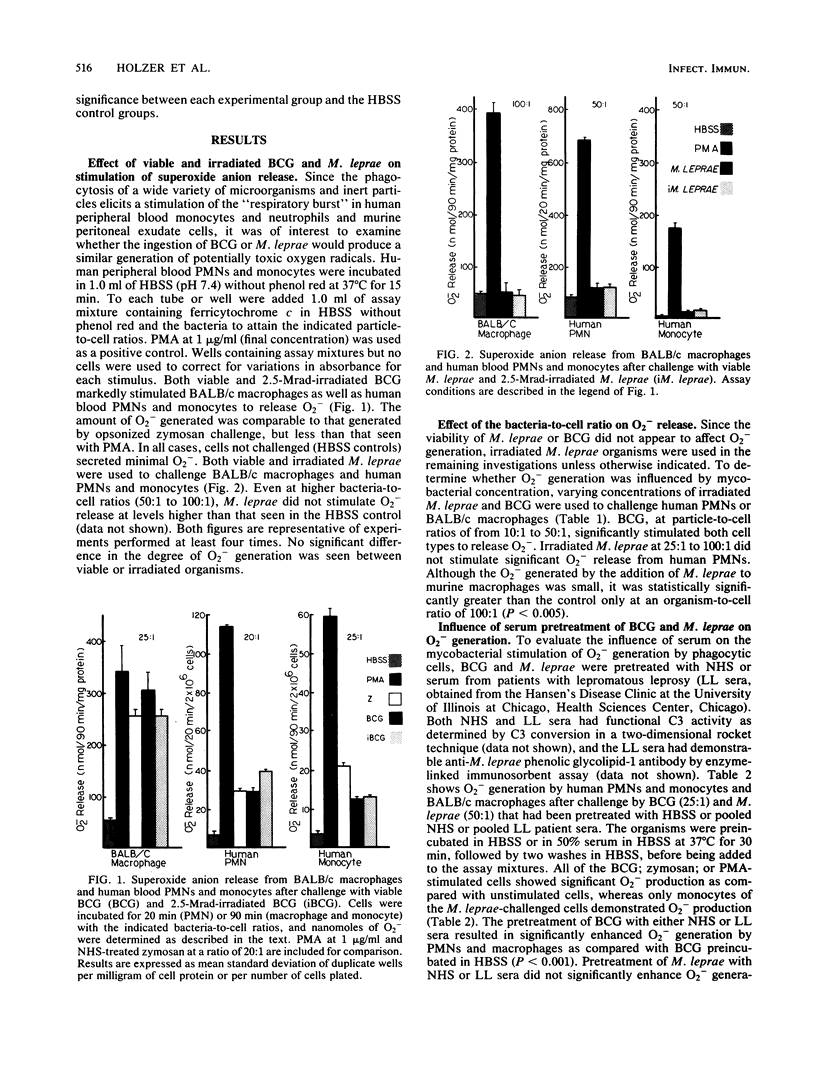
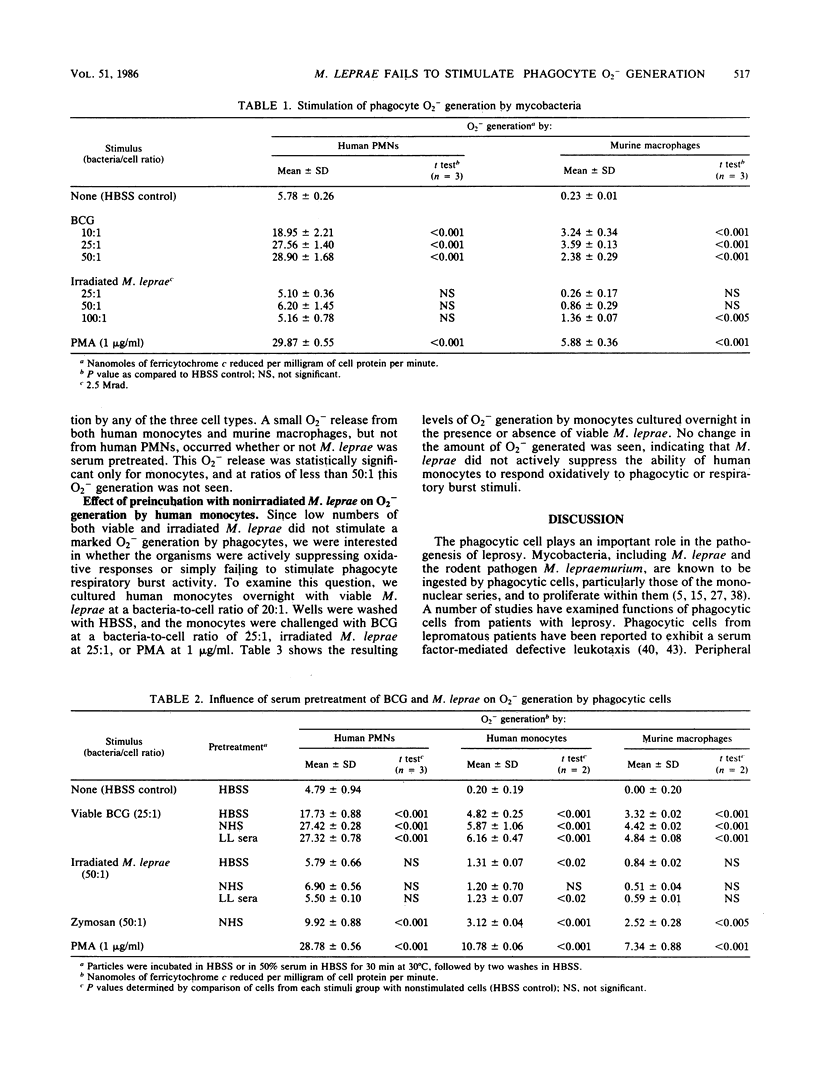
Abstract
Mycobacterium leprae is an intracellular pathogen that is ingested by and proliferates within cells of the monocyte/macrophage series. Mechanisms by which intracellular pathogens resist destruction may involve failure to elicit a phagocyte "respiratory burst" or resistance to toxic oxygen derivatives and lysosomal enzymes. We have studied the ability of M. leprae and Mycobacterium bovis BCG to stimulate the generation of superoxide anion (O2-) in vitro by human blood neutrophils and monocytes and murine peritoneal macrophages. M. leprae bacteria failed to stimulate significant O2- release except at high bacteria-to-cell ratios (greater than 50:1) whether or not they were pretreated with normal serum or serum from patients with lepromatous leprosy. Either viable or irradiated BCG; on the other hand, stimulated the three cell types to release significant amounts of O2- when challenged with as few as 10 organisms per cell. Serum pretreatment enhanced the release of O2- by the three cell types. Preincubation for 18 h with viable M. leprae did not inhibit the ability of monocytes to respond with an oxidative burst to phagocytic stimuli. The failure of M. leprae to stimulate phagocyte O2- generation may be an important factor in its pathogenicity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando M., Suga M., Sugimoto M., Tokuomi H. Superoxide production in pulmonary alveolar macrophages and killing of BCG by the superoxide-generating system with or without catalase. Infect Immun. 1979 May;24(2):404–410. doi: 10.1128/iai.24.2.404-410.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Mehra V. Immunological unresponsiveness in leprosy. Immunol Rev. 1984 Aug;80:5–28. doi: 10.1111/j.1600-065x.1984.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Dhople A. M. Effect of lysozyme on Mycobacterium leprae. Lepr India. 1983 Jul;55(3):455–464. [PubMed] [Google Scholar]
- Drutz D. J., Cline M. J., Levy L. Leukocyte antimicrobial function in patients with leprosy. J Clin Invest. 1974 Feb;53(2):380–386. doi: 10.1172/JCI107570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez M. E., Sen L., Vadez R., Baliña L. M. Defective blood mononuclear phagocyte function in patients with leprosy. Int J Lepr Other Mycobact Dis. 1979 Dec;47(4):575–579. [PubMed] [Google Scholar]
- Gangadharam P. R., Pratt P. E. Susceptibility of Mycobacterium intracellulare to hydrogen peroxide. Am Rev Respir Dis. 1984 Aug;130(2):309–311. doi: 10.1164/arrd.1984.130.2.309. [DOI] [PubMed] [Google Scholar]
- Goihman-Yahr M., Rodriguez-Ochoa G., Aranzazu N., Pinardi M. E., de Gomez M. E., Ocanto A., Convit J. In vitro activation of neutrophils by suspensions of Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1979 Dec;47(4):570–574. [PubMed] [Google Scholar]
- Ha D. K., Gardner I. D., Lawton J. W. Macrophage function in Mycobacterium lepraemurium infection: morphological and functional changes of peritoneal and splenic macrophages in vitro. Infect Immun. 1983 Jan;39(1):353–361. doi: 10.1128/iai.39.1.353-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haregewoin A., Godal T., Mustafa A. S., Belehu A., Yemaneberhan T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature. 1983 May 26;303(5915):342–344. doi: 10.1038/303342a0. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Mustafa A. S., Helle I., Waters M. F., Leiker D. L., Godal T. Reversal by interleukin-2 of the T cell unresponsiveness of lepromatous leprosy to Mycobacterium leprae. Immunol Rev. 1984 Aug;80:77–86. doi: 10.1111/j.1600-065x.1984.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg H. The role of macrophages in the lymphoproliferative response to Mycobacterium leprae in vitro. Clin Exp Immunol. 1978 Oct;34(1):46–51. [PMC free article] [PubMed] [Google Scholar]
- Hoffenbach A., Lagrange P. H., Bach M. A. Deficit of interleukin 2 production associated with impaired T-cell proliferative responses in Mycobacterium lepraemurium infection. Infect Immun. 1983 Jan;39(1):109–116. doi: 10.1128/iai.39.1.109-116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffenbach A., Lagrange P. H., Bach M. A. Influence of dose and route of Mycobacterium lepraemurium inoculation on the production of interleukin 1 and interleukin 2 in C57BL/6 mice. Infect Immun. 1984 Jun;44(3):665–671. doi: 10.1128/iai.44.3.665-671.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Fujiwara T., Brennan P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J Biol Chem. 1982 Dec 25;257(24):15072–15078. [PubMed] [Google Scholar]
- Jackett P. S., Aber V. R., Lowrie D. B. Virulence and resistance to superoxide, low pH and hydrogen peroxide among strains of Mycobacterium tuberculosis. J Gen Microbiol. 1978 Jan;104(1):37–45. doi: 10.1099/00221287-104-1-37. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Shepard C. C. Toxic effect of the peroxidase-hydrogen peroxide-halide antimicrobial system on Mycobacterium leprae. Infect Immun. 1984 May;44(2):534–536. doi: 10.1128/iai.44.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefford M. J., Mackaness G. B. Suppression of immunity to Mycobacterium lepraemurium infection. Infect Immun. 1977 Nov;18(2):363–369. doi: 10.1128/iai.18.2.363-369.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. D., Kim W. S., Kim C. S., Good R. A., Park B. H. NBT responses of neutrophils and monocytes in leprosy. Int J Lepr Other Mycobact Dis. 1974 Apr-Jun;42(2):150–153. [PubMed] [Google Scholar]
- Mahadevan P. R., Antia N. H. Biochemical alteration in cells following phagocytosis of M. leprae--the consequence--a basic concept. Int J Lepr Other Mycobact Dis. 1980 Jun;48(2):167–171. [PubMed] [Google Scholar]
- Mor N. Intracellular location of Mycobacterium leprae in macrophages of normal and immune-deficient mice and effect of rifampin. Infect Immun. 1983 Nov;42(2):802–811. doi: 10.1128/iai.42.2.802-811.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y., Sakane T., Miyachi Y., Ozaki M. Oxygen metabolism in phagocytes of leprotic patients: enhanced endogenous superoxide dismutase activity and hydroxyl radical generation by clofazimine. J Clin Microbiol. 1984 Nov;20(5):837–842. doi: 10.1128/jcm.20.5.837-842.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Kaplan G., Levy E., Sarno E. N., Kushner P., Granelli-Piperno A., Vieira L., Colomer Gould V., Levis W., Steinman R. Defective gamma interferon production in leprosy. Reversal with antigen and interleukin 2. J Exp Med. 1983 Dec 1;158(6):2165–2170. doi: 10.1084/jem.158.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Resistance of various strains of mycobacteria to killing by activated macrophages in vivo. J Immunol. 1983 Sep;131(3):1452–1454. [PubMed] [Google Scholar]
- Pearson R. D., Harcus J. L., Symes P. H., Romito R., Donowitz G. R. Failure of the phagocytic oxidative response to protect human monocyte-derived macrophages from infection by Leishmania donovani. J Immunol. 1982 Sep;129(3):1282–1286. [PubMed] [Google Scholar]
- Preston P. M. Macrophages and protective immunity in Mycobacterium lepraemurium infections in a 'resistant' (C57Bl) and a 'susceptible' (BALB/c) mouse strain. Clin Exp Immunol. 1982 Feb;47(2):243–252. [PMC free article] [PubMed] [Google Scholar]
- Rojas-Espinosa O. Phagocytosis in leprosy. 2. Production of superoxide by circulating blood leukocytes from lepromatous patients. Int J Lepr Other Mycobact Dis. 1978 Jul-Dec;46(3-4):337–341. [PubMed] [Google Scholar]
- Salgame P. R., Birdi T. J., Mahadevan P. R., Antia N. H. Role of macrophages in defective cell mediated immunity in lepromatous leprosy. I. Factor(s) from macrophages affecting protein synthesis and lymphocyte transformation. Int J Lepr Other Mycobact Dis. 1980 Jun;48(2):172–177. [PubMed] [Google Scholar]
- Salgame P. R., Mahadevan P. R., Antia N. H. Mechanism of immunosuppression in leprosy: presence of suppressor factor(s) from macrophages of lepromatous patients. Infect Immun. 1983 Jun;40(3):1119–1126. doi: 10.1128/iai.40.3.1119-1126.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel D. R., Godal T., Myrvang B., Song Y. K. Behavior of Mycobacterium leprae in human macrophages in vitro. Infect Immun. 1973 Sep;8(3):446–449. doi: 10.1128/iai.8.3.446-449.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Sher R., Anderson R., Glover A., Wadee A. A. Polymorphonuclear cell function in the various polar types of leprosy and erythema nodosum leprosum. Infect Immun. 1978 Sep;21(3):959–965. doi: 10.1128/iai.21.3.959-965.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Kelso A., Louis J. A. Intracellular destruction of Leishmania tropica by macrophages activated with macrophage activating factor/interferon. Clin Exp Immunol. 1984 Jan;55(1):157–165. [PMC free article] [PubMed] [Google Scholar]
- Walker L., Lowrie D. B. Killing of Mycobacterium microti by immunologically activated macrophages. Nature. 1981 Sep 3;293(5827):69–71. doi: 10.1038/293069a0. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Goralnick S., Bullock W. E. Defective leukotaxis in patients with lepromatous leprosy. Int J Lepr Other Mycobact Dis. 1976 Jan-Jun;44(1-2):243–249. [PubMed] [Google Scholar]
- Watson S., Bullock W., Nelson K., Schauf V., Gelber R., Jacobson R. Interleukin 1 production by peripheral blood mononuclear cells from leprosy patients. Infect Immun. 1984 Sep;45(3):787–789. doi: 10.1128/iai.45.3.787-789.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler P. R., Gregory D. Superoxide dismutase, peroxidatic activity and catalase in Mycobacterium leprae purified from armadillo liver. J Gen Microbiol. 1980 Dec;121(2):457–464. doi: 10.1099/00221287-121-2-457. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Johnston R. B., Jr Dissociation of phagocytosis from stimulation of the oxidative metabolic burst in macrophages. J Exp Med. 1984 Feb 1;159(2):405–416. doi: 10.1084/jem.159.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]


