Abstract
The p21-activated protein kinases (Paks) regulate cellular proliferation, differentiation, transformation, and survival through multiple downstream signals. Paks are activated directly by the small GTPases Rac and Cdc42 and several protein kinases including Akt and PDK-1. We found that Akt phosphorylated and modestly activated Pak1 in vitro. The major site phosphorylated by Akt on Pak1 mapped to serine 21, a site originally shown to be weakly autophosphorylated on Pak1 when Cdc42 or Rac activates it. A peptide derived from the region surrounding serine 21 was a substrate for Akt but not Pak1 in vitro, and Akt stimulated serine 21 phosphorylation on the full-length Pak1 much better than Rac did. The adaptor protein Nck binds Pak near serine 21, and its association is regulated by phosphorylation of this site. We found that either treatment of Pak1 in vitro with Akt or coexpression of constitutively active Akt with Pak1 reduced Nck binding to Pak1. In HeLa cells, green fluorescent protein-tagged Pak1 was concentrated at focal adhesions and was released when Akt was cotransfected. A peptide containing the Nck binding site of Pak1 fused to a portion of human immunodeficiency virus Tat to allow it to enter cells was used to test the functional importance of Nck/Pak binding in Akt-stimulated cell migration. This Tat-Nck peptide reduced Akt-stimulated cell migration. Together, these data suggest that Akt modulates the association of Pak with Nck to regulate cell migration.
Regulation of cell growth, differentiation, and survival requires the coordinated modulation of multiple cell signaling pathways. The signals from the small GTPase Ras are among the best characterized signaling pathways. Ras binds and activates several effectors including Raf, phosphatidylinositol (PI) 3-kinase and Ral GDS (34). Raf is a serine-threonine kinase that phosphorylates and activates Mek, another kinase. Mek then phosphorylates and activates the mitogen-activated protein kinase Erk. PI-3 kinase generates phospholipid second messengers that stimulate downstream signaling pathways to regulate the actin cytoskeleton and also promote cell survival by inhibiting apoptosis (18). PI-3 kinase regulates the actin cytoskeleton through the small GTPase Rac (5). PI-3 kinase also promotes cell survival by activating the Akt protein kinase, which phosphorylates targets such as Bad and forkhead (9, 14, 17). Recent studies have found that p21-activated protein kinase (Pak) protein kinases are key transducers of all of these signals (5).
Pak kinases are serine-threonine protein kinases identified because they bind and are activated by the small GTPases Rac and Cdc42. Paks are divided into two groups, A and B, based on sequence similarities. Group A consists of three closely related family members, Pak1 (Pakα), Pak2 (Pakγ), and Pak3 (Pakβ). Group B contains Pak4, Pak5, and Pak6 (1, 32). Expression of Pak in cells stimulates some of the changes in the actin cytoskeleton associated with Rac and Cdc42, including stimulation of cell ruffling and inhibition of stress fibers (31, 44, 45).
Because of their identification as Rac and Cdc42 effectors, Paks have most often been associated with cytoskeletal signaling. However, Paks also regulate protein kinase cascades. Pak expression stimulates Jun N-terminal kinase (JNK) (2, 8, 55), although Rac primarily uses alternative effectors to activate JNK (36, 49, 50). Paks also transduce signals to Erk which have been proposed to promote cell proliferation (19, 25, 49, 51). The role of Pak in Erk signaling is often regarded as an example of cross talk because Pak is a relatively weak activator of Erk by itself and only activates Erk in the presence of Raf. Nevertheless, the signal from Pak is physiologically relevant since dominant-negative mutants of Pak inhibit many activators of Erk, including Ras. The direct targets of Pak include Raf and Mek, both of which are phosphorylated by Pak (19, 25). Thus, Pak is necessary but not sufficient for Erk activation and sufficient but not necessary for JNK activation (19, 49-51).
Similarly, a role for Pak in promoting cell survival signals was revealed by the finding that Pak1, Pak2, and Pak4 all inhibit apoptosis and phosphorylate the cell survival factor Bad (21, 24, 43, 52). Thus, Pak plays an integral role in multiple signals from Ras through several downstream signaling pathways. These signals may play important roles in cell transformation since dominant-negative mutants of Pak can inhibit cell transformation (36, 41, 49, 50).
While most of the regulation of Pak is through small GTPases, Pak kinases are also regulated by GTPase-independent mechanisms. Growth factor receptors can recruit Pak to the membrane through the adaptor proteins Nck and Grb2 (6, 20, 30, 40). The interaction with Nck has been the most studied. Nck contains three SH3 domains and a C-terminal SH2 domain. When receptors are activated they undergo tyrosine phosphorylation, creating docking sites for the Nck SH2 domain. The second SH3 domain on Nck binds a proline-rich region near the N terminus of Pak. Through this mechanism, Nck translocates Pak to the membrane where it is then activated by small GTPases (30). The PIX (Pak-interacting exchange factor) proteins are another family of proteins that bind Pak through SH3 domains (3, 33). The various PIX isoforms are guanine exchangers for Rac and Cdc42 but can activate Pak through both GTPase-dependent and GTPase-independent mechanisms (12). Several protein kinases also regulate Pak. PDK-1 phosphorylates Pak at threonine 423, a site that is also autophosphorylated when Pak is activated by Rac or Cdc42 (26). Cdc2 also phosphorylates Pak at T212 (4, 53). Another protein kinase, Akt, can also stimulate Pak, but the mechanism of regulation is not known (52). In Dictyostelium, Akt phosphorylates Pak, but the phosphorylation site is not conserved on mammalian Pak homologs (11).
Both PIX and Nck binding can be regulated by Pak autophosphorylation. PIX binds near serine 199 while Nck binds near serine 21, both of which are autophosphorylated on Pak1 when it is activated in vitro. Phosphorylation of serine 21 reduces Nck binding and phosphorylation of serine 199 reduces PIX binding, modifications which ultimately release Pak from focal contacts (57). Recently, autophosphorylation of serine 21 was called into question because platelet-derived growth factor (PDGF) could activate Pak in attached cells without causing phosphorylation of Pak at serine 21. When Pak was isolated from suspended cells, however, serine 21 was phosphorylated but Pak was not activated (23). This study also found that Nck was released in detached cells, presumably because serine 21 was phosphorylated. Hence, it is possible to activate Pak1 without phosphorylating serine 21, and phosphorylation of serine 21 can be observed on inactive Pak. A likely explanation for these observations is that serine 21 can be phosphorylated by other kinases and is not exclusively autophosphorylated. We report here that Akt phosphorylates Pak1 at serine 21 and that expression of Akt can release Pak1 from Nck in vivo and in vitro. Evidence is presented to suggest that Akt modulation of the Nck/Pak complex is important for cell migration. These studies provide a molecular mechanism whereby Akt can regulate cell migration by releasing Pak1 from Nck to translocate Pak1.
MATERIALS AND METHODS
Materials.
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum, Lipofectamine, and recombinant human PDGF-BB were from Invitrogen (Carlsbad, Calif,). FuGENE 6 transfection reagent, complete protease inhibitor cocktail tablets, and fibronectin were from Roche (Indianapolis, Ind.). Mouse monoclonal anti-Nck antibody and active Akt were from Upstate Biotechnology, Inc. (Charlottesville, Va.). Rabbit polyclonal anti-Pak1 antibody and protein A/G PLUS agarose were from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Rabbit anti-Akt antibody and phospho-Akt Thr-308 antibody were from Cell Signaling (Beverly, Mass.). Mouse monoclonal anti-human vinculin was from Sigma (St. Louis, Mo.). Highly cross-absorbed Alexa Fluor 594 goat anti-mouse immunoglobulin G (heavy plus light chains) and 5-chloromethylfluorescein diacetate (CMFDA) were from Molecular Probes (Eugene, Oreg.). Transwell membranes (8-μm pore size) were from Costar (Corning, N.Y.). Ni-nitrilotriacetic acid (NTA) agarose was from Qiagen (Valencia, Calif.). Glutathione-Sepharose was from Amersham (Piscataway, N.J.). The Transformer site-directed mutagenesis kit was from CLONTECH Laboratories, Inc. (Palo Alto, Calif.). Peptides Pak S21 (PPAPPMRNTSTMIGA), Pak S21A (PPAPPMRNTATMIGA), Bad S136 (PFRGRSRSAP), and Bad S136A (PFRGRSRAAP) were synthesized by Invitrogen (targets for mutation and modification are underlined). The peptide Tat-Pak S21(YGRKKRRQRRRGKPPAPPMRNTSTM) and the serine 21-phosphorylated form Tat-Pak pS21 (YGRKKRRQRRRGKPPAPPMRNT[pS]TM), together with fluorescein isothiocyanate (FITC)-tagged versions of these peptides (with FITC fused to their N termini), were synthesized by ALPHA Diagnostic International (San Antonio, Tex.). To raise a Pak1 phospho-S21-specific antibody, rabbits were immunized with the peptide PPMRNT[pS]TMIGAG. Serum was collected and purified over a peptide column and then passed over a column consisting of the nonphosphorylated version of the peptide to increase the specificity (Invitrogen).
Plasmids.
Plasmids expressing a Myc-tagged wild-type (WT) Pak1 and a kinase-dead (KD) (K299R) version of Pak1 based on the pCMV6 vector have been described elsewhere (49). Akt constructs used in this study include plasmids expressing a membrane-targeted Akt with a deletion of the PH domain (MyrAkt) and a kinase-deficient, dominant-negative mutant (DNAkt) (K179M) protein (52). To express a green fluorescent protein (GFP) fusion protein of Pak, the WT Pak1 gene was cloned into the KpnI site of the pcDNA3 vector with a GFP gene fused to its C terminus. The plasmid pd2EGFP-N1, which expresses GFP alone, was also from CLONTECH.
Protein purification.
Cdc42, Rac, the second SH3 domain of Nck, the SH3 domain of PIX, full-length Pak1, and Pak1 fragments were purified as either glutathione S-transferase (GST) or His fusion proteins from Escherichia coli strain BL21 following standard procedures (47). To express and purify Pak1 proteins (WT and mutants) from baculovirus, constructs were made by using the Transformer site-directed mutagenesis kit. The constructs were sequenced by the Cell Center of the University of Pennsylvania. The infection of insect cells (Sf9) was carried out by a facility at the Wistar Institute. His-tagged Pak1 proteins were purified from insect cell extracts under native conditions with Ni-NTA agarose by following the procedures supplied by Qiagen. Briefly, the cells were lysed in lysis buffer containing 1% NP-40. After centrifugation, the supernatant was applied to a column filled with Ni-NTA agarose, the column was then washed, and the protein was eluted with elution buffer containing a higher concentration of imidazole.
Kinase assays.
Kinase assays were conducted by incubating a mixture of proteins, 20 μM ATP, and indicated substrates in kinase buffer (10 mM MgCl2, 40 mM HEPES [pH 7.4]) in a reaction volume of 25 μl. Reactions were initiated by adding 5 μCi of [γ-32P]ATP, and then the mixtures were incubated at 30°C for 30 min. The reaction was terminated by adding 25 μl of 2× sodium dodecyl sulfate (SDS) sample buffer. The samples were then boiled and resolved by SDS-polyacrylamide gel electrophoresis (PAGE) or on 10 to 20% Tris-Tricine gels (Bio-Rad), for peptide substrates, and visualized by autoradiography.
Mass spectrometry.
Samples corresponding to Akt-phosphorylated His-PAK1 K299A were identified and excised from SDS gels. The gel pieces were washed three times in a solution of 50% 0.1 M NH4HCO3-50% acetonitrile at 37°C for 30 min each. The gel pieces were dried in a Savant Speed-Vac and treated with 10 mM dithiothreitol in 0.1 M NH4HCO3 for 1 h at 56°C to oxidize methionine amino acids. This was followed by a 30-min incubation at room temperature (RT) with 100 mM iodoacetic acid to alkylate reduced cysteine to carboxymethyl cysteine. The gel piece was again dried in a Speed-Vac, and fresh 0.3 mg of trypsin (Promega) in 0.1 M NH4HCO3 was added to rehydrate the gel, which was then incubated at 37°C overnight. Peptides were extracted in 5% acetonitrile containing 0.1% acetyl HO. Prior to the phosphopurification of the peptides from the eluted mixture, a mini-column containing Pharmacia's Hi-Trap chelating resin was freshly prepared. The resin was charged a wash with 200 μl of 20 mM FeCl3 followed by three washes with 0.1 M NH4HCO3 and three washes with H2O. The column was equilibrated with buffer A (50 mM morpholinoethanesulfonic acid [sMES, pH 5.5], 1 M NaCl). The eluted peptides were diluted in buffer A and allowed to pass through the column by gravity flow. The column was then washed three times with buffer A, and nonphosphorylated peptides were eluted with buffer B (2 mM MES, pH 6.0). The phosphopeptides were eluted in 150 μl of 0.1 M NH4HCO3 (pH 8.0). The phosphopeptides were then purified and concentrated by using a C18 ZipTip (Millipore) and eluted in matrix containing 3,5-dihydroxybenzoic acid, 50% acetonitrile, and 25 mM diammonium citrate. Samples were analyzed on an Applied Biosystems DE STR time-of-flight mass spectrometer by delayed extraction in refractor mode.
Cell culture and transfection.
Cells were maintained in DMEM containing 10% heat-inactivated fetal bovine serum and supplemented with 2 mM glutamine, 100 U of penicillin G/ml and 100 μg of streptomycin/ml at 37°C in 5% CO2. For transfection of COS-7 cells, cells were split and grown for a day to 60 to 80% confluence and transfected with Lipofectamine as recommended. For each 100-mm-diameter plate, 5 μg of Pak1 plasmid (WT or K299R) along with 5 μg of Akt plasmid (or vector) was used. The cells were allowed to express the proteins for 40 to 48 h after DNA was added. To harvest, the cells were washed twice with ice-cold phosphate-buffered saline (PBS), washing was followed by the addition of 0.8 ml of ice-cold lysis buffer (40 mM HEPES [pH 7.4], 1% Nonidet P-40, 100 mM NaCl, 1 mM EDTA, 25 mM NaF, 1 mM sodium orthovanadate) containing the recommended concentration of complete protease inhibitor, and the mixture was put on ice for 5 min. The cells were then scraped from plates, transferred into centrifuge tubes, incubated on ice for 10 to 15 min, and then spun in a microcentrifuge at 14,000 rpm for 15 min at 4°C. The supernatant was collected as cell lysate and either used fresh or frozen at −80°C and used immediately.
Assays for Nck-Pak binding.
To test the in vivo association of Nck and Pak1, COS-7 cells were transfected with plasmids expressing Myc-tagged KD (K299R) Pak1 along with MyrAkt or DNAkt. Endogenous Nck protein was immunoprecipitated from about 500 μg of total protein in COS-7 cell lysates by rotation incubation for 2 h with mouse anti-Nck in 150 to 200 μl of lysis buffer at 4°C. After adding 30 μl of protein A/G PLUS agarose and further incubating the mixture for 2 h, the beads were collected by centrifugation in a microcentrifuge at full speed for 10 s at 4°C. The pellet was washed three times with lysis buffer. The Myc-tagged Pak1 coimmunoprecipitated with Nck was detected with mouse monoclonal antibody against Myc (9E10) on a Western blot. The cell lysates, including cells expressing both WT or KD Pak1 and Akt, were also used for GST-Nck pull-down experiments. The same amount of E. coli-originated GST-Nck bound to glutathione-Sepharose (8 μl of 50% glutathione-Sepharose saturated with GST-Nck) was added to cell lysates (about 500 μg of total protein) and rotation incubated at 4°C for 2 h. Then the beads were spun in a centrifuge and washed three times with lysis buffer. Pak1 bound to GST-Nck was detected by Western blotting with the Myc antibody. For in vitro pull-down experiments of PAK1 by Nck, 0.1 μg of baculovirus PAK1 (WT or KD) was mixed with or without 0.5 μg of Akt in kinase assay buffer with 20 μM ATP and incubated for 30 min at 30°C. GST-Nck bound to glutathione-Sepharose was then added to the reaction mixture as described above, the mixture was further incubated at 4°C for 2 h in lysis buffer, and the beads were collected and washed three times with lysis buffer. PAK1 proteins bound to GST-Nck were then detected with a Pak1 antibody.
Immunofluorescence.
HeLa cells were split and grown for about a day on six-well plates until subconfluent and then transfected with a plasmid expressing GFP-Pak1 along with Akt plasmids by using FuGENE 6 as recommended. Two micrograms of Akt (or vector) and 1 μg of Pak plasmids were used for each well. Seven hours after DNA was added, cells were trypsinized and replated into a Nunc chamber precoated with fibronectin; 24 h after adding the DNA, the cells were washed twice with PBS at RT, fixed with 3.7% paraformaldehyde for 30 min, and then permeabilized with PBS containing 0.5% Triton for 20 min. Following incubation with PBS containing 3% bovine serum albumin and goat serum for 30 min at 37°C, cells were incubated with a 1:400 dilution of vinculin antibody for 1 h at RT, washed with PBS containing 0.1% Triton three times, and then incubated with a 1:500 dilution of Alexa Fluor 594 goat anti-mouse antibody for 45 min at RT. After being washed several times with PBS containing 0.1% Triton X-100, the samples were mounted and observed under a Leica Polyvar 2 fluorescence microscope. Micrographs were taken with an Hamamatsu ORCA charge-coupled device camera operated with Improvision (Lexington, Mass.) OPENLAB 2 software.
Cell migration assays.
Transwell cell migration assays were performed with Rat1 stable cell lines. To establish the stable cell lines, Rat1 cells were transfected with MyrAkt or vector and were selected with 500 μg of G418/ml. The expression of MyrAkt was verified by Western blotting with both Akt and Phospho-T308 Akt antibodies. For migration assays, procedures modified from Stoletov et al. were used (48). Subconfluent cells were detached with trypsin-EDTA, collected in DMEM containing serum, and then washed once with warm DMEM containing 0.5% bovine serum albumin and kept in it until used. Transwell membranes were precoated with fibronectin for 20 min before use, and about 20,000 cells were added to the upper part of each well. Where indicated, 10 μg of PDGF/ml was added to the medium in the bottom part of the well. After incubation at 37°C with 5% CO2 for 2.5 h, the nonmigrating cells, which stay on the upper side of the membrane, were removed by gently wiping the membrane with a cotton swab. The membranes were then stained with CMFDA, and the cells were counted under a fluorescence microscope to score how many had migrated to the bottom side of the membrane. Cells were counted at four different random locations on the membrane, excluding the edge.
RESULTS
Akt phosphorylates and activates Pak1.
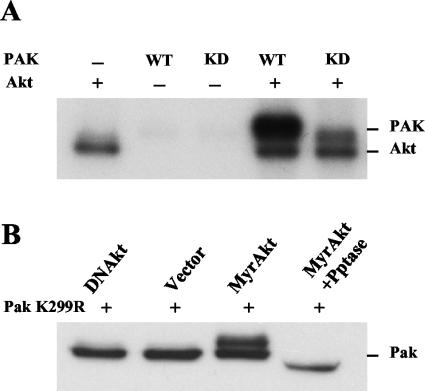
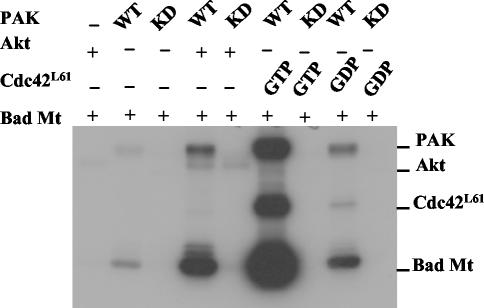
A previous study suggested that Akt regulates Pak1 through a novel pathway since Akt activation of Pak1 was not inhibited by dominant-negative mutants of either Rac or Cdc42 and a dominant-negative Akt mutant did not inhibit either Rac or Cdc42 activation of Pak1 (52). These data suggested that Akt signals independently of Rac and Cdc42, perhaps at the level of Pak itself. We hypothesized that Akt activates Pak1 through direct phosphorylation as it does in Dictyostelium (11). To test this, we incubated purified Pak1 with Akt and [γ-32P]ATP and then analyzed the reactions on an SDS-polyacrylamide gel (Fig. 1A). Because Pak1 autophosphorylates on multiple sites when it is activated, we also tested K299A, a KD mutant of Pak1. Akt stimulated phosphorylation of Pak1. There was a considerable increase (about fivefold more) in the phosphate incorporated into WT Pak1 than the KD Pak, suggesting that Pak1 is autophosphorylating itself after Akt phosphorylation. To test whether Akt stimulates phosphorylation of Pak in vivo, we transfected constitutively active Akt into cells along with KD Pak1 and analyzed Pak1 on Western blots (Fig. 1B). Akt stimulated the appearance of a slow-migrating form of Pak1. This was caused by phosphorylation of Pak1 because the slow-migrating band was sensitive to phosphatase treatment. Thus, Akt phosphorylates Pak in vitro and stimulates phosphorylation in vivo. To test whether Akt activated Pak1, we performed kinase assays with an exogenous substrate (Fig. 2). Akt also phosphorylates many of the commonly used substrates for Pak1, such as P47Phox, histone H4, and myelin basic protein (data not shown). However, we found that a fragment of Bad, which is a substrate for both Akt and Pak1, could be mutated to distinguish Pak1 from Akt in these assays. Akt phosphorylates Bad on serine 136, whereas other kinases, including Pak, phosphorylate Bad on serine 112 (43). However, Pak phosphorylates other sites on Bad, enabling us to use a mutant Bad as a substrate to measure Pak activity in the presence of Akt. We found that mutating the two known phosphorylation sites on Bad generated a Pak1 substrate, BadA112, A136, that was not phosphorylated by Akt. When we tested BadA112, A136 in Pak kinase assays, we found that Akt stimulated incorporation of phosphate into the substrate. Incorporation was dependent on a functional Pak1, since neither KD Pak1 nor Akt alone stimulated phosphorylation. Akt activation of Pak1 was not as strong as activation by Cdc42 or Rac (data not shown for Rac). We conclude from these experiments that Akt can phosphorylate Pak1 directly and that this phosphorylation modestly stimulates Pak1 activity.
FIG. 1.
Akt phosphorylates PAK1 in vitro and in vivo. (A) In vitro kinase assay. WT or KD PAK1 (0.1 μg) from baculovirus was incubated in the presence or absence of 0.4 μg of Akt and [γ-32P]ATP in an in vitro kinase assay. The reaction mixture was separated by SDS-10% PAGE, and incorporation of 32P was detected by autoradiography. (B) Cell transfection assay. COS-7 cells were transfected with KD Pak1 (Pak1 K299R) and constitutively active Akt (MyrAkt) or DNAkt. The Myc-Pak1 was immunoprecipitated with Myc antibody and then separated as above and blotted with Myc antibody. The sample labeled MyrAkt +Pptase was treated with calf intestinal alkaline phosphatase and potato phosphatase after immunoprecipitation and prior to electrophoresis. +, present; −, absent.
FIG. 2.
Akt modestly activates Pak1 in vitro. Baculovirus PAK1 (0.2 μg) was incubated with either 0.4 μg of Akt or 0.8 μg of GST-Cdc42L61 (GTP or GDP loaded). Four micrograms of GST-Bad (amino acids 104 to 141) S112/136A double mutant (labeled Bad Mt), a Pak-specific substrate, was used as the substrate in a kinase assay. The reaction mixture was separated by SDS-10% PAGE and detected by autoradiography. +, present; −, absent.
Serine 21 is an Akt phosphorylation site.
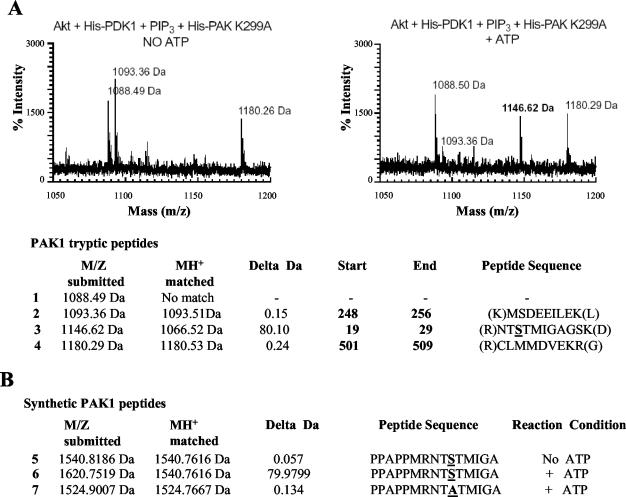
The recognition site for most Akt substrates is the motif RXRXXS/T (38, 54). This motif was not found by inspecting the sequence of Pak1. Therefore, we mapped the site(s) on Pak1 phosphorylated by Akt. We treated a KD Pak1 with Akt, isolated Pak1 by immunoprecipitation, digested the isolated protein with trypsin, and then subjected the proteolytic fragments to matrix-assisted laser desorption ionization (mass spectrometry) (Fig. 3). With this technology, we were able to identify 80% of the predicted peptides, although we were unable to detect a peptide at 1,067.2 Da, which would contain amino acids 19 to 29 of Pak1. However, when we treated Pak1 with activated Akt, we found a peak at 1,146.62 Da (Fig. 3A, right panel and line 3). This is the size predicted if the amino acid 19 to 29 peptide was phosphorylated. No differences were observed between the Akt-treated and untreated samples other than the appearance of this new peak. This experiment suggested that Akt could phosphorylate the full-length Pak1 between amino acids 19 and 29.
FIG. 3.
Mass spectroscopy identifies serine 21 as a phosphorylation site by Akt in vitro. Hemagglutinin-Akt (WT) was immunoprecipitated from COS-7 cell lysates and incubated with 0.1 μg of His-PDK-1, lipid vesicles containing phosphatidylcholine/phosphatidylinositol-3,4,5-triphosphate (PIP3) at a ratio of 99:1 and either catalytically inactive His-PAK1 K299A (1.5 μg/μl) (A) or synthetic PAK1 peptides (1 mg/ml) (B) in the absence or presence of 100 μM ATP for 30 min at 30°C. The appearance of a peak at 1,146.62 Da correlates with an 80-Da increase in the predicted MH+ corresponding to the phosphorylation of serine or threonine within amino acids 19 to 29 of PAK1. Observed peptides and their corresponding tryptic fragments in PAK1 K299A are shown in lines 1 to 4. The peptide corresponding to the unphosphorylated serine 21 (MH+ = 1,066.52 Da) was not observed under any conditions. (B) To determine whether serine 21 is the Akt phosphorylation site, synthetic PAK1 peptides were incubated with Akt as described above. No mass shift was observed when ATP was omitted (line 5); however, a shift of 80 Da was observed in the presence of ATP (line 6). Mutation of the serine corresponding to position 21 in PAK1 to alanine failed to become phosphorylated, indicating that serine 21 was the site of Akt phosphorylation (line 7).
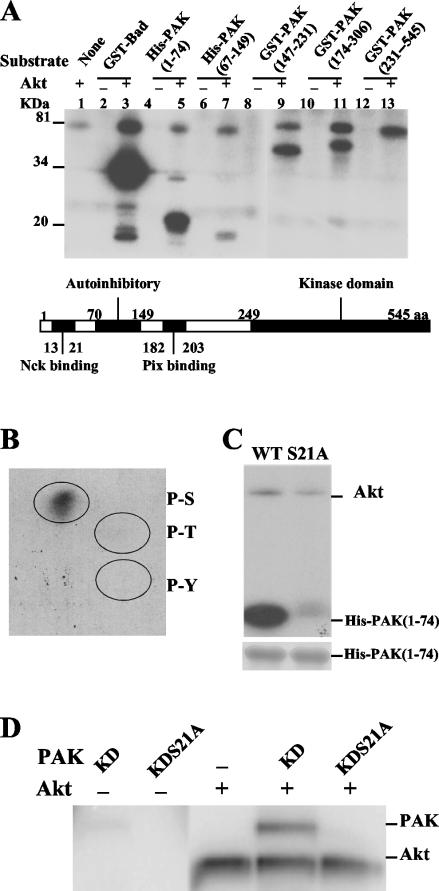
We also tested a number of Pak1 fragments for phosphorylation by Akt (Fig. 4A). Overlapping fragments of Pak1 covering the entire protein were tested. Akt did not phosphorylate a fragment from the catalytic region (constructed in a KD mutant to prevent autophosphorylation). Akt did phosphorylate two fragments from the central region of Pak1 encompassing amino acids 147 to 231 and 174 to 306, suggesting that there may be one or more sites in the region from amino acids 147 to 306. However, phosphorylation of these fragments was weak and they were not implicated by mass spectrometry, so they were not pursued. The fragment that was the best substrate for Akt expressed amino acids 1 to 74 of Pak1 (Fig. 4A). This fragment also encompassed amino acids 19 to 29, which were implicated by mass spectrometry as described above. Furthermore, a peak corresponding to the phosphorylated amino acid 19 to 29 peptide was observed when the phosphorylated amino acid 1 to 74 fragment was analyzed by matrix-assisted laser desorption ionization (mass spectrometry) (data not shown). We determined by a phospho-amino acid analysis that the amino acid residue phosphorylated on the amino acid 1 to 74 fragment was a serine (Fig. 4B). An inspection of the sequence revealed two serines in the amino acid 19 to 29 peptide at positions 21 and 28. Serine 21, but not serine 28, is a highly conserved amino acid found in all three group A Pak isoforms. Serine 21 is also phosphorylated when Pak1 is activated in vitro by autophosphorylation and in vivo through multiple mechanisms. Therefore, we performed mutagenesis to determine if mutating S21 affected phosphorylation by Akt. We found that the S21A mutation in the amino acid 1 to 74 fragment was phosphorylated much less readily than the original fragment (Fig. 4C). Moreover, the result that purified full-length KD S21A mutant Pak was not phosphorylated by Akt strongly indicates that S21 is the major phosphorylation site (Fig. 4D).
FIG. 4.
Serine 21 of Pak1 is the Akt phosphorylation site in vitro. (A) Four micrograms of each PAK1 fragment from E. coli was incubated in the presence or absence of 0.4 μg of Akt in a kinase assay. The reaction mixture was separated by SDS-12% PAGE, and the phosphorylation of those PAK fragments was detected by autoradiography. (B) Phosphoserine on His-PAK (amino acids 1 to 74) was detected by phosphoamino acid analysis after Akt treatment. His-PAK (amino acids 1 to 74) was phosphorylated with Akt in vitro and separated by SDS-12% PAGE. The protein was isolated, subjected to acid hydrolysis, and analyzed on a two-dimensional thin-layer chromatography plate (7). The thin-layer chromatography plate was first stained with ninhydrin to visualize phosphoamino acid markers and then exposed to X-ray film. P-S, P-T, and P-Y represent phosphoserine, phosphothreonine, and phosphotyrosine, respectively. (C) Mutation of serine 21 to alanine prevents Akt phosphorylation of the PAK fragment in vitro. Four micrograms of purified WT or S21A His-PAK (amino acids 1 to 74) was used as the substrate in the presence of 0.4 μg of Akt in a kinase assay. The reaction mixture was separated by SDS-12% PAGE, the gel was stained with Coomassie brilliant blue to confirm equalloading (lower panel), and phosphorylation of His-PAK (amino acids 1 to 74) was detected by autoradiography (upper panel). (D) Baculovirus KD PAK (0.1 μg) or its S21A mutant was incubated with 0.4 μg of Akt in a kinase assay, the reaction mixture was separated by SDS-10% PAGE, and incorporation of 32P was detected by autoradiography. +, present; −, absent.
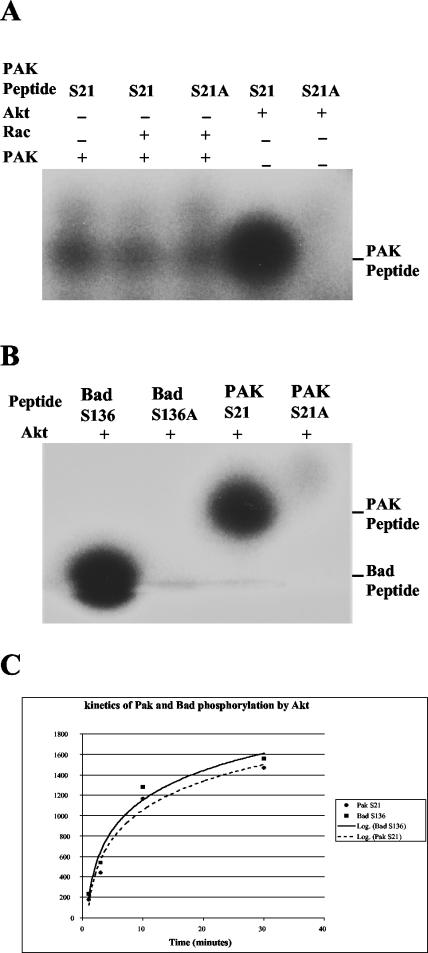
To compare Pak with another Akt substrate, we synthesized a peptide corresponding to amino acids 11 to 26 (PPAPPMRNTSTMIGA) and compared it with a peptide derived form Bad. Akt phosphorylated the S21 peptide to levels comparable to a peptide substrate derived from the Akt recognition site in Bad surrounding amino acid 136 (Fig. 5B). Control peptides with serine-to-alanine changes (S21A and S136A) were both very poor substrates for Akt. A mass spectrometry analysis found that the phosphorylated peptide shifted by 80.10 Da, demonstrating that only a single site is phosphorylated on the S21 peptide (Fig. 3B, lines 5 to 7). Interestingly, Pak1 phosphorylated neither the WT peptide nor the S21A peptide, although S21 has been reported to be autophosphorylated on Pak (Fig. 5A) (31). To exclude the possibility that the result shown in Fig. 5B was from saturation of the assay, a kinetics assay with less Akt (0.2 μg) was done to test the relative efficiency of phosphorylation by Akt for the Pak and Bad peptides. The results from the time course indicated that both peptides followed similar kinetics (Fig. 5C).
FIG. 5.
Serine 21 of PAK1 is a weak autophosphorylation site but a good phosphorylation site for Akt. (A) PAK S21 peptides were incubated with Rac, PAK1, or Akt. Only Akt phosphorylated the peptides efficiently. Twenty micrograms of peptides was incubated with 0.4 μg of Akt, 0.1 μg of PAK1, or 0.4 μg of Rac together with [γ-32P]ATP. The reaction mixture was separated on a 10 to 20% Tris-Tricine gel, and phosphorylation of the peptides was detected by autoradiography. (B) Comparison of a Pak S21 peptide with Bad S136, a known Akt substrate. Twenty micrograms of Bad S136 peptides (PFRGRSRSAP or S136A mutant PFRGRSRAAP) or Pak S21 peptides (PPAPPMRNTSTMIGA or S21A mutant PPAPPMRNTATMIGA) were used as substrates, along with [γ-32P]ATP and 0.4 μg of active Akt in a kinase assay conducted as described for panel A. (C) Kinetics of phosphorylation of PAK and Bad peptides by Akt. Twenty micrograms of Pak S21 or Bad S136 peptides was incubatedwith 0.2 μg of Akt in a kinase assay through a time course of 1, 3, 10, and 30 min. The samples were run on a 10 to 20% Tricine gel, and the signals were quantified with a phosphorimager and processed with Excel. +, present; −, absent.
We also raised a phosphospecific antibody to serine 21. The antibody had a >10,000-fold-higher titer to a phosphorylated peptide. When either WT or KD recombinant Pak1 was treated with Akt, the antibody recognized the protein (Fig. 6, lanes 5 and 7). No band was observed when serine 21 was mutated to alanine (Fig. 6, lanes 8 and 9). Interestingly, the antibody showed only a marginal recognition of Rac- and Cdc42-treated Pak1 (Fig. 6, lanes 1 and 2; data not shown for Cdc42). Kinase-active Pak was more readily recognized than KD Pak, suggesting a possibility that kinase-active Pak1 is in a conformation more available to Akt than the KD protein. From this mapping study we conclude that Akt phosphorylates Pak1 primarily at serine 21, although it may also phosphorylate other minor sites on Pak1. Additionally, most, but not all, of the phosphate on serine 21 is a consequence of exogenous phosphorylation.
FIG. 6.
Analysis of PAK1 serine 21 phosphorylation with a phospho-S21-specific antibody. Antibody specific for the phosphorylated form of PAK S21 was raised as described in Materials and Methods. The phosphorylation of S21 was detected in a Western blot with a 1:1,000 dilution of the phosphospecific antibody from samples incubated in kinase buffer with 20 μM cold ATP. PAK1 (WT, KD, or S21A mutants) (0.1 μg) and Rac or Akt (0.4 μg) were incubated for 30 min at 30°C, separated on a 10% gel, and blotted. +, present; −, absent.
Akt phosphorylation of Pak reduces Nck binding.
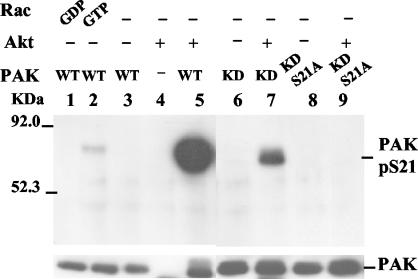
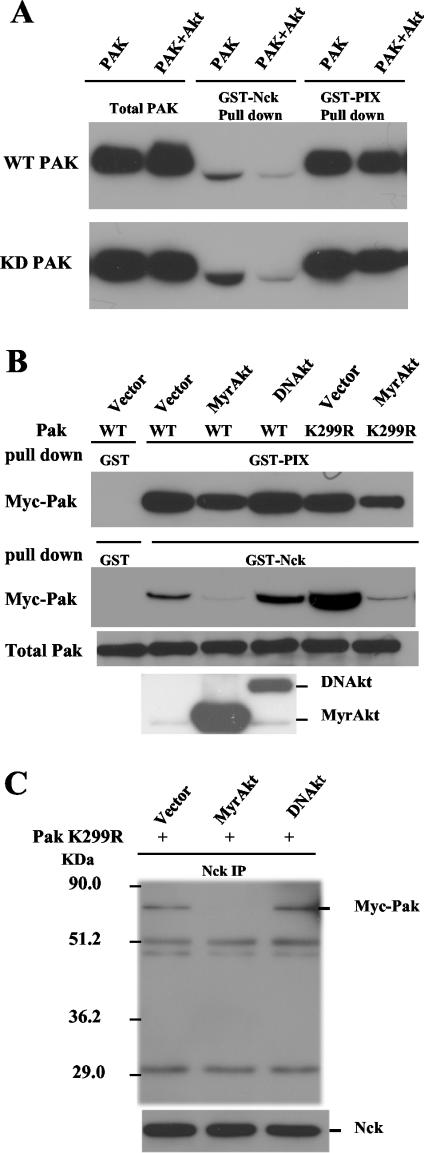
Phosphorylation of serine 21 prevents association of Pak1 with the adaptor protein Nck (57). Nck binds Pak1 at a proline-rich SH3 binding site in the region near serine 21. Since Akt phosphorylates Pak1 on serine 21, we reasoned that Akt might influence Nck binding. To test this possibility, we treated Pak1 with Akt and then tested whether it still bound to Nck. Nck binding was determined by performing Western blots for Pak1 after it was subjected to a GST pull-down assay with the SH3 domain from Nck. We found greatly reduced binding to Nck after recombinant Pak1 was incubated with Akt (Fig. 7A). Similar results were observed when Pak1 was expressed in COS-7 cells (Fig. 7B). In both experiments we were able to rule out the possibility that the effects were caused indirectly after autophosphorylation of Pak1 because we observed reduced Nck binding when the KD Pak was used instead of WT Pak. To determine whether the reduction in the Nck binding efficiency caused by Akt could change the level of Nck associated with Pak1 in cells, we determined whether Akt expression could release endogenous Nck from Pak1. We performed immunoprecipitations with an anti-Nck antibody from extracts of cells transfected with Pak1 and Akt. When the cells were transfected with the KD Pak1, we observed Pak1 in the Nck immunoprecipitates. The amount of Pak1 coprecipitating with the Nck antibody was greatly reduced when active Akt was cotransfected (Fig. 7C). The DNAkt had no effect on Pak1 association with Nck. Together, these studies suggest that Akt stimulates Pak1 by direct phosphorylation at serine 21 and that one consequence of phosphorylation at serine 21 by Akt is to release the Nck adaptor protein from Pak1.
FIG. 7.
Akt reduces Pak binding with Nck both in vitro and in vivo. (A) Akt treatment of PAK1 reduced the amount of PAK1 pulled down by GST-Nck. Baculovirus PAK1 (WT or KD) (0.1 μg) was incubatedwith 0.5 μg of Akt in kinase assay buffer in the presence of ATP, then PAK was pulled down with GST-Nck. The PAK bound to GST-Nck was detected with a Pak1 antibody on a Western blot. GST-PIX, a GST fusion SH3 domain of PIX responsible for binding PAK1 at a distinct site was used as a control. (B) Akt expression in COS-7 cells leads to reduced binding of Myc-Pak1 with Nck in a GST-Nck pull-down assay with cell lysates. Myc-Pak was pulled down with GST-Nck from cells expressing Myc-Pak (or Myc-Pak1 K299R) along with either MyrAkt or DNAkt. The Myc-Pak bound to GST-Nck was detected with Myc antibody on a Western blot. Akt expression was confirmed with anti-hemagglutinin antibody on the bottom blot. (C) Coexpression of MyrAkt in COS-7 cells reduced Myc-Pak association with endogenous Nck in vivo. COS-7 cells were transfected with Myc-Pak K299R along with either MyrAkt or DNAkt. The endogenous Nck was then immunoprecipitated from the cell lysates, and the coprecipitated Myc-Pak was detected with Myc antibody on a Western blot. +, present.
Akt translocates Pak from focal adhesions.
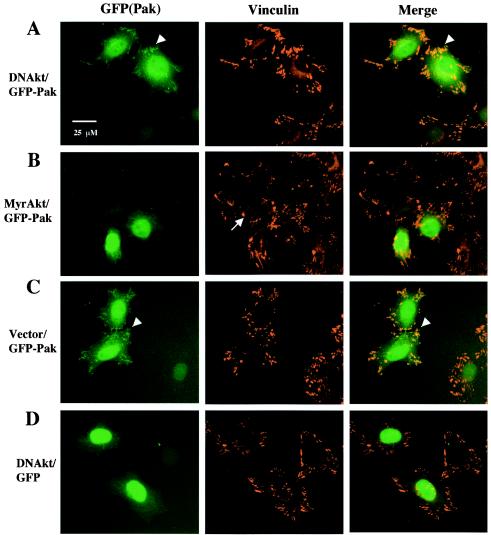
In vivo, the association of Pak with Nck and PIX is thought to translocate Pak to cell membranes and focal adhesions. To test whether Akt may regulate the localization of Pak, we used a Pak1 construct expressing a GFP-tagged protein to localize Pak (Fig. 8). In HeLa cells cotransfected with DNAkt (Fig. 8A) or vector (Fig. 8C) along with GFP-Pak, we consistently observed that in most cells a portion of GFP-Pak localized to focal adhesions (41 of 50 cells expressing GFP-Pak with DNAkt and 27 of 34 expressing GFP-Pak with vector), though a significant fraction of Pak was in the cytoplasm as well. In contrast, in cells cotransfected with MyrAkt (Fig. 8B), the percentage of cells with colocalization of Pak and vinculin was significantly reduced (4 of 37 cells expressing GFP-Pak1 with MyrAkt). Even in cells where there was some localization of GFP-Pak to focal adhesions, the colocalization of GFP-Pak and vinculin was generally less complete for MyrAkt cotransfected cells when compared with DNAkt and vector cotransfected cells (data not shown). GFP alone primarily localized to the nucleus area, and its localization was not changed by DNAkt or MyrAkt cotransfection (Fig. 8D; data not shown for MyrAkt). In addition, about half of the MyrAkt cotransfected cells (either with GFP-Pak or GFP alone, data not shown for latter) were also smaller and less spread out (Fig. 8B) than cells transfected with vector or DNAkt along with GFP-Pak or GFP. As documented above, we hypothesized that Akt was affecting Nck binding in vivo. To test whether effects of Akt on Pak localization might be caused by changes in Nck association, we used peptides to block the association of Pak with Nck. We synthesized peptides corresponding to the Nck binding site on Pak as well as an S21 phosphorylated form of the peptide. The peptides were synthesized as fusions to a sequence derived from the human immunodeficiency virus Tat protein which enables the peptides to penetrate membranes. The S21 peptide was more efficient than its phosphorylated form in interfering with Nck/Pak binding as confirmed in a GST pull-down assay (Fig. 9A). A fraction of the two Nck peptides were conjugated with FITC and tested with cells to confirm transport through membranes; after the addition of 20 μg of peptides/ml and incubation for 30 min, their entrance into HeLa and Rat1 cells was confirmed under a fluorescence microscope (data not shown). We found that the WT Nck binding peptide was more effective at reducing GFP-Pak localization to focal adhesions than the phosphopeptide; we found that the phosphopeptide was weakly active (data not shown). The partial activity may be because it still maintains some binding to Nck, even an S21A mutant peptide maintains some binding to Nck (57), so the S21 phosphopeptide may also maintain some binding. Another possibility is that the phosphopeptide was partially dephosphorylated in vivo by phosphatases.
FIG. 8.
Akt reduces the colocalization of GFP-Pak1 with focal adhesions in HeLa cells. HeLa cells were transfected with GFP-tagged Pak1 WT along with Akt. Vinculin was stained with vinculin antibody followed by Alexa-Fluor 594 goat anti-mouse antibody to localize the focal adhesions. In the cells expressing MyrAkt (B), GFP-Pak typically has a cytoplasmic localization, and some cells were smaller and did not spread as well (indicated by the arrow). In cells cotransfected with DNAkt (A) or vector (C), more GFP-Pak colocalizes to the focal adhesions as indicated by arrowheads (yellow in the merged images), whereas GFP alone primarily localized to the nucleus area and its localization was not changed by DNAkt (D) or MyrAkt (data not shown). The cells were observed under a Leica Polyvar 2 fluorescence microscope, and micrographs were taken with a Hamamatsu ORCA charge-coupled device camera operated with Improvision OPENLAB 2 software. Bar, 25 μm.
FIG. 9.
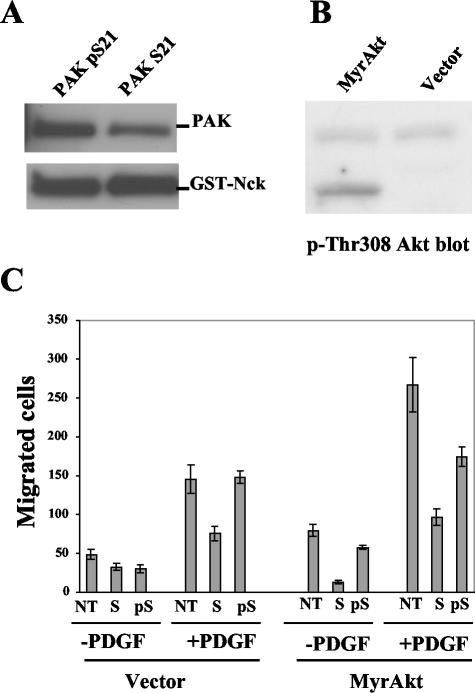
Effects of phospho- and dephospho-Pak S21 peptides on Nck-Pak binding and cell migration. (A) Pak S21 peptide is more efficient in breaking up Nck-Pak binding than its phospho form. Baculovirus Pak1 (0.1 μg) was incubated with 8 μl of 50% glutathione-Sepharose saturated with GST-Nck in the presence of 50 μg of the peptides/ml in lysis buffer and incubated overnight at 4°C. The beads were spun down and washed three times with the same buffer, and this was followed by the detection of Pak pulled down by GST-Nck on a Western blot with Pak antibody. The GST-Nck (bottom) was detected with a GST antibody. (B) Confirmation of MyrAkt expression in a MyrAkt stable cell line by Western blotting with a phospho-Thr 308 Akt antibody. Note that the transfected MyrAkt is smaller than the endogenous Akt. (C) Effects of peptides on cell migration. Rat1 stable cell lines were used in a transwell cell migration assay. An human immunodeficiency virus Tat domain was fused to the N terminus of Pak peptides to facilitate their entry into cells. About 20,000 cells were applied to each well precoated with fibronectin, and where indicated, the medium contains 25 μg of the Pak peptides/ml. Ten micrograms of PDGF/ml was added to the medium in the bottom part of the well, where indicated. After incubation for 2.5 h, cells were wiped off the upper side of the membrane with a cotton swab, then the membrane was stained with CMFDA, and migrating cells were counted under a Leica Polyvar 2 fluorescence microscope. Cell migration data were plotted as bar graphs (mean + standard deviation; n = 4). NT, no treatment; S, Pak S21 peptide; pS, Pak phospho-S21 peptide.
Role of Nck/Pak association in Akt-stimulated cell migration.
Our data so far suggest that Akt affects the association of Nck with Pak. While much is known about mechanisms by which Akt regulates cell survival by phosphorylation of specific targets, several recent studies have shown that Akt plays a role in cell migration as well (22, 37). Since Pak and Nck are both required for cell migration, we sought to test whether Akt uses the Nck/Pak complex to stimulate cell migration. We used a transwell assay system to test this model (Fig. 9C). Cells were transferred to the upper chamber of the transwell and allowed to migrate for 2.5 h. After the migration period, the cells were stained, and the cells that migrated to the lower chamber were counted. PDGF stimulated migration about threefold. We found, as previously reported, that cells expressing Akt had about a 50% higher migration rate (22, 37). PDGF stimulated the migration of the Akt expressing cells about 2.5-fold more than the nonexpressing cells. Importantly, migration of both cell lines was inhibited from two- to fivefold by the Nck peptides. The phosphopeptide was much less effective in inhibiting migration. Taken together, these data suggest that the Nck/Pak complex has a role in Akt-stimulated cell migration.
DISCUSSION
Akt has long been studied for its role in promoting cell survival through targets such as Bad and Forkhead, whereas Pak has been studied for its role in cell motility and the actin cytoskeleton. More recently, Pak was shown to promote cell survival, also through Bad, while Akt has been implicated in cell motility. However, the signaling events downstream of Akt that regulate cell motility are poorly defined. Activated Akt stimulates cell motility and dominant-negative Akt mutants inhibit cell motility, suggesting that Akt phosphorylates important regulators of the cytoskeleton (22, 37). One possible target is Rac; however, Akt phosphorylation of Rac1 inhibits GTP-binding activity and would inhibit motility (28). Our study suggests that Pak is an Akt target. Since many of our experiments were carried out with kinase-inactive forms of Pak and recombinant proteins, we can rule out indirect effects of Akt on Pak through small GTPases.
Effects of Akt on cell polarity.
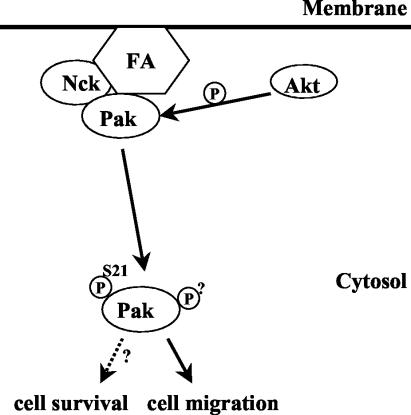
When neutrophils are treated with chemoattractants, PI 3-kinase rapidly synthesizes phospholipids at the leading edge (46). Akt then rapidly translocates to the leading edge because it contains a PH domain that binds the PI 3-kinase-derived phospholipids. The relevance of this recruitment is not known. One mechanism suggested by this study is that Akt phosphorylates Pak on serine 21 to direct Pak to new targets away from focal adhesions. In mammalian cells, a substantial body of evidence suggests that regulation of SH3 binding can modulate localization of Pak1 to direct cell polarity. Two known SH3 ligands of Pak1, Nck and PIX, both bind close to autophosphorylation sites, and phosphorylation of these sites prevents SH3 binding as well as Pak localization (57). Both Nck and PIX are required to direct Pak1 to focal contacts (33). Several observations suggest that the association of Nck with Pak is important for the effects of Pak on cell motility and polarity. Microinjection of Pak causes an asymmetric ruffling in fibroblasts, which was termed polarized cell ruffling. But microinjection of a Pak mutant that fails to bind Nck does not induce the polarized cell ruffling (45). Furthermore, the Tat-Nck fusion peptides can inhibit cell migration and prevent Pak from localizing to the leading edge of endothelial cells (27). Thus, Nck binding is apparently required to direct the effects of Pak1 to the leading edge where it participates in orienting cell polarity (Fig. 10).
FIG. 10.
A model for Akt regulation of Pak. Pak can form complexes with Nck and PIX through a distinct SH3 binding domain, which leads to the localization of Pak in focal adhesions (FA). Phosphorylation of serine 21 on Pak by Akt releases Pak from Nck binding and translocates Pak to the cytosol; this may limit the time Pak spends at the leading edge or facilitate recycling of Pak to and from the leading edge, thus stimulating cell migration.
The mechanism described above draws on the model proposed for cell motility in Dictyostelium, where an Akt→Pak signal regulates responses to the chemoattractant cyclic AMP (cAMP). In this organism, Akt phosphorylates Pak and, through some unknown mechanism, translocates Pak from the leading edge to the trailing edge. This is thought to be necessary for polarizing cell movement towards the chemoattractant. Akt- and Pak-null mutants of Dictyostelium have a strikingly similar phenotype (11, 35). While both respond to cAMP by extending lamellipodia, the lamellipodia are not polarized to the leading edge, and subsequently, neither mutant orients its movements towards the cAMP source. Moreover, Akt knockout cells fail to translocate Pak from the cell membrane. However, the mechanism of redirection of Pak remains to be determined in Dictyostelium. It may involve SH3 interactions with homologs of Nck and PIX, because the Akt phosphorylation site is located in a proline-rich region, but no SH3 proteins have been reported. In this model, Akt and Pak may act by directing cell polarity and not necessarily chemokinesis.
Effects of Akt on cell spreading and adhesion.
Another mechanism that may contribute to the effects of Akt on cell motility may be through the regulation of cell spreading by Pak. When cells are treated with trypsin to detach them from plates, they become round but spread again when they reattach. Cell spreading is blocked when Pak is overexpressed (42). Paks can phosphorylate myosin light chain kinase, which has been proposed as a mechanism to explain the effects of Pak on cell spreading. It may be relevant to the effects of Akt on cell motility that we find that Akt expression reduces cell spreading somewhat, consistent with an interaction with Pak (Fig. 8B). Cell spreading correlates in a biphasic way with cell motility. Cells that spread poorly do not adhere to substrate tightly enough to extend stable lamellae, while cells that spread well adhere too tightly to easily release their adhesions from the trailing edge. Cells that spread moderately well move the fastest (39). As activated Pak1 stimulates stress fiber rearrangement and dissolution, Pak1 may also depolymerize actin in the trailing parts of the cell during cell motility to help them detach as they move. Alternatively, Pak may stimulate contraction at the trailing edge by regulating the phosphorylation of myosin (10, 15). At the other extreme of the effects of Pak on cell motility, membrane targeting of Pak stimulates polarized ruffling and neurite extension in PC12 cells (13). Thus, the net effect of Akt may be to limit the time Pak spends at the leading edge or facilitate recycling of Pak to and from the leading edge in such a way as to maintain an optimal amount of cell spreading for cell motility. In this model, Akt stimulates motility by stimulating chemokinesis and not necessarily cell polarity.
Mechanism of Akt regulation of Pak.
Serine 21 was originally identified as an autophosphorylation site. However, several observations suggest that it is a relatively poor autophosphorylation site. First, when compared to other sites, it is weakly autophosphorylated on Rac- and Cdc42-stimulated Pak, and in some constructs of Pak1, serine 21 autophosphorylation is not observed at all (31). Second, a peptide derived from the region is a very poor substrate for Pak (Fig. 5A). Third, growth factors can activate Pak1 without stimulating phosphorylation of serine 21, and Pak1/Nck complexes can be isolated from many cells stimulated with growth factors or attached via integrins to matrixes (16, 57). Conversely, detachment can stimulate phosphorylation of serine 21 and release Nck without activating Pak (23). Together, these suggest that phosphorylation of serine 21 may only come about when very high levels of Rac are used to activate Pak1 and that, under physiological conditions, Akt is the primary way that Pak1 is phosphorylated at this site. Similarly, threonine 423 is another autophosphorylation site found in Pak1 that is probably not autophosphorylated in vivo but by PDK-1 (26).
Pak1 is activated by Akt in vitro, although not as strongly as it is activated by Rac or Cdc42. Amino acids 70 to 149 comprise a region of Pak called the autoinhibitory region, because this portion binds and inhibits catalytic activity. Small GTPases bind near the autoinhibitory region to release it from the catalytic domain. Considering serine 21 is near but outside the autoinhibitory domain, the weak activation is not surprising. While phosphorylation of other minor sites might contribute to activation, these sites were not detected in the full-length protein. In vivo, the situation is likely to be more complicated, a major effect of Akt may be to recruit PDK-1 to Pak. Thus, Akt is not likely to stimulate the activity of Pak on its own, and in some cases it may even eventually reduce Pak activity by driving it away from small GTPases in the membrane. Interestingly, we observed that Akt stimulates phosphorylation of threonine 423 in the presence of PDK-1 (data not shown). Threonine 423 is in the kinase domain of Pak1 and is usually blocked by the amino regulatory domain (29, 56). Since Akt can potentiate phosphorylation at threonine 423, it is likely that phosphorylation at serine 21 and perhaps other sites also relieve inhibition to allow more efficient access to PDK-1.
Several studies have defined an optimal motif for Akt phosphorylation sites as RXRXXS/T (38, 54). This sequence is not present in the region surrounding serine 21. The sequence surrounding S21 is PMRNTS. This sequence has a proline instead of the arginine at the −5 position. Nevertheless, we provide data with the full-length Pak1, Pak1 fragments, and also synthetic peptides that Akt phosphorylates this site. Phosphorylation of a synthetic peptide was comparable to a substrate derived from Bad, a well-documented target of Akt. Thus, the sequence PXRXXS may be another sequence motif recognized by Akt. Finally, although we provide evidence in this study that Akt is a serine 21 kinase, Akt is member of a group of kinases collectively known as the AGC kinases, which consist of protein kinases A, C, and G. Studies are under way to determine whether other family members also regulate Pak through the mechanism described in this study.
Acknowledgments
We thank Alex Toker for the baculovirus Akt and PDK-1 expression systems and Jonathan Chernoff for WT and KD baculovirus Pak constructs. We also thank Amita Sehgal and members of the lab for helpful discussions and for comments on the manuscript.
This work was supported by grants from the NIH to G.B. (GM44428 and GM39434) and to J.F. (GM48241), and by grants to J.F. from the DOD neurofibromatosis program (DAMD17-01-1-0719) and the American Cancer Society (RPG-99-102-01-TBE). C.C.K. was supported by the Heme and Blood Proteins training grant. B.H.F. was supported by NIH training grant CA09677.
REFERENCES
- 1.Abo, A., J. Qu, J. Cammarano, C. Dan, A. Fritsch, V. Baud, B. Belisle, and A. Minden. 1998. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 17:6527-6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagrodia, S., B. Derijard, R. J. Davis, and R. A. Cerione. 1995. Cdc42 and Pak-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 270:27995-27998. [DOI] [PubMed] [Google Scholar]
- 3.Bagrodia, S., S. J. Taylor, K. A. Jordan, L. Van Aelst, and R. A. Cerione. 1998. A novel regulator of p21-activated kinases. J. Biol. Chem. 273:23633-23636. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee, M., D. Worth, D. M. Prowse, and M. Nikolic. 2002. Pak1 phosphorylation on T212 affects microtubules in cells undergoing mitosis. Curr. Biol. 12:1233-1239. [DOI] [PubMed] [Google Scholar]
- 5.Bar-Sagi, D., and A. Hall. 2000. Ras and Rho GTPases: a family reunion. Cell 103:227-238. [DOI] [PubMed] [Google Scholar]
- 6.Bokoch, G. M., Y. Wang, B. P. Bohl, M. Sells, L. A. Quilliam, and U. G. Knaus. 1996. Interaction of the Nck adapter protein with p21-activated kinase (PAK1). J. Biol. Chem. 271:25746-25749. [DOI] [PubMed] [Google Scholar]
- 7.Boyle, W. J., P. Van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110-149. [DOI] [PubMed] [Google Scholar]
- 8.Brown, J. L., L. Stowers, M. Baer, J. Trejo, S. Coughlin, and J. Chant. 1996. Human Ste20 homologue hPAK1 links GTPases to the JNK MAP kinase pathway. Curr. Biol. 6:598-605. [DOI] [PubMed] [Google Scholar]
- 9.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 10.Brzeska, H., U. G. Knaus, Z.-Y. Wang, G. M. Bokoch, and E. D. Korn. 1997. p21-activated kinase has substrate specificity similar to Acanthamoeba myosin I heavy chain kinase and activates Acanthamoeba myosin I. Proc. Natl. Acad. Sci. USA 94:1092-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung, C. Y., G. Potikyan, and R. A. Firtel. 2001. Control of cell polarity and chemotaxis by Akt/PKB and PI3 kinase through the regulation of PAKα. Mol. Cell 7:937-947. [DOI] [PubMed] [Google Scholar]
- 12.Daniels, H., F. T. Zenke, and G. M. Bokoch. 1999. αPix stimulates p21-activated kinase activity through exchange factor-dependent and independent mechanisms. J. Biol. Chem. 274:6047-6050. [DOI] [PubMed] [Google Scholar]
- 13.Daniels, H. R., P. S. Hall, and G. M. Bokoch. 1998. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 17:754-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta, S. R., H. Dudek, X. Tao, S. Master, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-341. [DOI] [PubMed] [Google Scholar]
- 15.De la Roche, M. A., and G. P. Cote. 2001. Regulation of Dictyostelium myosin I and myosin II. Biochim. Biophys. Acta 1525:245-261. [DOI] [PubMed] [Google Scholar]
- 16.Del Pozo, M. A., L. S. Price, N. B. Alderson, X.-D. Ren, and M. A. Schwartz. 2000. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 19:2008-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Downward, J. 1999. How BAD phosphorylation is good for survival. Nat. Cell Biol. 1:E33-E35. [DOI] [PubMed] [Google Scholar]
- 18.Downward, J. 2001. The ins and outs of signalling. Nature 411:759-762. [DOI] [PubMed] [Google Scholar]
- 19.Frost, J. A., H. Steen, P. Shapiro, T. Lewis, N. Ahn, P. E. Shaw, and M. H. Cobb. 1997. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 16:6426-6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galisteo, M. L., J. Chernoff, Y.-C. Su, E. Y. Skolnik, and J. Schlessinger. 1996. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J. Biol. Chem. 271:20997-21000. [DOI] [PubMed] [Google Scholar]
- 21.Gnesutta, N., J. Qu, and A. Minden. 2001. The serine/threonine kinase PAK4 prevents caspase activation and protects cells from apoptosis. J. Biol. Chem. 276:14414-14419. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi, M., N. Masuyama, Y. Fukui, A. Suzuki, and Y. Gotoh. 2001. Akt mediates Rac/Cdc42-regulated cell motility in growth factor-stimulated cells and in invasive PTEN knockout cells. Curr. Biol. 11:1958-1962. [DOI] [PubMed] [Google Scholar]
- 23.Howe, A. 2001. Cell adhesion regulates the interaction between Nck and p21-activated kinase. J. Biol. Chem. 276:14541-14544. [DOI] [PubMed] [Google Scholar]
- 24.Jakobi, R., E. Moertl, and M. A. Koeppel. 2001. p21-activated protein kinase γ-PAK suppresses programmed cell death of BALB3T3 fibroblasts. J. Biol. Chem. 276:16624-16634. [DOI] [PubMed] [Google Scholar]
- 25.King, A. J., H. Sun, B. Diaz, D. Barnard, W. Miao, S. Bagrodia, and M. S. Marshall. 1998. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 396:180-183. [DOI] [PubMed] [Google Scholar]
- 26.King, C. C., M. M. Gardiner, F. T. Zenke, B. P. Bohl, A. C. Newton, B. A. Hemmings, and G. M. Bokoch. 2000. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1). J. Biol. Chem. 275:41201-41209. [DOI] [PubMed] [Google Scholar]
- 27.Kiosses, W. B., J. Hood, S. Yang, M. E. Gerritsen, D. A. Cheresh, N. Alderson, and M. A. Schwartz. 2002. A dominant-negative p65 PAK peptide inhibits angiogenesis. Circ. Res. 90:697-702. [DOI] [PubMed] [Google Scholar]
- 28.Kwon, T., D. Y. Kwon, J. Chun, J. H. Kim, and S. S. Kang. 2000. Akt protein kinase inhibits Rac1-GTP binding through phosphorylation of serine 71 of Rac1. J. Biol. Chem. 275:423-428. [DOI] [PubMed] [Google Scholar]
- 29.Lei, M., W. Lu, W. Meng, M.-C. Parrini, M. J. Eck, B. J. Mayer, and S. C. Harrison. 2000. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell 102:387-397. [DOI] [PubMed] [Google Scholar]
- 30.Lu, W., S. Katz, R. Gupta, and B. J. Mayer. 1997. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr. Biol. 7:85-94. [DOI] [PubMed] [Google Scholar]
- 31.Manser, E., H.-Y. Huang, T.-H. Loo, X.-Q. Chen, J.-M. Dong, T. Leung, and L. Lim. 1997. Expression of constitutively active α-Pak reveals effects of the kinase on actin and focal complexes. Mol. Cell. Biol. 17:1129-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manser, E., T. Leung, H. Salihuddin, Z.-S. Zhao, and L. Lim. 1994. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367:40-46. [DOI] [PubMed] [Google Scholar]
- 33.Manser, E., T.-S. Loo, C.-G. Koh, Z.-S. Zhao, X.-Q. Chen, L. Tan, T. Leung, and L. Lim. 1998. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell 1:183-192. [DOI] [PubMed] [Google Scholar]
- 34.Marshall, M. S. 1995. Ras target proteins in eukaryotic cells. FASEB J. 9:1311-1318. [DOI] [PubMed] [Google Scholar]
- 35.Meili, R., C. Ellsworth, S. Lee, T. B. K. Reddy, H. Ma, and R. A. Firtel. 1999. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 18:2092-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mira, J.-P., V. Benard, J. Grofen, L. C. Sanders, and U. G. Knaus. 2000. Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc. Natl. Acad. Sci. USA 97:185-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morales-Ruiz, M., D. Fulton, G. Sowa, L. R. Languino, Y. Fujio, K. Walsh, and W. C. Sessa. 2000. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ. Res. 86:892-896. [DOI] [PubMed] [Google Scholar]
- 38.Obata, T., M. B. Yaffe, G. G. Leparc, E. T. Piro, H. Maegawa, A. Kashiwagi, R. Kikkawa, and L. C. Cantley. 2000. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J. Biol. Chem. 275:36108-36115. [DOI] [PubMed] [Google Scholar]
- 39.Palecek, S. P., J. C. Loftus, M. H. Ginsberg, D. A. Laffenburger, and A. F. Horwitz. 1997. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 385:537-540. [DOI] [PubMed] [Google Scholar]
- 40.Puto, L. A., K. Pestonjamasp, C. C. King, and G. M. Bokoch. 2003. p21-activated kinase 1 (PAK1) interacts with the Grb2 adapter protein to couple to growth factor signaling. J. Biol. Chem. 278:9388-9393. [DOI] [PubMed] [Google Scholar]
- 41.Qu, J., M. S. Cammarano, Q. Shi, K. C. Ha, P. Lanerolle, and A. Minden. 2001. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol. Cell. Biol. 21:3523-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanders, L. C., F. Matsumura, G. M. Bokoch, and P. Lanerolle. 1999. Inhibition of myosin light chain kinase by p21-activated kinase. Science 283:2083-2085. [DOI] [PubMed] [Google Scholar]
- 43.Schurmann, A., A. F. Mooney, L. C. Sanders, M. A. Sells, H.-G. Wang, J.-C. Reed, and G. M. Bokoch. 2000. p21-activated kinase (PAK1) phosphorylates the death agonist Bad and protects cells from apoptosis. Mol. Cell. Biol. 20:453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sells, M. A., and J. Chernoff. 1997. Emerging from the Pak: p21-activated protein kinase family. Trends Cell Biol. 7:162-167. [DOI] [PubMed] [Google Scholar]
- 45.Sells, M. A., U. G. Knaus, S. Bagrodia, D. M. Ambrose, G. M. Bokoch, and J. Chernoff. 1997. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 7:202-210. [DOI] [PubMed] [Google Scholar]
- 46.Servant, G., A. D. Weiner, P. Herzmark, T. Balla, J. W. Sedat, and H. R. Bourne. 2000. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287:1037-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith, D. B., and K. S. Johnson. 1988. Single step purification of polypeptides expressed in Escherichia coli as fusion proteins with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 48.Stoletov, K. V., K. E. Ratcliffe, S. C. Spring, and B. L. Terman. 2001. NCK and PAK participate in the signaling pathway by which vascular endothelial growth factor stimulates the assembly of focal adhesions. J. Biol. Chem. 276:22748-22755. [DOI] [PubMed] [Google Scholar]
- 49.Tang, Y., Z. Chen, D. Ambrose, J. Liu, J. B. Gibbs, J. Chernoff, and J. Field. 1997. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol. Cell. Biol. 17:4454-4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang, Y., S. Marwaha, J. L. Rutkowski, G. I. Tennekoon, P. C. Phillips, and J. Field. 1998. A role for Pak protein kinases in Schwann cell transformation. Proc. Natl. Acad. Sci. USA 95:5139-5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang, Y., J. Yu, and J. Field. 1999. Signals from the Ras, Rac and Rho GTPases converge on the Pak protein kinase in Rat-1 fibroblasts. Mol. Cell. Biol. 19:1881-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang, Y., H. Zhou, A. Chen, R. N. Pittman, and J. Field. 2000. The Akt proto-oncogene links Ras to Pak and cell survival signals. J. Biol. Chem. 275:9106-9109. [DOI] [PubMed] [Google Scholar]
- 53.Thiel, D. A., M. K. Reeder, A. Pfaff, T. R. Coleman, M. A. Sells, and J. A. Chernoff. 2002. Cell cycle regulated phosphorylation of p21-activated kinase 1. Curr. Biol. 12:1227-1232. [DOI] [PubMed] [Google Scholar]
- 54.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346:561-575. [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, S., J. Han, M. A. Sells, J. Chernoff, U. G. Knaus, R. J. Ulevitch, and G. M. Bokoch. 1995. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J. Biol. Chem. 270:23934-23936. [DOI] [PubMed] [Google Scholar]
- 56.Zhao, Z.-S, E. Manser, X. Chen, C. Chong, T. Leung, and L. Lim. 1998. A conserved negative regulatory region in αPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol. Cell. Biol. 18:2153-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao, Z.-S., E. Manser, and L. Lim. 2000. Interaction between PAK and Nck: a template for Nck targets and role of PAK autophosphorylation. Mol. Cell. Biol. 20:3906-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]