Abstract
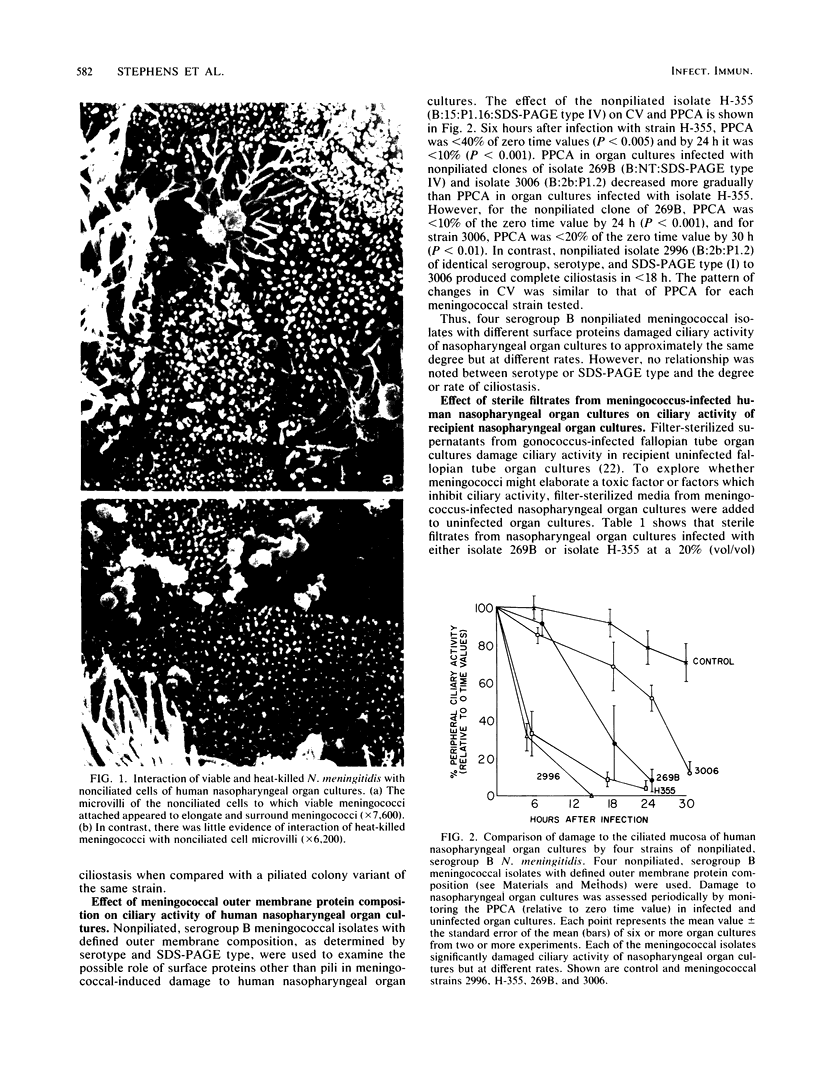
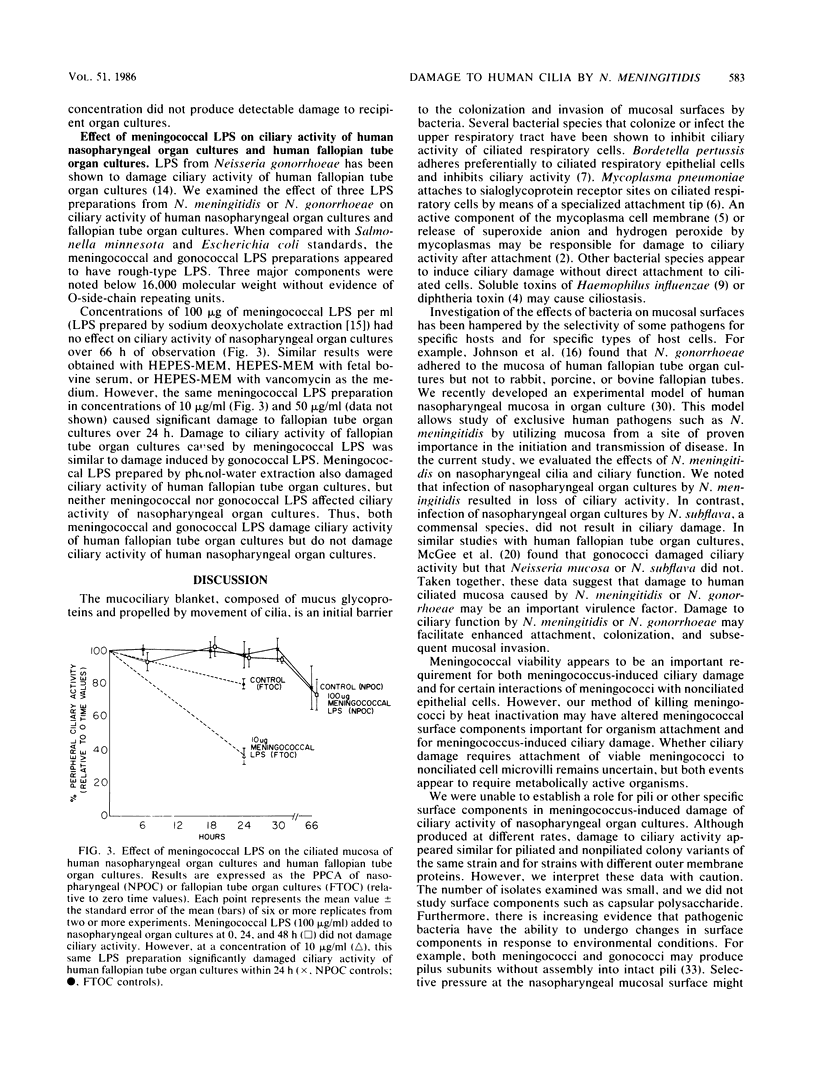
We used an in vitro model of human nasopharyngeal tissue in organ culture to evaluate the effects of Neisseria meningitidis on human cilia and ciliary function. Encapsulated, viable meningococci damaged ciliated epithelium of nasopharyngeal organ cultures, whereas Neisseria subflava, a commensal species, did not. Meningococcus-induced ciliary damage was due to loss of ciliated cells to which meningococci were not attached. Damage was seen with piliated and nonpiliated meningococci and did not appear to require the presence of other specific meningococcal surface proteins. Meningococcal viability was a requirement for both ciliary damage and interactions of meningococci with microvilli of nonciliated epithelial cells. That is, filter-sterilized supernatants from meningococcus-infected organ cultures, heat-killed meningococci at high inoculum, and purified meningococcal or gonococcal lipopolysaccharide at concentrations of 100 micrograms/ml did not damage ciliary activity of nasopharyngeal organ cultures. In contrast, meningococcal lipopolysaccharide at 10 micrograms/ml markedly damaged ciliary activity of human fallopian tube organ cultures, suggesting a selective toxicity of lipopolysaccharide for specific human ciliated cells. Damage to nasopharyngeal ciliated epithelium by N. meningitidis may be an important first step in meningococcal colonization of the human nasopharynx, but meningococcal lipopolysaccharide does not appear to be directly responsible for this toxicity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M. Y. Histology of the human nasopharyngeal mucosa. J Anat. 1965 Jul;99(Pt 3):657–672. [PMC free article] [PubMed] [Google Scholar]
- Almagor M., Kahane I., Yatziv S. Role of superoxide anion in host cell injury induced by mycoplasma pneumoniae infection. A study in normal and trisomy 21 cells. J Clin Invest. 1984 Mar;73(3):842–847. doi: 10.1172/JCI111279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Collier A. M. Diphtheria pathogenesis in Guinea pig tracheal organ culture. Infect Immun. 1974 Nov;10(5):1146–1151. doi: 10.1128/iai.10.5.1146-1151.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Barile M. F. Ciliostatic, hemagglutinating, and proteolytic activities in a cell extract of Mycoplasma pneumoniae. Infect Immun. 1980 Sep;29(3):1111–1116. doi: 10.1128/iai.29.3.1111-1116.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A. M. Attachment by mycoplasmas and its role in disease. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S685–S691. doi: 10.1093/clinids/5.supplement_4.s685. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Peterson L. P., Baseman J. B. Pathogenesis of infection with Bordetella pertussis in hamster tracheal organ culture. J Infect Dis. 1977 Aug;136 (Suppl):S196–S203. doi: 10.1093/infdis/136.supplement.s196. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Peppler M. S., Frasch C. E., Mocca L. F., McGrath P. P., Washington G. Adherence of isolates of Neisseria meningitidis from patients and carriers to human buccal epithelial cells. J Infect Dis. 1980 Oct;142(4):556–568. doi: 10.1093/infdis/142.4.556. [DOI] [PubMed] [Google Scholar]
- Denny F. W. Effect of a toxin produced by Haemophilus influenzae on ciliated respiratory epithelium. J Infect Dis. 1974 Feb;129(2):93–100. doi: 10.1093/infdis/129.2.93. [DOI] [PubMed] [Google Scholar]
- Frasch C. E., Zollinger W. D., Poolman J. T. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985 Jul-Aug;7(4):504–510. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- Goldman W. E., Klapper D. G., Baseman J. B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect Immun. 1982 May;36(2):782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C. R., Johnson A. P., Taylor-Robinson D., Melly M. A., McGee Z. A. Host species-specific damage to oviduct mucosa by Neisseria gonorrhoeae lipopolysaccharide. Infect Immun. 1981 Dec;34(3):1056–1058. doi: 10.1128/iai.34.3.1056-1058.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C. R., Melly M. A., Hellerqvist C. G., Coniglio J. G., McGee Z. A. Toxic activity of purified lipopolysaccharide of Neisseria gonorrhoeae for human fallopian tube mucosa. J Infect Dis. 1981 Mar;143(3):432–439. doi: 10.1093/infdis/143.3.432. [DOI] [PubMed] [Google Scholar]
- Hoff G. E., Frasch C. E. Outer membrane antigens of Neisseria meningitidis group B serotype 2 studied by crossed immunoelectrophoresis. Infect Immun. 1979 Sep;25(3):849–856. doi: 10.1128/iai.25.3.849-856.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. P., Taylor-Robinson D., McGee Z. A. Species specificity of attachment and damage to oviduct mucosa by Neisseria gonorrhoeae. Infect Immun. 1977 Dec;18(3):833–839. doi: 10.1128/iai.18.3.833-839.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- KAUHTIO J., RANTASALO I. Acute bacterial meningitis in children. I. A review of 257 cases admitted to Children's Hospital, University of Helsinki, in 1946-55. Ann Paediatr Fenn. 1958;4(1):42–48. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGee Z. A., Johnson A. P., Taylor-Robinson D. Human fallopian tubes in organ culture: preparation, maintenance, and quantitation of damage by pathogenic microorganisms. Infect Immun. 1976 Feb;13(2):608–618. doi: 10.1128/iai.13.2.608-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Johnson A. P., Taylor-Robinson D. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J Infect Dis. 1981 Mar;143(3):413–422. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- Melly M. A., Gregg C. R., McGee Z. A. Studies of toxicity of Neisseria gonorrhoeae for human fallopian tube mucosa. J Infect Dis. 1981 Mar;143(3):423–431. doi: 10.1093/infdis/143.3.423. [DOI] [PubMed] [Google Scholar]
- Melly M. A., McGee Z. A., Rosenthal R. S. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J Infect Dis. 1984 Mar;149(3):378–386. doi: 10.1093/infdis/149.3.378. [DOI] [PubMed] [Google Scholar]
- Morrison D. C. Bacterial endotoxins and pathogenesis. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S733–S747. doi: 10.1093/clinids/5.supplement_4.s733. [DOI] [PubMed] [Google Scholar]
- Peltola H. Meningococcal disease: still with us. Rev Infect Dis. 1983 Jan-Feb;5(1):71–91. doi: 10.1093/clinids/5.1.71. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Morton G. Adherence of Neisseria meningitidis to human epithelial cells. Infect Immun. 1981 Jan;31(1):430–435. doi: 10.1128/iai.31.1.430-435.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Van Melle E., Tomalty L. Experimental meningococcal infection in neonatal animals: models for mucosal invasiveness. Can J Microbiol. 1984 Aug;30(8):1022–1029. doi: 10.1139/m84-159. [DOI] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., Hoffman L. H., McGee Z. A. Interaction of Neisseria meningitidis with human nasopharyngeal mucosa: attachment and entry into columnar epithelial cells. J Infect Dis. 1983 Sep;148(3):369–376. doi: 10.1093/infdis/148.3.369. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A. Association of virulence of Neisseria meningitidis with transparent colony type and low-molecular-weight outer membrane proteins. J Infect Dis. 1983 Feb;147(2):282–292. doi: 10.1093/infdis/147.2.282. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A. Attachment of Neisseria meningitidis to human mucosal surfaces: influence of pili and type of receptor cell. J Infect Dis. 1981 Apr;143(4):525–532. doi: 10.1093/infdis/143.4.525. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., Whitney A. M., Rothbard J., Schoolnik G. K. Pili of Neisseria meningitidis. Analysis of structure and investigation of structural and antigenic relationships to gonococcal pili. J Exp Med. 1985 Jun 1;161(6):1539–1553. doi: 10.1084/jem.161.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]



