NZ9000(pSEC:Blg41-60:Nuc) (hatched bars) or NZ9000(pSEC:Blg) (black bars) (7) or nonrecombinant control strain NZ9000(pVE3655) (gray bars) for five consecutive days. Results for naïve mice are also shown (white bars). Three weeks after J1, anti-Blg41-60 and anti-Blg IgA levels were assayed in fecal pellets as described in Materials and Methods.
DISCUSSION
This study describes the production in L. lactis of a fusion protein between a major epitope of Blg and the mature part of staphylococcal Nuc, called Blg41-60::Nuc, and the characterization of its immunogenicity in mice. Several prokaryotic proteins have already been produced in L. lactis and are generally active. In contrast, results with eukaryotic proteins are more variable: hen egg white lysozyme can be produced but is inactive (49), whereas biologically active bovine plasmin (3) and murine interleukins (44) have been produced. A bovine coronavirus epitope was also expressed as a fusion protein with Nuc in L. lactis (24) and was efficiently secreted and detected by specific Nuc and bovine coronavirus antibodies. Blg has also been produced in L. lactis, and most of Blg was found to be in a denatured form (7). The addition of a lactococcal signal peptide drastically enhanced the production of Blg. Nevertheless, only 3% of the Blg produced was secreted (7). Recently, Bernasconi et al. showed that the Lactobacillus bulgaricus proteinase PrtB is a potential efficient carrier to export entire Blg protein or a fragment of Blg (5).
SE (up to 75%) and quantities (∼30 mg/liter) of Blg41-60::Nuc are higher than those previously observed for Nuc (∼60% and ∼15 mg/ml) (24, 27). These differences could be due to the total net charge of −1 in the first 10 amino acids (VYVEELKPTP) of the 41-60 sequence, with two negative charges at positions 4 and 5 (underlined; positive charge is in italics). The presence of negative charges just after the cleavage site of the SP is known to be favorable for efficient protein secretion (50). The same results were previously demonstrated by using LEISSTCDA synthetic propeptide, which enhances both SE and the quantity of Nuc (24, 35).
Reactivities with anti-Blg41-60 MAbs of synthetic Blg41-60 peptide and recombinant fusion protein Blg41-60::Nuc are similar in competitive EIA and identical in competitive ELISA. In EIA, the signal followed is the binding of the tracer Blg41-60-AChE. In solution, synthetic or recombinant peptide does not compete with tracer in the same way. Blg41-60 binds with more affinity to MAbs than Blg41-60::Nuc, in which Blg41-60 could be less accessible for MAbs. This difference in reactivity between the two peptides in EIA could result in an underestimation of Blg41-60::Nuc. These problems disappear when the plate is directly coated with the recombinant peptide. It is known that direct coating of wells elicits a total or partial denaturation of the protein (1, 42, 51). Therefore, denaturation of Blg41-60::Nuc can enhance the accessibility to the Blg41-60 epitope.
L. lactis has been previously described as an antigen delivery system (52; see reference 29 for a review). Preliminary experiments on mice of subcutaneous administration of the killed Blg41-60::Nuc-producing L. lactis strain were performed, and anti-Blg41-60 IgG1, IgG2a, and IgE levels were monitored. After administration of this L. lactis strain, we could not detect a significant specific immune response compared to control mice. In contrast, a Th1-type specific response was induced after two injections of the purified Blg41-60::Nuc protein coadministered with killed control strain or with CFA. The IgG1/IgG2a ratio, weaker with lactococci than with CFA, suggests a Th1-type response (12), confirmed by the lack of IgE. This result shows that (i) subcutaneously administered lactococci are a potent adjuvant, preferentially inducing a nonallergic response, and (ii) Nuc is a good carrier for the Blg41-60 peptide, inducing a specific immune response against this major IgE epitope. In our experiments, lysis of subcutaneously administered killed lactococci is probably insufficient to allow induction of a specific immune response, while subcutaneous administration of a recombinant strain expressing tetanus toxin fragment C (TTFC) has been shown to elicit a specific IgG response (33). This could be due to a stronger immunogenicity of TTFC. Our hypothesis could explain the discrepancy observed between the present results and our previous experiments in which oral administration of the recombinant strain producing whole Blg led to the lysis of lactococci in the gastrointestinal tract and consequently to the release of Blg, allowing the induction of a specific mucosal IgA response (7). Indeed, for the present study, mice intragastrically administered the live recombinant strain developed a specific IgA response detected in fecal pellets, demonstrating a mucosal immune response. Nasal and oral immunization with a strain expressing TTFC elicits specific IgA in mucosa and specific IgG1 and IgG2a in serum (34, 36). This difference also is probably due to a higher immunogenicity of TTFC.
Peptides of Der p 1 (a house dust mite allergen) were produced as fusion proteins in E. coli, and they induced oral tolerance once administered to mice by feeding (20). A recombinant fusion protein containing multiple linked T-cell epitopes from chain 1 of Fel d 1 (a major cat allergen) can also induce peripheral T-cell tolerance in mice when delivered subcutaneously (37). Our food-grade recombinant strain could also be tested for immunotherapy by using the high IgE-responder mouse model of allergy to bovine Blg that has already been developed (1).
Acknowledgments
J.-M.C. and S.N. contributed equally to this work.
We are grateful to the members of URLGA and UIAA groups for helpful discussions during the course of this work.
Sébastien Nouaille was the recipient of a MENRT grant from the French government.
REFERENCES
- 1.Adel-Patient, K., C. Creminon, H. Bernard, G. Clement, L. Negroni, Y. Frobert, J. Grassi, J. M. Wal, and J. M. Chatel. 2000. Evaluation of a high IgE-responder mouse model of allergy to bovine beta-lactoglobulin (BLG): development of sandwich immunoassays for total and allergen-specific IgE, IgG1 and IgG2a in BLG-sensitized mice. J. Immunol. Methods 235:21-32. [DOI] [PubMed] [Google Scholar]
- 2.Adel-Patient, K., M. A. Nahori, B. Proust, E. S. J. R. Lapa, C. Creminon, J. M. Wal, and B. B. Vargaftig. 2003. Elicitation of the allergic reaction in beta-lactoglobulin-sensitized Balb/c mice: biochemical and clinical manifestations differ according to the structure of the allergen used for challenge. Clin. Exp. Allergy 33:376-385. [DOI] [PubMed] [Google Scholar]
- 3.Arnau, J., E. Hjerl-Hansen, and H. Israelsen. 1997. Heterologous gene expression of bovine plasmin in Lactococcus lactis. Appl. Microbiol. Biotechnol. 48:331-338. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez-Humaran, L. G., P. Langella, A. Miyoshi, A. Gruss, R. T. Guerra, R. Montes de Oca-Luna, and Y. Le Loir. 2002. Production of human papillomavirus type 16 E7 protein in Lactococcus lactis. Appl. Environ. Microbiol. 68:917-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernasconi, E., J. E. Germond, M. Delley, R. Fritsche, and B. Corthesy. 2002. Lactobacillus bulgaricus proteinase expressed in Lactococcus lactis is a powerful carrier for cell wall-associated and secreted bovine beta-lactoglobulin fusion proteins. Appl. Environ. Microbiol. 68:2917-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatel, J. M., P. Langella, K. Adel-Patient, J. Commissaire, J. M. Wal, and G. Corthier. 2001. Induction of mucosal immune response after intranasal or oral inoculation of mice with Lactococcus lactis producing bovine beta-lactoglobulin. Clin. Diagn. Lab. Immunol. 8:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clement, G., D. Boquet, Y. Frobert, H. Bernard, L. Negroni, J. M. Chatel, K. Adel-Patient, C. Creminon, J. M. Wal, and J. Grassi. 2002. Epitopic characterization of native bovine beta-lactoglobulin. J. Immunol. Methods 266:67-78. [DOI] [PubMed] [Google Scholar]
- 9.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellman, G. L., K. D. Courtney, V. Andres, and R. M. Featherstone. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7:88-95. [DOI] [PubMed]
- 11.Enouf, V., P. Langella, J. Commissaire, J. Cohen, and G. Corthier. 2001. Bovine rotavirus nonstructural protein 4 produced by Lactococcus lactis is antigenic and immunogenic. Appl. Environ. Microbiol. 67:1423-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feltquate, D. M., S. Heaney, R. G. Webster, and H. L. Robinson. 1997. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J. Immunol. 158:2278-2284. [PubMed] [Google Scholar]
- 13.Ford, R. P., D. J. Hill, and C. S. Hosking. 1983. Cows' milk hypersensitivity: immediate and delayed onset clinical patterns. Arch. Dis. Child 58:856-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaeng, S., S. Scherer, H. Neve, and M. J. Loessner. 2000. Gene cloning and expression and secretion of Listeria monocytogenes bacteriophage-lytic enzymes in Lactococcus lactis. Appl. Environ. Microbiol. 66:2951-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson, T. J. 1984. Ph.D. thesis. University of Cambridge, Cambridge, England.
- 16.Halstensen, T. S. 1997. Why are we not all allergic: basic mechanisms for tolerance development. Environ. Toxicol. Pharmacol. 4:25-31. [DOI] [PubMed] [Google Scholar]
- 17.Hill, D. J., M. A. Firer, G. Ball, and C. S. Hosking. 1989. Recovery from milk allergy in early childhood: antibody studies. J. Pediatr. 114:761-766. [DOI] [PubMed] [Google Scholar]
- 18.Host, A., and S. Halken. 1998. Epidemiology and prevention of cow's milk allergy. Allergy 53:111-113. [DOI] [PubMed] [Google Scholar]
- 19.Host, A., H. P. Jacobsen, S. Halken, and D. Holmenlund. 1995. The natural history of cow's milk protein allergy/intolerance. Eur. J. Clin. Nutr. 49:13-18. [PubMed] [Google Scholar]
- 20.Hoyne, G. F., M. G. Callow, M. C. Kuo, and W. R. Thomas. 1994. Inhibition of T-cell responses by feeding peptides containing major and cryptic epitopes: studies with the Der p I allergen. Immunology 83:190-195. [PMC free article] [PubMed] [Google Scholar]
- 21.Hoyne, G. F., N. M. Kristensen, H. Yssel, and J. R. Lamb. 1995. Peptide modulation of allergen-specific immune responses. Curr. Opin. Immunol. 7:757-761. [DOI] [PubMed] [Google Scholar]
- 22.Hoyne, G. F., R. E. O'Hehir, D. C. Wraith, W. R. Thomas, and J. R. Lamb. 1993. Inhibition of T cell and antibody responses to house dust mite allergen by inhalation of the dominant T cell epitope in naive and sensitized mice. J. Exp. Med. 178:1783-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuipers, O. P., P. G. de Ruyters, M. Kleerezen, and W. M. de Vos. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15-21. [Google Scholar]
- 24.Langella, P., and Y. Le Loir. 1999. Heterologous protein secretion in Lactococcus lactis: a novel antigen delivery system. Braz. J. Med. Biol. Res. 32:191-198. [DOI] [PubMed] [Google Scholar]
- 25.Langella, P., Y. Le Loir, S. D. Ehrlich, and A. Gruss. 1993. Efficient plasmid mobilization by pIP501 in Lactococcus lactis subsp. lactis. J. Bacteriol. 175:5806-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Loir, Y., A. Gruss, S. D. Ehrlich, and P. Langella. 1994. Direct screening of recombinants in gram-positive bacteria using the secreted staphylococcal nuclease as a reporter. J. Bacteriol. 176:5135-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Loir, Y., A. Gruss, S. D. Ehrlich, and P. Langella. 1998. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J. Bacteriol. 180:1895-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin, L. L., Y. F. Wei, P. T. Stockmann, K. M. Leahy, P. Needleman, J. Grassi, and P. Pradelles. 1987. Development, validation and application of an enzyme immunoassay (EIA) of atriopeptin. Biochem. Biophys. Res. Commun. 144:469-476. [DOI] [PubMed] [Google Scholar]
- 29.Mercenier, A., H. Muller-Alouf, and C. Grangette. 2000. Lactic acid bacteria as live vaccines. Curr. Issues Mol. Biol. 2:17-25. [PubMed] [Google Scholar]
- 30.Miller, K., C. Meredith, I. Selo, and J. M. Wal. 1999. Allergy to bovine beta-lactoglobulin: specificity of immunoglobulin E generated in the Brown Norway rat to tryptic and synthetic peptides. Clin. Exp. Allergy 29:1696-1704. [DOI] [PubMed] [Google Scholar]
- 31.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348-2357. [PubMed] [Google Scholar]
- 32.Negroni, L., H. Bernard, G. Clement, J. M. Chatel, P. Brune, Y. Frobert, J. M. Wal, and J. Grassi. 1998. Two-site enzyme immunometric assays for determination of native and denatured beta-lactoglobulin. J. Immunol. Methods 220:25-37. [DOI] [PubMed] [Google Scholar]
- 33.Norton, P. M., H. W. Brown, J. M. Wells, A. M. Macpherson, P. W. Wilson, and R. W. Le Page. 1996. Factors affecting the immunogenicity of tetanus toxin fragment C expressed in Lactococcus lactis. FEMS Immunol. Med. Microbiol. 14:167-177. [DOI] [PubMed] [Google Scholar]
- 34.Norton, P. M., J. M. Wells, H. W. Brown, A. M. Macpherson, and R. W. Le Page. 1997. Protection against tetanus toxin in mice nasally immunized with recombinant Lactococcus lactis expressing tetanus toxin fragment C. Vaccine 15:616-619. [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro, L. A., V. Azevedo, Y. Le Loir, S. C. Oliveira, Y. Dieye, J. C. Piard, A. Gruss, and P. Langella. 2002. Production and targeting of the Brucella abortus antigen L7/L12 in Lactococcus lactis: a first step towards food-grade live vaccines against brucellosis. Appl. Environ. Microbiol. 68:910-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson, K., L. M. Chamberlain, K. M. Schofield, J. M. Wells, and R. W. Le Page. 1997. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat. Biotechnol. 15:653-657. [DOI] [PubMed] [Google Scholar]
- 37.Rogers, B. L., J. F. Bond, S. J. Craig, A. K. Nault, D. B. Segal, J. P. Morgenstern, M. S. Chen, C. B. Bizinkauskas, C. M. Counsell, and A. M. Lussier. 1994. Potential therapeutic recombinant proteins comprised of peptides containing recombined T cell epitopes. Mol. Immunol. 31:955-966. [DOI] [PubMed] [Google Scholar]
- 38.Rolland, J., and R. O'Hehir. 1998. Immunotherapy of allergy: anergy, deletion, and immune deviation. Curr. Opin. Immunol. 10:640-645. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 40.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 41.Selo, I., G. Clement, H. Bernard, J. Chatel, C. Creminon, G. Peltre, and J. Wal. 1999. Allergy to bovine beta-lactoglobulin: specificity of human IgE to tryptic peptides. Clin. Exp. Allergy 29:1055-1063. [DOI] [PubMed] [Google Scholar]
- 42.Selo, I., L. Negroni, C. Creminon, J. Grassi, and J. M. Wal. 1996. Preferential labeling of alpha-amino N-terminal groups in peptides by biotin: application to the detection of specific anti-peptide antibodies by enzyme immunoassays. J. Immunol. Methods 199:127-138. [DOI] [PubMed] [Google Scholar]
- 43.Steidler, L., W. Hans, L. Schotte, S. Neirynck, F. Obermeier, W. Falk, W. Fiers, and E. Remaut. 2000. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289:1352-1355. [DOI] [PubMed] [Google Scholar]
- 44.Steidler, L., K. Robinson, L. Chamberlain, K. M. Schofield, E. Remaut, R. W. Le Page, and J. M. Wells. 1998. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect. Immun. 66:3183-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terzaghi, B., and W. E. Sandine. 1975. Improved medium for lactic acid streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuji, N. M., J. Kurisaki, and K. Mizumachi. 1993. Establishment of CD4+ T cell clones specific to bovine beta-lactoglobulin and analysis of their specificity. J. Biochem. (Tokyo) 113:545-548. [DOI] [PubMed] [Google Scholar]
- 48.Tsuji, N. M., J. Kurisaki, K. Mizumachi, and S. Kaminogawa. 1993. Localization of T-cell determinants on bovine beta-lactoglobulin. Immunol. Lett. 37:215-221. [DOI] [PubMed] [Google Scholar]
- 49.van de Guchte, M., F. J. van der Wal, J. Kok, and G. Venema. 1992. Lysozyme expression in Lactococcus lactis. Appl. Microbiol. Biotechnol. 37:216-224. [DOI] [PubMed] [Google Scholar]
- 50.von Heijne, G. 1986. The distribution of positively charged residues in bacteria inner membrane proteins correlates with trans-membrane topology. EMBO J. 5:3021-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wal, J. M., H. Bernard, C. Creminon, C. Hamberger, B. David, and G. Peltre. 1995. Cow's milk allergy: the humoral immune response to eight purified allergens. Adv. Exp. Med. Biol. 371B:879-881. [PubMed] [Google Scholar]
- 52.Wells, J. M., K. Robinson, L. M. Chamberlain, K. M. Schofield, and R. W. Le Page. 1996. Lactic acid bacteria as vaccine delivery vehicles. Antonie Leeuwenhoek 70:317-330. [DOI] [PubMed] [Google Scholar]