Abstract
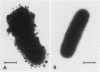
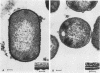
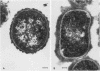
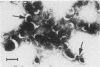
Capsular structure and biochemical composition varied between two isolates (virulent and avirulent) of Haemophilus pleuropneumoniae serotype 5. The presence of capsule was determined by transmission electron microscopy with glutaraldehyde-osmium, ruthenium red, alcian blue, and phosphotungstic acid staining procedures. The virulent isolate of H. pleuropneumoniae had a distinct, adherent capsule. The avirulent isolate had a fragile, easily removed capsule. Capsular material (CM) and a lipopolysaccharide (LPS) were isolated from each bacterial isolate and were compared biochemically and biologically. CM from both isolates contained carbohydrates, no detectable protein, and no detectable to trace amounts of lipid A. Each LPS contained heptose, hexose, galactose, glucosamine, 2-keto-3-deoxyoctonate, and lipid A. Biological responses to CM and LPS from both isolates were demonstrated in the proclotting enzyme of Limulus polyphemus amebocyte lysate activation and in serological cross-reactions by immunofluorescence and immunodiffusion precipitation. The virulent isolate contained approximately 10 mg of LPS per g more on an original dry weight basis than the avirulent isolate. LPS from the virulent isolate contained approximately 13 times more galactose than LPS from the avirulent isolate. The differences of capsular structure and biochemical composition may contribute to the role of CM in porcine H. pleuropneumoniae infections.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Baltimore R. S., Mitchell M. Immunologic investigations of mucoid strains of Pseudomonas aeruginosa: comparison of susceptibility to opsonic antibody in mucoid and nonmucoid strains. J Infect Dis. 1980 Feb;141(2):238–247. doi: 10.1093/infdis/141.2.238. [DOI] [PubMed] [Google Scholar]
- Bendixen P. H., Shewen P. E., Rosendal S., Wilkie B. N. Toxicity of Haemophilus pleuropneumoniae for porcine lung macrophages, peripheral blood monocytes, and testicular cells. Infect Immun. 1981 Sep;33(3):673–676. doi: 10.1128/iai.33.3.673-676.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram T. A., Jensen A. E. Responses of equine neutrophils to contagious equine metritis organism and its lipopolysaccharides. Am J Vet Res. 1984 Jun;45(6):1099–1104. [PubMed] [Google Scholar]
- Bertram T. A. Quantitative morphology of peracute pulmonary lesions in swine induced by Haemophilus pleuropneumoniae. Vet Pathol. 1985 Nov;22(6):598–609. doi: 10.1177/030098588502200615. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- Demarco de Hormaeche R., Thornley M. J., Glauert A. M. Demonstration by light and electron microscopy of capsules on gonococci recently grown in vivo. J Gen Microbiol. 1978 May;106(1):81–91. doi: 10.1099/00221287-106-1-81. [DOI] [PubMed] [Google Scholar]
- Gunnarsson A., Biberstein E. L., Hurvell B. Serologic studies on porcine strains of Haemophilus parahaemolyticus (pleuropneumoniae): agglutination reactions. Am J Vet Res. 1977 Aug;38(8):1111–1114. [PubMed] [Google Scholar]
- Heddleston K. L., Gallagher J. E., Rebers P. A. Fowl cholera: gel diffusion precipitin test for serotyping Pasteruella multocida from avian species. Avian Dis. 1972 Jul-Sep;16(4):925–936. [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häni H., König H., Nicolet J., Scholl E. Zur Haemophilus-Pleuropneumonie beim Schwein. V. Pathomorphologie. Schweiz Arch Tierheilkd. 1973 May;115(5):191–203. [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Kiley P., Holt S. C. Characterization of the lipopolysaccharide from Actinobacillus actinomycetemcomitans Y4 and N27. Infect Immun. 1980 Dec;30(3):862–873. doi: 10.1128/iai.30.3.862-873.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. The haemolytic activity of Haemophilus species. Acta Pathol Microbiol Scand B. 1976 Dec;84B(6):339–341. doi: 10.1111/j.1699-0463.1976.tb01950.x. [DOI] [PubMed] [Google Scholar]
- Lyman M. B., Stocker B. A., Roantree R. J. Comparison of the virulence of O:9,12 and O:4,5,12 Salmonella typhimurium his+ transductants for mice. Infect Immun. 1977 Feb;15(2):491–499. doi: 10.1128/iai.15.2.491-499.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris E. R., Rees D. A., Thom D., Welsh E. J. Conformation and intermolecular interactions of carbohydrate chains. J Supramol Struct. 1977;6(2):259–274. doi: 10.1002/jss.400060211. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Apicella M. A. Isolation of a lipopolysaccharide mutant of Neisseria gonorrhoeae: an analysis of the antigenic and biologic difference. J Infect Dis. 1982 Feb;145(2):206–216. doi: 10.1093/infdis/145.2.206. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Valtonen V. V., Valtonen M. Role of O-antigen (lipopolysaccharide) factors in the virulence of Salmonella. J Infect Dis. 1973 Jul;128(Suppl):81–85. doi: 10.1093/infdis/128.supplement_1.s81. [DOI] [PubMed] [Google Scholar]
- Nakai T., Sawata A., Kume K. Pathogenicity of Haemophilus pleuropneumoniae for laboratory animals and possible role of its hemolysin for production of pleuropneumonia. Nihon Juigaku Zasshi. 1984 Dec;46(6):851–858. doi: 10.1292/jvms1939.46.851. [DOI] [PubMed] [Google Scholar]
- Nicolet J. Sur l'hémophilose du porc. I. Identification d'un agent fréquent: Haemophilus parahaemolyticus. Pathol Microbiol (Basel) 1968;31(4):215–225. [PubMed] [Google Scholar]
- Nielsen R., O'Connor P. J. Serological characterization of 8 Haemophilus pleuropneumoniae strains and proposal of a new serotype: serotype 8. Acta Vet Scand. 1984;25(1):96–106. doi: 10.1186/BF03547283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORSKOV I., ORSKOV F., JANN B., JANN K. ACIDIC POLYSACCHARIDE ANTIGENS OF A NEW TYPE FROM E. COLI CAPSULES. Nature. 1963 Oct 12;200:144–146. doi: 10.1038/200144a0. [DOI] [PubMed] [Google Scholar]
- Rosendal S., Boyd D. A., Gilbride K. A. Comparative virulence of porcine Haemophilus bacteria. Can J Comp Med. 1985 Jan;49(1):68–74. [PMC free article] [PubMed] [Google Scholar]
- Sawata A., Kume K. Relationships between virulence and morphological or serological properties of variants dissociated from serotype 1 Haemophilus paragallinarum strains. J Clin Microbiol. 1983 Jul;18(1):49–55. doi: 10.1128/jcm.18.1.49-55.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Bacterial exopolysaccharides. Adv Microb Physiol. 1972;8:143–213. doi: 10.1016/s0065-2911(08)60190-3. [DOI] [PubMed] [Google Scholar]
- TACHIBANA D. K. CHARACTERIZATION OF THE BACTERICIDAL ACTION OF PRECOLOSTRAL PIGLET AND CALF SERA AGAINST GRAM NEGATIVE ENTERIC BACTERIA IN ROUGH PHASE. Folia Microbiol (Praha) 1965 May;10:145–155. doi: 10.1007/BF02881005. [DOI] [PubMed] [Google Scholar]
- Valtonen M. V. Role of phagocytosis in mouse virulence of Salmonella typhimurium recombinants with O antigen 6,7 or 4,12. Infect Immun. 1977 Dec;18(3):574–582. doi: 10.1128/iai.18.3.574-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]
- Yin E. T., Galanos C., Kinsky S., Bradshaw R. A., Wessler S., Lüderitz O., Sarmiento M. E. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972 Jan 28;261(1):284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]
- Zwahlen A., Winkelstein J. A., Moxon E. R. Surface determinants of Haemophilus influenzae pathogenicity: comparative virulence of capsular transformants in normal and complement-depleted rats. J Infect Dis. 1983 Sep;148(3):385–394. doi: 10.1093/infdis/148.3.385. [DOI] [PubMed] [Google Scholar]