Abstract
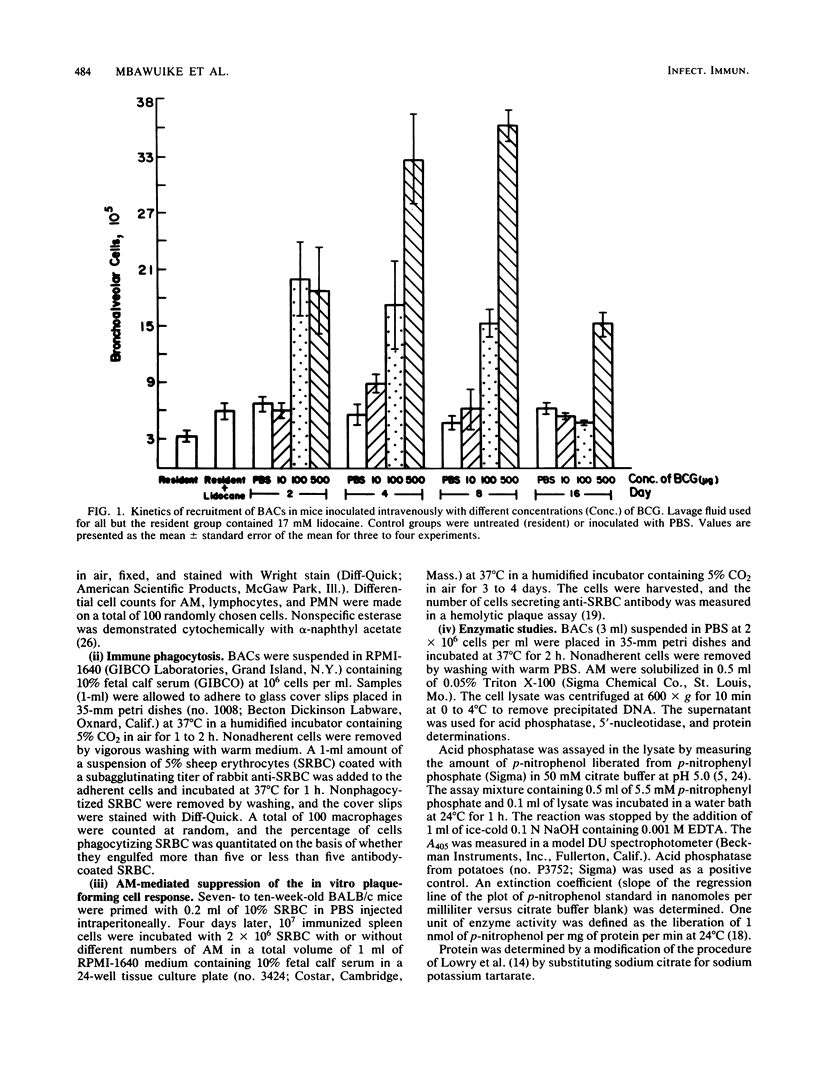
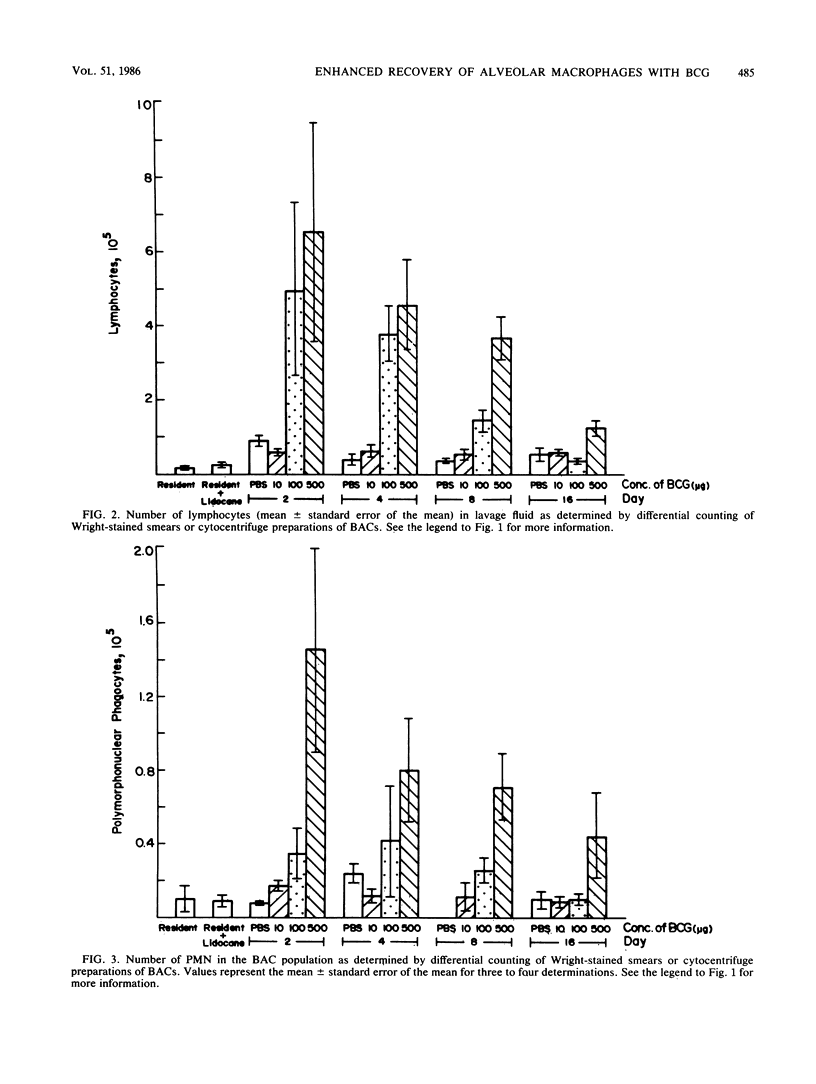
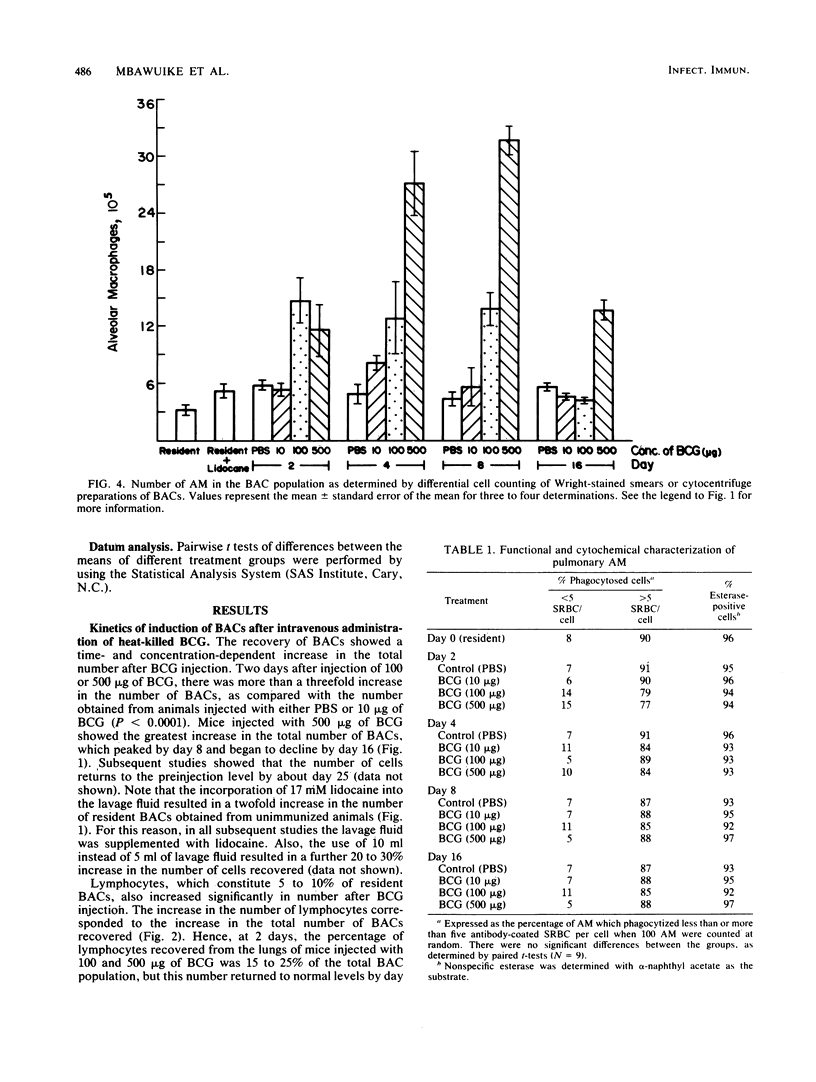
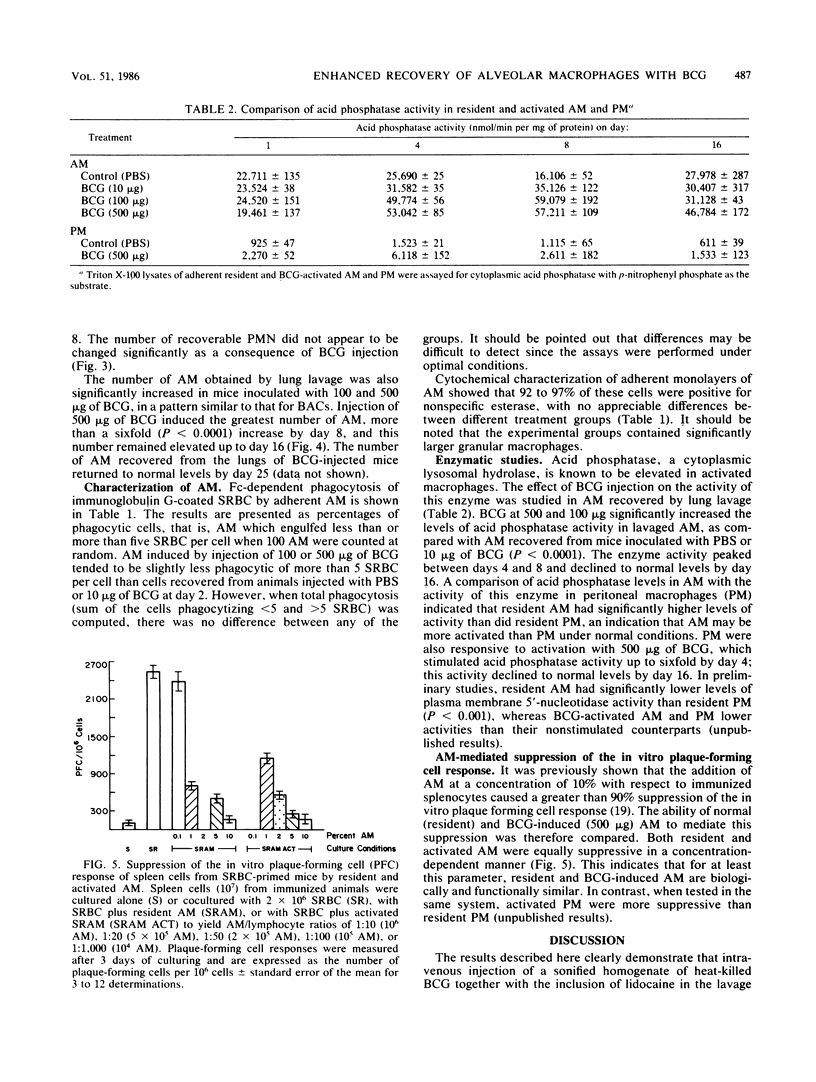
The kinetics of induction of the bronchoalveolar cell population (i.e., alveolar macrophages [AM], lymphocytes, and polymorphonuclear leukocytes) was studied in mice inoculated intravenously with heat-killed Mycobacterium bovis BCG. Injection of BCG at 100 and 500 micrograms but not at 10 micrograms per mouse resulted in an increase in the total number of bronchoalveolar cells (threefold) and in the number of AM (sixfold) recovered by bronchoalveolar lavage in a time-dependent manner, as compared with control mice. A significant increase in the number of lymphocytes was also observed between days 2 and 4 after injection, but this number returned to normal levels by day 8, whereas the number of polymorphonuclear leukocytes was not significantly altered. AM were characteristically phagocytic and stained positively for nonspecific esterase. AM recruited in response to BCG injection were activated, as indicated by elevated levels of acid phosphatase activity and decreased levels of membrane 5'-nucleotidase activity. However, both resident and BCG-induced AM suppressed the in vitro plaque-forming cell response of sheep erythrocyte-primed mice to the same extent. These results indicate that injection of heat-killed BCG induced increased numbers of activated AM, which appeared to be functionally similar to resident AM in their ability to phagocytize and modulate in vitro immune responses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baser Y., deShazo R. D., Barkman H. W., Jr, Nordberg J. Lidocaine effects on immunocompetent cells. Implications for studies of cells obtained by bronchoalveolar lavage. Chest. 1982 Sep;82(3):323–328. doi: 10.1378/chest.82.3.323. [DOI] [PubMed] [Google Scholar]
- Blussé van Oud Alblas A., van der Linden-Schrever B., van Furth R. Origin and kinetics of pulmonary macrophages during an inflammatory reaction induced by intravenous administration of heat-killed bacillus Calmette-Guérin. J Exp Med. 1981 Aug 1;154(2):235–252. doi: 10.1084/jem.154.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain J. D. Free cells in the lungs. Some aspects of their role, quantitation, and regulation. Arch Intern Med. 1970 Sep;126(3):477–487. doi: 10.1001/archinte.126.3.477. [DOI] [PubMed] [Google Scholar]
- Cohen A. B., Batra G., Petersen R., Podany J., Nguyen D. Size of the pool of alveolar neutrophils in normal rabbit lungs. J Appl Physiol Respir Environ Exerc Physiol. 1979 Aug;47(2):440–444. doi: 10.1152/jappl.1979.47.2.440. [DOI] [PubMed] [Google Scholar]
- Crofton R. W., Diesselhoff-den Dulk M. M., van Furth R. The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J Exp Med. 1978 Jul 1;148(1):1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscowitz H. B. In defense of the lung: paradoxical role of the pulmonary alveolar macrophage. Ann Allergy. 1985 Nov;55(5):634–650. [PubMed] [Google Scholar]
- Hoidal J. R., White J. G., Repine J. E. Influence of cationic local anesthetics on the metabolism and ultrastructure of human alveolar macrophages. J Lab Clin Med. 1979 May;93(5):857–866. [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. I. A simple technique for the preparation of high numbers of viable alveolar macrophages from small laboratory animals. J Immunol Methods. 1979;27(2):189–198. doi: 10.1016/0022-1759(79)90264-3. [DOI] [PubMed] [Google Scholar]
- Hu W. S., Muscoplat C. C. Lidocaine: effect on phagocytosis and purification of monocytes in bovine peripheral blood. Am J Vet Res. 1980 Mar;41(3):447–449. [PubMed] [Google Scholar]
- Johnson W. J., Marino P. A., Schreiber R. D., Adams D. O. Sequential activation of murine mononuclear phagocytes for tumor cytolysis: differential expression of markers by macrophages in the several stages of development. J Immunol. 1983 Aug;131(2):1038–1043. [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J. K. Biochemical criteria for activated macrophages. J Immunol. 1978 Sep;121(3):809–813. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MYRVIK Q. N., LEAKE E. S., OSHIMA S. A study of macrophages and epitheloid-like cells from granulomatous (BCG-induced) lungs of rabbits. J Immunol. 1962 Nov;89:745–751. [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- McCarron R. M., Goroff D. K., Luhr J. E., Murphy M. A., Herscowitz H. B. Methods for the collection of peritoneal and alveolar macrophages. Methods Enzymol. 1984;108:274–284. doi: 10.1016/s0076-6879(84)08092-7. [DOI] [PubMed] [Google Scholar]
- Pennline K. J., Conrad R. E., Gerber H. R., Herscowitz H. B. Suppressive effect of alveolar macrophages on the in vitro immune response of rabbit lymphocytes. J Reticuloendothel Soc. 1979 May;25(5):495–512. [PubMed] [Google Scholar]
- Rabinovitch M., DeStefano M. J. Use of the local anesthetic lidocaine for cell harvesting and subcultivation. In Vitro. 1975 Nov-Dec;11(6):379–381. doi: 10.1007/BF02616374. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Newball H. H. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974 Oct;84(4):559–573. [PubMed] [Google Scholar]
- Schnyder J., Baggiolini M. Secretion of lysosomal hydrolases by stimulated and nonstimulated macrophages. J Exp Med. 1978 Aug 1;148(2):435–450. doi: 10.1084/jem.148.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield J. D., Hammarström L., Smith E. The effect of membrane stabilizing agents on induction of the immune response. I. Effect of lymphocyte activation in mixed lymphocyte reactions. J Exp Med. 1976 Aug 1;144(2):562–567. doi: 10.1084/jem.144.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van oud Alblas A. B., van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979 Jun 1;149(6):1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]


