Abstract
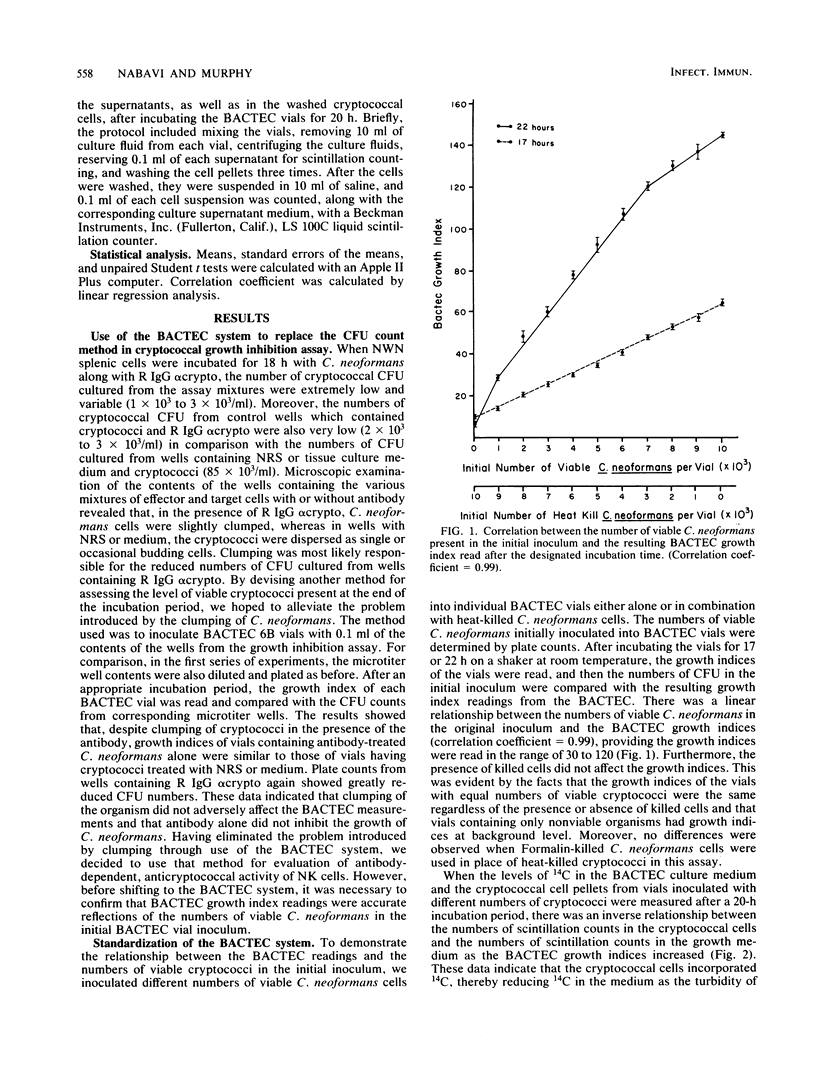
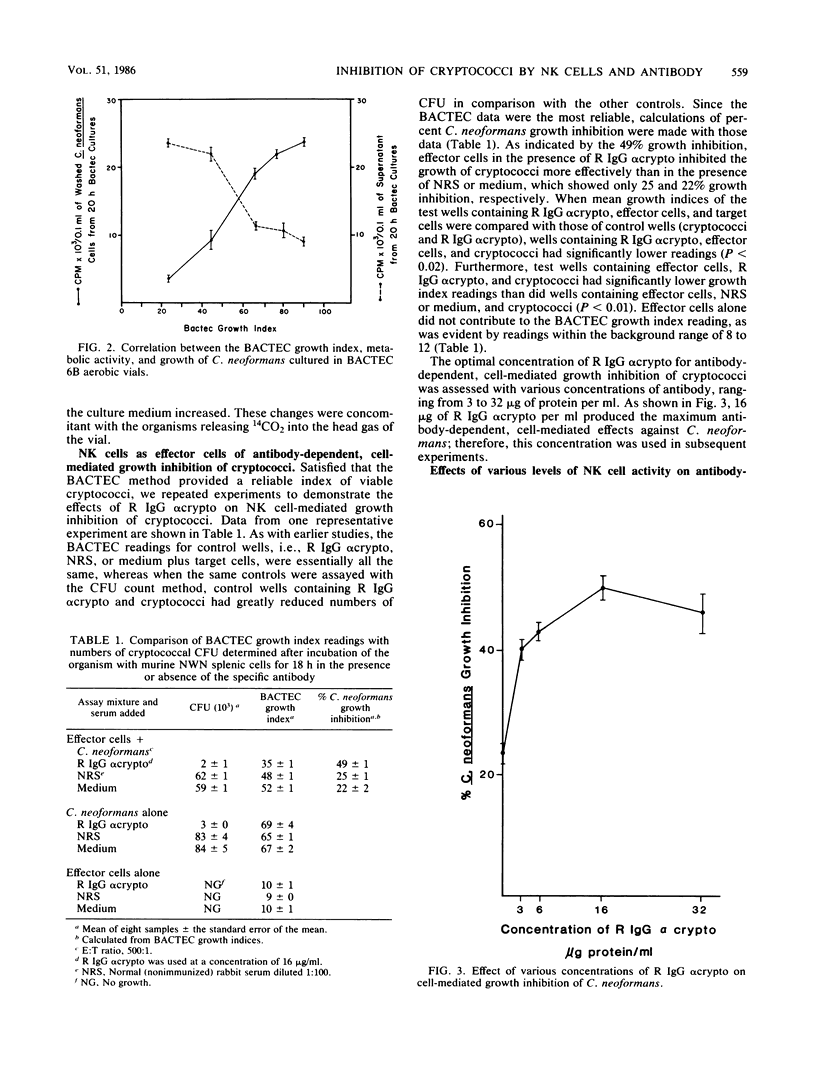
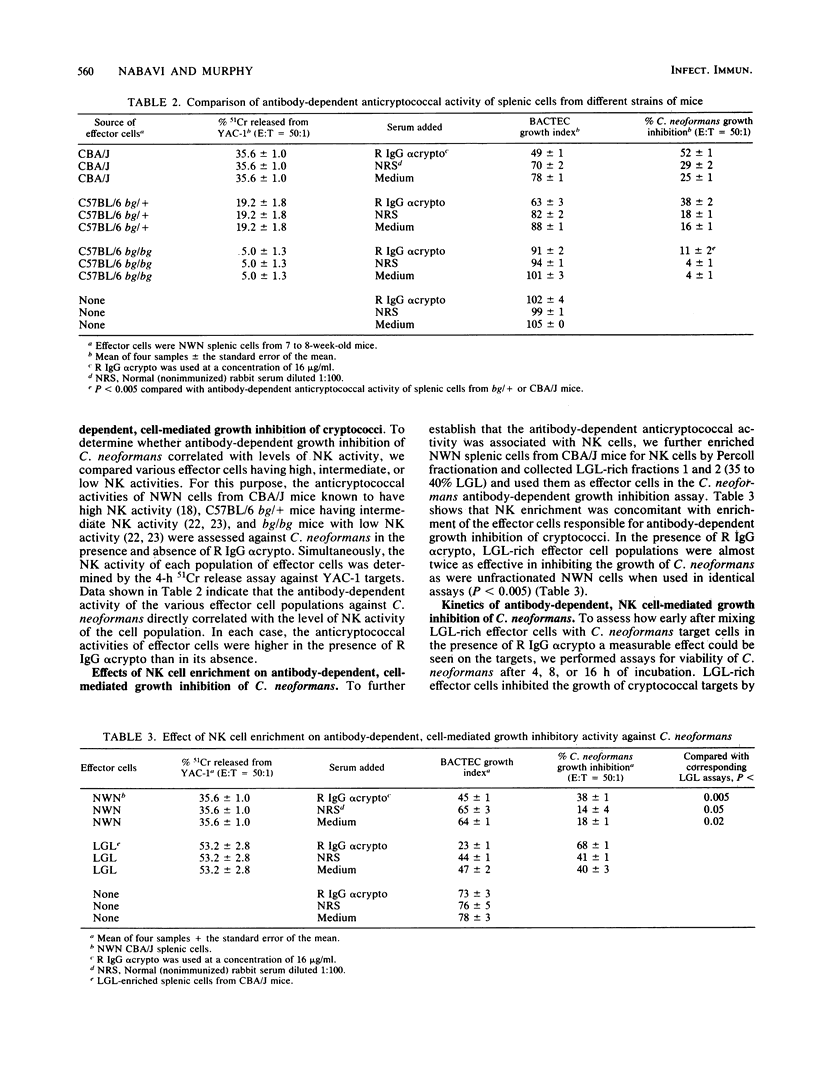
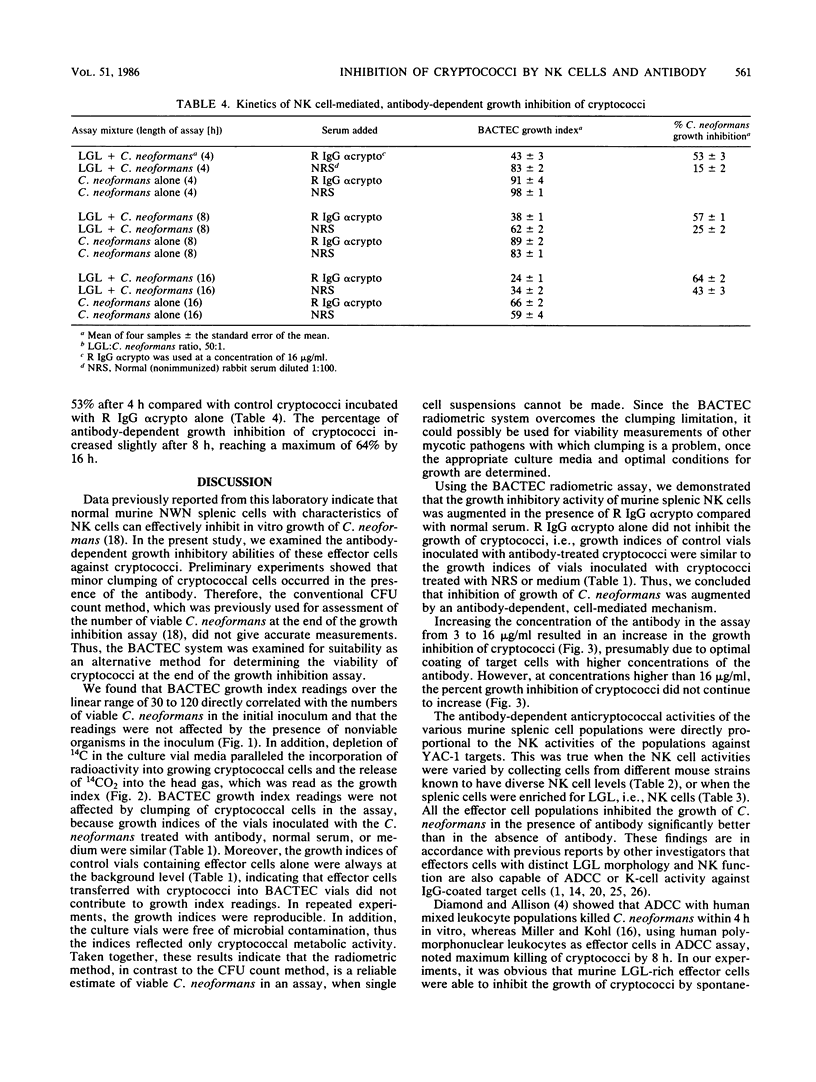
Previous data from this laboratory indicate that normal murine nylon wool nonadherent splenic cells with characteristics of natural killer (NK) cells effectively inhibit in vitro growth of Cryptococcus neoformans, a yeastlike pathogen. Since NK cells have been shown to be involved in antibody-dependent, cell-mediated cytotoxicity against immunoglobulin G (IgG)-coated tumor cells and xenogenic erythrocytes, we were interested in assessing the effects of the IgG fraction of rabbit anticryptococcal serum on NK cell-mediated inhibition of C. neoformans growth. Early in the study it became apparent that the conventional method of determining the numbers of CFU that was used previously for assessment of viable cryptococci at the end of the growth inhibition assay was not reliable for these studies, owing to minor clumping of the organisms in the presence of anticryptococcal antibody. Therefore, the BACTEC radiometric system was evaluated and determined to be a reliable replacement for the CFU count method. Using the BACTEC methodology, we showed that the anticryptococcal antibody significantly augmented the in vitro ability of NK cells to inhibit the growth of C. neoformans compared with normal rabbit serum or tissue culture medium. Furthermore, the antibody alone did not have an adverse effect on the organism, confirming that reduced growth indices obtained from test wells containing antibody, NK cells, and cryptococci were due to the effects of the NK cells. Maximum anticryptococcal activity of the NK cells was observed in the presence of 16 micrograms of IgG per ml; however, significant augmentation of anticryptococcal activity was seen with antibody concentrations as low as 3 micrograms/ml. Using different populations of murine splenic cells which had varying degrees of NK cell activity, we were able to show that NK cell activities, as determined by 51Cr release from YAC-1 targets, directly correlated with antibody-dependent, cell-mediated growth inhibition against cryptococci, suggesting that NK cells were effector cells in the antibody-dependent assays. Furthermore, in every case, the antibody-dependent activity of NK cells against C. neoformans was higher than the spontaneous activity of NK cells against the organism, emphasizing that NK cell activity against cryptococci can be augmented by specific antibody. When NK cell numbers were enriched by Percoll fractionation of nylon wool nonadherent splenic cells, antibody-dependent and spontaneous growth inhibitory activities of the effector cells were concomitantly augmented, confirming that NK cells were the effector cells in antibody-dependent growth inhibition of cryptococci.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Bradley T. P., Bonavida B. Mechanism of cell-mediated cytotoxicity at the single cell level. IV. Natural killing and antibody-dependent cellular cytotoxicity can be mediated by the same human effector cell as determined by the two-target conjugate assay. J Immunol. 1982 Nov;129(5):2260–2265. [PubMed] [Google Scholar]
- Diamond R. D., Allison A. C. Nature of the effector cells responsible for antibody-dependent cell-mediated killing of Cryptococcus neoformans. Infect Immun. 1976 Sep;14(3):716–720. doi: 10.1128/iai.14.3.716-720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D. Antibody-dependent killing of Cryptococcus neopormans by human peripheral blood mononuclear cells. Nature. 1974 Jan 18;247(5437):148–150. doi: 10.1038/247148a0. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974 Feb;80(2):176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Experimental murine cryptococcosis: effect of hyperimmunization to capsular polysaccharide. J Immunol. 1967 May;98(5):914–922. [PubMed] [Google Scholar]
- Graybill J. R., Hague M., Drutz D. J. Passive immunization in murine cryptococcosis. Sabouraudia. 1981 Dec;19(4):237–244. doi: 10.1080/00362178185380411. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Bartram S., Haskill J. S., Nunn M., Holden H. T., West W. H. Fc receptors on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1977 Jul;119(1):322–326. [PubMed] [Google Scholar]
- Kozel T. R., Follette J. L. Opsonization of encapsulated Cryptococcus neoformans by specific anticapsular antibody. Infect Immun. 1981 Mar;31(3):978–984. doi: 10.1128/iai.31.3.978-984.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Gotschlich E. C. The capsule of cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982 Oct;129(4):1675–1680. [PubMed] [Google Scholar]
- Kozel T. R., Highison B., Stratton C. J. Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect Immun. 1984 Feb;43(2):574–579. doi: 10.1128/iai.43.2.574-579.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Suzuki R., Hinuma S., Saitoh F. Studies of murine large granular lymphocytes. I. Identification as effector cells in NK and K cytotoxicities. J Immunol. 1982 Jul;129(1):388–394. [PubMed] [Google Scholar]
- Luini W., Boraschi D., Alberti S., Aleotti A., Tagliabue A. Morphological characterization of a cell population responsible for natural killer activity. Immunology. 1981 Aug;43(4):663–668. [PMC free article] [PubMed] [Google Scholar]
- Miller G. P., Kohl S. Antibody-dependent leukocyte killing of Cryptococcus neoformans. J Immunol. 1983 Sep;131(3):1455–1459. [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Nabavi N., Murphy J. W. In vitro binding of natural killer cells to Cryptococcus neoformans targets. Infect Immun. 1985 Oct;50(1):50–57. doi: 10.1128/iai.50.1.50-57.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo E., Wigzell H. Natural killer cells may be the only cells in normal mouse lymphoid cell populations endowed with cytolytic ability for antibody-coated tumour target cells. Scand J Immunol. 1978 Apr;7(4):297–306. doi: 10.1111/j.1365-3083.1978.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Perfect J. R., Lang S. D., Durack D. T. Influence of agglutinating antibody in experimental cryptococcal meningitis. Br J Exp Pathol. 1981 Dec;62(6):595–599. [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Lohmann-Matthes M. L., Domzig W., Wigzell H. The beige mutation in the mouse. II. Selectivity of the natural killer (NK) cell defect. J Immunol. 1979 Nov;123(5):2174–2181. [PubMed] [Google Scholar]
- Roder J. C. The beige mutation in the mouse. I. A stem cell predetermined impairment in natural killer cell function. J Immunol. 1979 Nov;123(5):2168–2173. [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J. E., Jones R. D. Serodiagnosis of clinical cryptococcosis. Am Rev Respir Dis. 1968 Feb;97(2):275–282. doi: 10.1164/arrd.1968.97.2.275. [DOI] [PubMed] [Google Scholar]
- Wåhlin B., Perlmann P. Characterization of human K cells by surface antigens and morphology at the single cell level. J Immunol. 1983 Nov;131(5):2340–2347. [PubMed] [Google Scholar]


